Abstract
Background
Retrospective studies and randomized controlled trials support the safety of laparoscopic complete mesocolic excision (CME) for the treatment of right-sided colon cancer (RSCC). Few studies, however, examine the learning curve of this operation and its impact on safety during an implementation period. We aim to evaluate the learning curve and safety of the implementation of laparoscopic CME with intracorporeal anastomosis for RSCC.
Methods
Consecutive patients undergoing a laparoscopic right colectomy with intracorporeal anastomosis for RSCC between January 2016 and June 2023 were included. Clinical, perioperative, and histopathological variables were collected. Correlation and cumulative sum (CUSUM) analyses between the operating time and case number were performed. Breakpoints of the learning curve were estimated using the broken-line model. CME and conventional laparoscopic right colectomy outcomes were compared after propensity score matching (PSM).
Results
Two hundred and ninety patients underwent laparoscopic right colectomy during study period. One hundred and eight met inclusion criteria. After PSM, 56 non-CME and 28 CME patients were compared. CME group had a non-statistically significant tendency to a longer operating time (201 versus 195 min; p = 0.657) and a shorter hospital stay (3 versus 4 days; p = 0.279). No significant differences were found in total complication rates or their profile. Correlation analysis identified a significant trend toward operating time reduction with increasing case numbers (Pearson correlation coefficient = − 0.624; p = 0.001). According to the CUSUM analysis, an institutional learning curve was deemed completed after 13 cases and the broken-line model identified three phases: learning (1–6 cases), consolidation (7–13 cases), and mastery (after 13 cases).
Conclusion
The learning curve of laparoscopic CME for RSCC can be achieved after 13 cases in centers with experience in advanced laparoscopic surgery and surgeons with familiarity with this technique. Its implementation within this setting seems to be as safe as performing a conventional right colectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Complete mesocolic excision (CME) has been popularized worldwide since its description by Höhenberger et al. in 2009 [1]. Since then, retrospective studies have consistently reported a better overall and disease-free survival in favor of this technique over conventional surgery for right-sided colon cancer [2, 3]. Moreover, recent randomized studies have also confirmed its feasibility and safety compared to conventional resections [4, 5]. Nevertheless, the dissemination of CME has been hindered due to its technical difficulty.
CME involves a careful dissection of major vascular structures such as the superior mesenteric vessel which has been a major limitation to its widespread dissemination in the minimally invasive era. Additionally, the vascular anatomy of the right and transverse colon is complex and highly variable [6]. Although concerning rates of intraoperative vascular injuries were described initially, recent systematic reviews and meta-analyses of prospective studies now highlight the strong safety profile of CME when performed by experienced groups [3, 7, 8].
The safety of CME in the context of its implementation, however, has not been extensively evaluated in the literature. Furthermore, scarce publications evaluate its learning curve [9, 10]. Hence, the objective of this study is to evaluate the learning curve of laparoscopic CME for right-sided colon cancer and to evaluate its safety during an implementation period.
Materials and methods
Study design
A retrospective cohort study was conducted. Consecutive patients undergoing a laparoscopic resection for right hemicolectomy between January 2016 and June 2023 were included. Patients who underwent surgery for a non-neoplastic surgical indication, had distal transverse colon tumors, or an extracorporeal anastomosis were excluded. Demographic, clinical, perioperative, and histopathological variables were collected, Patients were then divided into two groups according to the surgical technique (CME and non-CME). Learning curves were obtained using the operating time and CUSUM plot analyses. Given the retrospective nature of this study, to ensure comparability between groups by reducing selection bias, a propensity score matching (PSM) was performed.
Definitions and outcomes of interest
Comorbidities were recorded individually and by calculating the Charlson comorbidity index [11]. Perioperative complications, reoperation, unplanned readmissions, and mortality were collected up to 30 days postoperatively; postoperative complications were classified according to the Clavien–Dindo classification [12]. Severe complications were defined as any Clavien–Dindo III or above complication. Ileus was defined as a functional obstruction of the gastrointestinal tract, accompanied by abdominal distension, nausea, and vomiting, requiring a nasogastric tube insertion for more than 24 h postoperatively. Anastomotic leak was defined as clinical or radiologic evidence of a defect of the intestinal wall at the anastomosis communicating the intra- and extra-luminal compartments. Histopathological data were recorded according to the TNM classification (AJCC 8th Edition for Cancer Staging) [13]. Conventional right hemicolectomy is defined as standard D2 lymph nodes dissection.
Team and procedure
All surgeries were performed by two senior attending colorectal surgeons or by a surgical resident under direct supervision. Each senior surgeon had prior training in advanced colorectal laparoscopic surgery, having completed a minimum of 500 procedures each. One surgeon had previous exposure to 5 open and 30 robotic CME cases during an overseas fellowship and assisted in the initial 5 procedures performed by the other attending surgeon.
All patients underwent mechanical bowel preparation with oral antibiotics before surgery. Preoperative imaging was carefully reviewed to elucidate the vascular anatomy of the patient. The initial approach was the same for every case: pneumoperitoneum up to 15 mmHg was established using a Veress needle in a peri-umbilical position. A four-trocar technique was employed with the trocar location as follows: peri-umbilical, left upper quadrant, left iliac fossa, and right iliac fossa. For extended right colectomies, an additional right upper quadrant trocar was utilized.
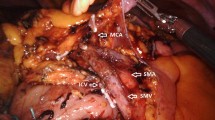
Patients were positioned in a slight Trendelenburg position with left lateralization. The major omentum was reflected above the transverse colon and the small bowel lateralized to the left to expose the right mesocolon. From there, an inferior approach was initiated, identifying the duodenum and the uncinate process of the pancreas. This procedure allowed a clear identification of the mesenteric window in most cases. Subsequently, the superior mesenteric vein (SMV) was exposed below the ileocolic vessels. The ileocolic vessels were then dissected and centrally ligated (Fig. 1). Dissection proceeded on the anterior surface of the SMV. In the rare presence of a right colic artery, it was ligated and divided. Dissection then progressed toward the gastrocolic trunk identifying, ligating, and dividing the superior right colic vein and any accessory colonic branches. Gastric and pancreatic branches stemming from the gastrocolic trunk were identified and preserved. The right branch of the middle colic artery was ligated and divided. For extended right colectomies, a central ligation of the middle colic artery and vein was performed. Additionally, the omentum was resected preserving the gastroepiploic arcade and infrapancreatic lymph nodes were also resected. After achieving vascular control, the colonic mobilization was completed with a medial to lateral approach.
Operative field during complete mesocolic excision for right side colon cancer. Duo Duodenum, UP uncinate process of the pancreas, ICV ileocolic vessels stump, SMV Superior mesenteric vein, GCT gastrocolic trunk MCV middle colic vessels stump, TC transverse colon, RCV Right colic vein, RBMCA right branch or middle colic artery
The extraction of the specimen was performed through a Pfannenstiel incision. An isoperistaltic latero-lateral intracorporeal ileocolonic anastomosis was performed utilizing stay sutures, enterotomies and a 60-mm laparoscopic linear stapler. Enterotomies were closed with a running 3–0 absorbable suture (barbed or not). Mesenteric defects were not routinely closed.
Statistical analysis
An institutional learning curve analysis was planned. Operating time, defined as the time elapsed between the skin incision and closure, was chosen as the primary outcome for assessing the LC. The relationship between operating time and cumulative cases was primarily evaluated using a linear regression model. Combined procedures such as concurrent liver resection or cholecystectomy were excluded from the LC analysis. To evaluate the progression of the LC, a cumulative sum analysis (CUSUM) was performed. All surgical procedures were sequenced chronologically from the earliest to the most recent. The CUSUM value is established as the difference between any case (Xi) and mean surgical time of the series (µ) and is represented by the subsequent equation:
Afterward, a CUSUM curve was plotted and analyzed employing the broken-linemodel to identify breakpoints defining distinct phases within the learning curve [14]. Confidence intervals of 95% were calculated for these breakpoints.
Moreover, to compare CME with standard resections, a PSM was performed utilizing age, sex, the Charlson comorbidity index, extent of resection, and pTNM stage. The nearest-neighbor matching method with a 2:1 ratio was used to select cases. Normal distribution was assessed using the Shapiro–Wilk test. Categorical variables were summarized using relative frequency and percentage; median and interquartile range or mean and standard deviation were used for numerical values. Then, the groups were compared using Mann–Whitney for continuous variables and Fisher exact test for categorical variables. A two-sided p < 0.05 indicated statistical significance.
Ethics
A local institutional ethics committee approval was obtained to conduct this study (ID 230716001).
Results
Demographic and baseline variables
Two hundred and ninety consecutive patients underwent laparoscopic right hemicolectomy between January 2016 and June 2023. Of these, 108 meet the inclusion criteria. The non-CME group consisted of 80 patients operated between 2016 and June 2023, while CME group consisted of 28 patients operated between May 2022 and June 2023. PSM resulted in 56 non-CME patients matched to 28 CME patients. Baseline characteristic of these patients are shown in Table 1.
After PSM, clinically relevant demographic and pathologic characteristic were similar. There was no significant difference between age (p = 0.856), sex (p = 0.938), the Charlson comorbidity index (p = 0.872), or the extent of resection (p = 1.00). An initial non-significant disparity in cancer staging was optimized (p = 0.959). The preoperative CEA levels were statistically, but not clinically significantly different between groups (2.4 vs 3.1; p = 0.03). An extended right colectomy was performed in 17.9% of patients in both groups (p = 1.00). Other variables as prior abdominal surgery (p = 0.817) and BMI (p = 0.563) were also similar.
Perioperative outcomes
Perioperative outcomes are summarized in Table 2. Operative time tended to be longer and the length of stay tended to be shorter in the CME group, but these differences were not statistically significant. Overall complication rates tended to be more frequent in the CME group (32.1% vs 26.8%; p = 0.081); however, severe complications tended to be less frequent in CME group (3.6% vs 5.4%; p = 1.00). No differences were found in other outcomes, such as mortality, reoperation, or readmission rates between groups.
Learning curves
Four cases were excluded from the learning curve analysis because they were associated to other procedures (combined), and the operating time of CME could not be assessed (two patients had a cholecystectomy, one patient had a hernia repair and another one had an en bloc splenectomy). Our correlation analysis revealed that operating time decreased significantly with the increasing number of cases (Fig. 2) (p = 0.001). Then, a CUSUM analysis and broken-line model identified two cut-off points: at case 6 [95% CI (4.6–7.8)] and 13 [95% CI (10.8–15.2)]. This allowed for the identification of three periods in our learning curve: learning phase (1–6), consolidation phase (7–13), and mastery phase (after 14 cases) as shown in Fig. 3.
Discussion
Our findings indicate that the institutional learning curve of laparoscopic CME for right-sided colon cancer can be achieved after 13 cases when members of the team have had prior exposure to CME. Furthermore, this study suggests that the implementation of CME is safe when performed by surgeons with experience in complex minimally invasive colorectal surgery.
Existing literature suggests that the completion of the learning curve of a laparoscopic right colectomy with CME can be achieved after 24–33 consecutive cases [9, 10]. Recently, Giani et al. conducted a multidimensional evaluation of the learning curve of three surgeons performing a laparoscopic right colectomy with CME [9]. They reported a median operative time of 195 min, a median lymph node harvest of 23 lymph nodes, and an overall complication rate of 22%. Interestingly, their definition of learning curve completion did not rely in operating time, but rather on a risk-adjusted CUSUM (RA-CUSUM) analysis of a composite outcome of surgical success or failure. They defined surgical failure as the occurrence of at least one of the following: conversion, prolonged operative time, reoperation, major complication, prolonged LOS, death, readmission, inadequate lymph node sampling, or positive margin. The RA-CUSUM included an adjustment based on an estimated probability of surgical failure (depending on preoperative patients’ factors). Although the interpretation of results could be less intuitive in this scenario, the assessment of proficiency takes into account different levels of the surgical procedure. For the different surgeons evaluated in this study, operative time CUSUM analyses showed a peak at cases number 27, 26, and 17, while the RA-CUSUM analyses determined peaks at cases number 24, 33, and 22. Hence, the authors suggested 24 to 34 cases as the range of cumulative cases in which surgeons can achieve proficiency in laparoscopic CME for right colon cancer. In another two-center retrospective study, Cuk et al. employed an operating time CUSUM analysis to assess the learning curve of laparoscopic CME for right-sided resections [10]. They used the CUSUM analysis to determine peak in operative time which was used to conduct a comparison between pre-peak and post-peak perioperative outcomes, finding a significant decrease in the mean operative time (217.2 vs 191.6 min; p = 0.05) and a non-statistically significant reduction in severe surgical complications (defined as Clavien–Dindo ≥ 3) (13% vs 7%; p = 0.67). These post-peak results include an overall complication rate of 26% and a mean operating time of 191 min which are similar to our findings. They identified the peak in operative time at the 32nd case, leading them to conclude that this number of cases is required to reach the plateau phase in the learning curve.
Discrepancies between our results and the existing literature can be attributed to several factors. First, the definition of a learning curve completeness can differ across studies. As pointed earlier, previous studies do not use a homogeneous methodology. Learning curve completeness interpretations are based on the peak of the CUSUM curve considering operating times or composite outcomes. Although no specific methodology is universally accepted to evaluate learning curves, we aimed to determine this cut-off point using the broken-line model, which is a technique that has been employed in learning curve analyses in other studies [15]. Secondly, there might be significant variability among the surgeons performing the procedures and their degree of prior exposure to CME across different reports. In our study, the participants were consultant colorectal surgeons with an extensive experience in advanced laparoscopic colorectal surgery. Each had completed an intracorporeal anastomosis learning curve [16], but only one was familiar with the CME technique. Conversely, Giani et al. included three surgeons characterized as having “some degree of experience in minimally invasive colorectal surgery” with 34,110 and 95 previous cases, respectively. Cuk et al. analyzed “certified colorectal and experienced laparoscopic surgeons” each having completed a training program specific to CME surgery. Thirdly, we proposed the analysis of an institutional rather than an individual learning curve. We posit that this more accurately represents the learning process of the whole surgical team and not just the individual surgeon. As discussed in other studies, surgeons may have participated as an assistant in the same procedure, which could subsequently influence their learning curve. Which metrics better represent proficiency or whether learning curves should be assessed at individual or institutional levels remains a debatable matter. Nevertheless, all efforts contribute to a better understanding of this phenomenon and should be considered when examining the various aspects of a learning curve.
For right colon tumors, a D3 dissection involves the clearance of lymphatic tissue overlying the superior mesenteric vessels, which is considered a challenging territory for most colorectal surgeons. This technical aspect becomes relevant since some studies suggest a survival benefit of CME over conventional surgery at the expense of higher vascular morbidity rates [17]. Therefore, in the era of minimally invasive surgery, there has been reservation with regards to performing radical lymphatic clearance due to the perceived increased risk in high body mass index Western patients [18]. Nevertheless, recent reports from randomized clinical trials demonstrate no increase of intraoperative and postoperative surgical complications [4, 5, 19]. Our findings are consistent with previous reports indicating that when CME is executed by experienced laparoscopic colorectal surgeons it is as safe as performing a conventional resection (even during an implementation period).
Several limitations of this study need to be considered. Its retrospective nature may induce bias. Furthermore, the small sample size may potentially compromise the statistical power of our findings. Additionally, our decision to describe an institutional learning curve can also limit the generalizability of the outcomes and conclusions, when compared to individuals’ learning curves. Finally, relying solely on surgical time as a surrogate of a learning curve may be restrictive. Potentially, with increasing numbers more comprehensive methodologies can be applied to elucidate both individual and institutional realities regarding the learning process.
A more widespread adoption of minimally invasive CME may happen with evolving technology. While our findings, along with prior studies, have demonstrated the safety of this technique in the laparoscopic setting, the safety of a robotic approach to CME has concurrently been confirmed in a recent meta-analysis of retrospective studies [8]. Robotic surgery provides a stable three-dimensional, surgeon-controlled laparoscope and wristed instruments. These features allow for a more precise dissection along vascular structures which may facilitate the performance of these operations [20, 21]. However, the impact of these theoretical benefits is yet to be demonstrated on learning curves and objective evaluations of surgeons performing these complex procedures. Hence, future research should also focus on comparing the learning curve of robotic and laparoscopic approaches to CME. Finally, training within workshops, simulation models (utilizing cadaveric or synthetic tissues) and fellowships should also be investigated. Strategies to shorten learning curves and enhance the surgeons’ confidence and competencies related to vascular dissections in this context will be required before expecting a more widespread dissemination of CME.
Conclusion
The learning curve of laparoscopic CME can be achieved after 13 cases in centers with experience in advanced laparoscopic surgery and when surgeons have had exposure to this technique. Additionally, the implementation of CME within this setting is as safe as performing a conventional right colectomy. Further studies will be required to confirm these results.
References
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 11:354–364. https://doi.org/10.1111/J.1463-1318.2008.01735.X
De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara G, Toniato A, Pilati P, Franzato B (2021) Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:881–892. https://doi.org/10.1007/S00384-020-03797-3
Kong JC, Prabhakaran S, Choy KT, Larach JT, Heriot A, Warrier SK (2021) Oncological reasons for performing a complete mesocolic excision: a systematic review and meta-analysis. ANZ J Surg 91:124–131. https://doi.org/10.1111/ans.16518
Gao Z, Wang C, Cui Y, Shen Z, Jiang K, Shen D, Wang Y, Zhan S, Guo P, Yang X, Liu F, Shen K, Liang B, Yin M, Xie Q, Wang Y, Wang S, Ye Y (2020) Efficacy and safety of complete mesocolic excision in patients with colon cancer: three-year results from a prospective, nonrandomized, double-blind, controlled trial. Ann Surg 271:519–526. https://doi.org/10.1097/SLA.0000000000003012
Di Buono G, Buscemi S, Cocorullo G, Sorce V, Amato G, Bonventre G, Maienza E, Galia M, Gulotta L, Romano G, Agrusa A (2021) Feasibility and safety of laparoscopic complete mesocolic excision (CME) for right-sided colon cancer: short-term outcomes. A Randomized Clinical Study. Ann Surg 274:57–62. https://doi.org/10.1097/SLA.0000000000004557
Kuzu MA, Ismail E, Çelik S, Şahin MF, Güner MA, Hohenberger W, Açar HI (2017) Variations in the vascular anatomy of the right colon and implications for right-sided colon surgery. Dis Colon Rectum 60:290–298. https://doi.org/10.1097/DCR.0000000000000777
Emmanuel A, Haji A (2016) Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis 31:797–804. https://doi.org/10.1007/S00384-016-2502-0
Xu J, Mohan HM, Fleming C, Larach JT, Apte SS, Cohen LCL, Miskovic D, Jiang W, Heriot AG, Warrier SK (2023) Complete mesocolic excision versus standard resection for colon cancer: a systematic review and meta-analysis of perioperative safety and an evaluation of the use of a robotic approach. Tech Coloproctol. https://doi.org/10.1007/S10151-023-02838-7/METRICS
Giani A, Veronesi V, Bertoglio CL, Mazzola M, Bernasconi DP, Grimaldi S, Gualtierotti M, Magistro C, Ferrari G (2022) Multidimensional evaluation of the learning curve for laparoscopic complete mesocolic excision for right colon cancer: a risk-adjusted cumulative summation analysis. Colorectal Dis 24:577–586. https://doi.org/10.1111/CODI.16075
Cuk P, Simonsen RM, Sherzai S, Buchbjerg T, Andersen PV, Salomon S, Pietersen PI, Möller S, Al-Najami I, Ellebæk MB (2023) Surgical efficacy and learning curves of laparoscopic complete mesocolic excision with intracorporeal anastomosis for right-sided colon cancer: a retrospective two-center cohort study. J Surg Oncol 127:1152–1159. https://doi.org/10.1002/JSO.27230
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Brierley JD Gospodarowicz MK, Wittekind C (2017) TNM Classification of Malignant Tumours, 8th edn due December 2016. Union for International Cancer Control pp 1–272
Muggeo VMR (2003) Estimating regression models with unknown break-points. Stat Med 22:3055–3071. https://doi.org/10.1002/SIM.1545
Broering DC, Berardi G, El Sheikh Y, Spagnoli A, Troisi RI (2020) Learning curve under proctorship of pure laparoscopic living donor left lateral sectionectomy for pediatric transplantation. Ann Surg 271:542–548. https://doi.org/10.1097/SLA.0000000000002948
Jarry C, Cárcamo L, González JJ, Bellolio F, Miguieles R, Urrejola G, Zúñiga A, Crovari F, Molina ME, Larach JT (2021) Implementation of intracorporeal anastomosis in laparoscopic right colectomy is safe and associated with a shorter hospital stay. Updates Surg 73:93–100. https://doi.org/10.1007/s13304-020-00840-4
Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y (2017) Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis 19:962–972. https://doi.org/10.1111/codi.13900
Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gögenur I (2016) Short-term outcomes after complete mesocolic excision compared with “conventional” colonic cancer surgery. Br J Surg 103:581–589. https://doi.org/10.1002/bjs.10083
Xu L, Su X, He Z, Zhang C, Lu J, Zhang G, Sun Y, Du X, Chi P, Wang Z, Zhong M, Wu A, Zhu A, Li F, Xu J, Kang L, Suo J, Deng H, Ye Y, Ding K, Xu T, Zhang Z, Zheng M, Xiao Y, Chen L, Feng B, Zang L, Ma J, Feng Y, Ji D, He C, Fu Z, Huang Y, Jiang W, Wu Q, Yu M, Chen P, Guan W, Wu B, Li A, He G, He X, Wang D, Wang Y, Shen K, Lin G, Yao H, Qiu H, Liang Z, Zhou W, Xue H, Li B (2021) Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol 22:391–401. https://doi.org/10.1016/S1470-2045(20)30685-9
Yozgatli TK, Aytac E, Ozben V, Bayram O, Gurbuz B, Baca B, Balik E, Hamzaoglu I, Karahasanoglu T, Bugra D (2019) Robotic complete mesocolic excision versus conventional laparoscopic hemicolectomy for right-sided colon cancer. J Laparoendosc Adv Surg Tech 29:671–676. https://doi.org/10.1089/lap.2018.0348
Spinoglio G, Bianchi PP, Marano A, Priora F, Lenti LM, Ravazzoni F, Petz W, Borin S, Ribero D, Formisano G, Bertani E (2018) Robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol 25:3580–3586. https://doi.org/10.1245/s10434-018-6752-7
Funding
None reported.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Javier Vela, Christophe Riquoir, Cristián Jarry, Felipe Silva, Nicolás Besser, Gonzalo Urrejola, María Elena Molina, Rodrigo Miguieles, Felipe Bellolio, and José Tomás Larach have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vela, J., Riquoir, C., Jarry, C. et al. Learning curve and safety of the implementation of laparoscopic complete mesocolic excision with intracorporeal anastomosis for right-sided colon cancer: results from a propensity score-matched study. Surg Endosc 38, 5114–5121 (2024). https://doi.org/10.1007/s00464-024-11086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-024-11086-1