Abstract
Background
New York University Langone Health has three accredited bariatric centers, with altogether ten different bariatric surgeons. This retrospective analysis compares individual surgeon techniques in laparoscopic or robotic Roux-en-Y gastric bypass (RYGB) to identify potential associations with perioperative morbidity and mortality.
Methods
All adult patients who underwent RYGB between 2017 and 2021 at NYU Langone Health campuses were evaluated via electronic medical records and MBSAQIP 30-day follow-up data. We surveyed all ten practicing bariatric surgeons to analyze the relationship between their techniques and total adverse outcomes. Bleeding, SSI, mortality, readmission, and reoperation were specifically sub-analyzed via logistic regression.
Results
54 (7.59%) out of 711 patients who underwent laparoscopic or robotic RYGB encountered an adverse outcome. Lower adverse outcomes were observed with laparoscopic approach, creating the JJ anastomosis first, flat positioning, division of the mesentery, Covidien™ laparoscopic staplers, gold staples, unidirectional JJ anastomosis, hand-sewn common enterotomy, 100-cm Roux limb, 50-cm biliopancreatic limb, and routine EGD. Lower bleeding rates were observed with flat positioning, gold staples, hand-sewn common enterotomy, 50-cm biliopancreatic limb, and routine EGD. Lower readmission rates were observed in laparoscopic, flat positioning, Covidien™ staplers, unidirectional JJ anastomosis, and hand-sewn common enterotomy. Gold staples had lower reoperation rates. Otherwise, there was no statistically significant difference in SSI.
Conclusion
Certain surgical techniques in RYGB within our bariatric surgery group had significant effects on the rates of total adverse outcomes, bleeding, readmission, and reoperation. Our findings warrant further investigation into the aforementioned techniques via multivariate regression models or prospective study design.
Limitations
This study was limited by the inherent nature of its retrospective and univariate statistical design. We did not account for the interaction between techniques. The sample size of surgeons was small, and follow-up of 30 days was relatively short. We did not include patient characteristics in the model or control for surgeon skill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Roux-en-Y gastric bypass (RYGB) is regarded as a safe, effective weight loss surgery. RYGB promotes long-term weight loss as well as improvement in obesity-related comorbidities. However, several cross-sectional studies demonstrate huge variability in technique and perioperative practices for RYGB [1, 2].
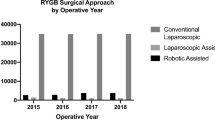
There is wide variability in almost every step of RYGB. To begin, a growing number of surgeons have adopted the robotic platform, which provides an opportunity to compare outcomes between the laparoscopic and robotic approaches. Even in patient positioning, some surgeons opt for flat positioning while others prefer reverse Trendelenburg. Stapler types, including height and color, widely vary among surgeons. The preoperative workup, specifically whether or not to routinely perform upper GI series or esophagogastroduodenoscopy is variable.
When it comes to intraoperative steps, there is no data on ordinality with performing either gastrojejunostomy or jejunojejunostomy anastomosis first, and routine division of mesentery remains highly variable among surgeons. In fact, even performing a unidirectional versus bidirectional jejunojejunostomy, and whether or not to sew or staple the common enterotomy differs between surgeons. The ideal length of Roux and biliopancreatic limbs remains in question. This variability is also observed within the group of bariatric surgeons at New York University (NYU). To date, there is minimal high-quality data on the impact of technique variability and outcomes on morbidity.
The goal of this retrospective study is to investigate different RYGB techniques and their potential associations with adverse outcomes, such as bleeding, surgical site infections, readmission, reoperation, and mortality. New York University (NYU) Langone Health has three accredited bariatric centers, with altogether ten different bariatric surgeons. As an accredited center, NYU Langone semi-annually reports complication data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) registry. Thus, the NYU Langone Health system provides a unique opportunity to study pre- and intraoperative techniques for RYGB and their impact on adverse events. To our knowledge, there is no other existing study that investigates perioperative outcomes and bariatric surgeon techniques using MBSAQIP data within the same health system (search strategy detailed in Appendix 1).
Methods
This is a retrospective study with data on all adult patients who underwent laparoscopic or robotic RYGB between 2017 and 2021 at the NYU Langone Health campuses. Data collected by each bariatric center at NYU Langone by direct chart review and submitted to the MBSAQIP registry, which in turn provides semi-annual reporting, comparing each of the NYU bariatric centers’ data for 30-day morbidity and mortality to outcomes of bariatric centers nationwide. Only first-time laparoscopic or robotic RYGB (not revisional) was included. Any prior bariatric surgery and other bypass variations were excluded. Total adverse outcomes included mortality, readmission, reoperation, and morbidity. Morbidity included bleeding, SSI, urinary tract infection, postoperative pneumonia, severe postoperative nausea and vomiting, deep vein thrombosis, and pulmonary embolism. We surveyed all 10 practicing bariatric surgeons regarding the following surgical techniques in RYGB, which are based on Delphi consensus statements on crucial technical steps [3]:
-
(1)
Surgical approach (laparoscopic vs. robotic vs. both)
-
(2)
Performing gastrojejunostomy (GJ) vs. jejunojejunostomy (JJ) anastomosis first
-
(3)
Patient positioning (flat, Trendelenburg, reverse Trendelenburg)
-
(4)
Division of mesentery (yes or no)
-
(5)
If yes to division of mesentery, what distance (does not apply vs. “x”-cm)
-
(6)
Type of stapler
-
(7)
Staple length
-
(8)
Staple color
-
(9)
Typical number of cartridges fired
-
(10)
Bi-directional or uni-directional jejunojejunostomy
-
(11)
Sewing vs. stapling the enterotomy for closure
-
(12)
Closure of the JJ mesenteric defect
-
(13)
Length of Roux (alimentary) limb
-
(14)
Length of biliopancreatic limb
-
(15)
Routine preoperative UGI series prior to bariatric surgery? (yes or no)
-
(16)
Routine preoperative EGD prior to bariatric surgery? (yes or no)
We analyzed the relationship between surgeon techniques and total adverse outcomes (including bleeding, surgical site infections (SSI), and any other morbidity, mortality, readmission, or reoperation). The incidence of any of the sub-outcomes is treated statistically as a “yes” occurrence for the total adverse outcomes. Complication data was collected at the surgeon level. Collected variables and surgeon characteristics, including total operations and adverse events, were obtained and anonymized in Table 1. Logistic regression was performed to analyze the impact of surgeon techniques on the risk of different adverse outcomes. There was no missing data for the covariates used in the data analysis. For the findings that were statistically significant in logistic regression models, a Pearson Chi-square test was used to confirm statistical significance between the two groups.
We also performed a comprehensive literature review on each of the techniques above (see Appendix 2–11).
Results
Data from 3815 adult patients were collected. Among them, 54 (7.59%) out of 711 patients who were included underwent laparoscopic or robotic RYGB and encountered an adverse outcome (Table 1).
Lower adverse event rates were observed in laparoscopic approach (vs. robotic), creating the JJ (vs. GJ first), flat positioning (vs. reverse Trendelenburg), routine division of the mesentery (vs. not), Covidien™ laparoscopic staplers (vs. Intuitive™ robotic), gold stapler loads (vs. blue/white), unidirectional JJ anastomosis (vs. bidirectional JJ), hand-sewn common enterotomy (vs. stapled), 100-cm Roux limb, 50-cm biliopancreatic limb and routine EGD.
Lower readmission rates were observed in flat positioning (vs. reverse Trendelenburg), gold stapler loads (vs. white robotic or tan laparoscopic), hand-sewn common enterotomy (vs. stapled), and routine EGD and less bleeding events. Additionally, laparoscopic (vs. robotic), flat (vs. reverse Trendelenburg), Covidien™ (vs. Intuitive™ robotic), unidirectional JJ anastomosis (vs. bidirectional), 50-cm biliopancreatic limb, and hand-sewn common enterotomy (vs. stapled) were associated with lower readmission rates.
Lower reoperation rates were observed with gold stapler loads versus (purple/tan; blue/white). All statistically significant outcomes were documented in Tables 2, 3, 4 and 5. Otherwise, there were no statistically significant differences in SSI among the surgical techniques (Supplementary Material). It was not feasible to test for mortality given the low incidence.
Discussion
We found that RYGB could be safely performed in various ways. While there may be some differences between certain techniques on adverse events, bleeding rates, and readmission rates, ultimately, each of the surgeons’ techniques did not result in differences in SSI rates. A pertinent discussion regarding each technique is detailed below:
Surgical approach
Our results demonstrated that a robotic approach had higher adverse outcomes (coefficient = 0.902, p-value = 0.002) and higher readmission rates (coefficient = 0.602, p-value = 0.043) when compared to laparoscopic. Other high-quality studies have shown that robotic and laparoscopic approaches demonstrate comparable rates of leak, length of hospital stay, and reoperation [4]. In a 2018 meta-analysis by Wang et al., there was no difference in clinical outcomes or weight loss at 2-year follow-up between laparoscopic and robotic approaches [5]. However, the robotic approach was found to have a longer operative time, which could be attributed to tasks like docking the robot [5]. Worse outcomes with a robotic approach may be attributable to learning curve and inherent issues with haptic feedback. Notably, some of our surgeons adopted the robotic approach as recently as 2020, which may explain these results.
Performing gastrojejunostomy (GJ) versus jejunojejunostomy (JJ) anastomosis first
Performing the GJ anastomosis first was correlated with higher adverse outcomes than performing the JJ anastomosis first (coefficient = 0.911, p-value = 0.002). From our literature search, there have been no studies on anastomosis creation ordinality (Appendix 3). We posit that creating the GJ first reduces flexibility, thus making it more probable to miss an iatrogenic injury to the small bowel at the end of the linear stapler deployment. Completing the JJ before gastric pouch creation also gives the surgeon a bail-out option if the patient becomes unstable. Additionally, during GJ creation, carrying the cut end of the jejunum remaining after a jejunojejunostomy, rather than a small bowel loop, may allow for more flexibility.
Patient positioning
Flat positioning had lower adverse outcomes (coefficient = 0.789, p-value = 0.015), bleeding (coefficient = 1.609, p-value = 0.039), and readmission rates (coefficient = 0.680, p-value = 0.039) when compared to reverse Trendelenburg. This may be controversial, as the purported utility in reverse Trendelenburg is both physiologic and anatomic with visualization of the hiatus and fundus when addressing the gastric pouch. In a RYGB case series by Artuso et al., physiologic changes during RYGB include pulmonary vascular resistance, reduced cardiac index after deflation, and persistent decrease in pH and bicarbonate intraoperatively during bypass surgery [6]. Notably, patients’ pH and PaCO2 continued to decrease and increase, respectively; this was true even in reverse Trendelenburg positioning when intrathoracic pressure should be lower and thus allowing better ventilation [6]. Flat positioning may offer more neutral physiology that should be studied further.
Division of mesentery
Dividing the mesentery was correlated with fewer adverse outcomes (coefficient = -0.816, p-value = 0.023). The driving principle of mesenteric division is to achieve adequate, tension-free length for the limbs in anastomotic creation. However, there is concern about complications related to vascular compromise, adhesions, and inadvertent creation of mesenteric defects at risk of internal herniation. The majority of studies related to the routine mesenteric division are retrospective. Backman et al. in 2019 reported that anastomotic leak rates in the first 30 days, along with ulceration and stenosis were significantly higher in patients who had their mesenteries routinely divided [7]. Further studies are needed to evaluate whether or not a routine division of mesentery leads to better patient outcomes.
Staplers
Intuitive™ staplers were associated with higher adverse outcomes (coefficient = 0.829, p-value = 0.005) and readmission rates (coefficient = 0.667, p-value = 0.032) when compared to Covidien™ staplers. In this univariate analysis, Intuitive™ staplers are confounded by the robotic approach, as these are only used robotically in this hospital system. The proposed benefits of an endoscopic stapler may be cost, as well as avoiding the need for repositioning and unsuccessful clamping during robotic cases [8]. In a non-systematic review of previous literature, Ghosh et al. found that stapler misfires were a common cause of leaks and bleeding [9]. Gold stapler loads (when compared to both higher and lower staple height colors) were lower in total adverse outcome rates, bleeding rates, and reoperation rates. In a Swedish retrospective study by Lundvall et al., low staple heights (≤ 1.0 cm tissue thickness when closed) compared to high staple heights (≥ 1.5 tissue thickness when closed) used in gastrojejunostomy creation were significantly correlated with fewer overall postoperative complications in the first 30 days after surgery [10]. Gold staplers (taller than blue/white but lower than black/purple) may be the middle-of-the-road height ideal for bypass and should be investigated.
Creation and technique for jejunojejunostomy
Our results showed that closure versus non-closure of the JJ mesentery did not have any statistically significant difference in adverse outcomes (coefficient = 0.034, p-value = 0.945). Several contemporary high-quality studies investigate JJ mesenteric closure. These previous studies overall suggest that routine closure reduces rates of internal hernias, while there is no definitive evidence of adverse outcomes, such as adhesions, kinking of the small bowel, early small bowel obstruction, and potential injury to the mesentery [11,12,13,14,15]. This study does not cover Petersen’s defect or other mesenteric defects, and this remains an area to be further explored.
Our study showed that bidirectional jejunojejunostomy had higher adverse outcome rates (coefficient = 0.659, p-value = 0.022) and lower readmission rates (coefficient = 0.681, p-value = 0.022) when compared to unidirectional. Some contrary studies have shown that bidirectional stapling of the JJ leads to a significantly reduced risk of early small bowel obstruction [16, 17].
Stapling the common enterotomy led to higher adverse outcomes (coefficient = 0.653, p-value = 0.024), bleeding (coefficient = 1.056, p-value = 0.044), and readmission rates when compared to hand-sewing (coefficient = 0.711, p-value = 0.017). Meta-analysis data demonstrates no significant difference between sewing versus stapling in RYGB [18]. However, stapling the common enterotomy may result in more bleeding [18].
Length of Roux (alimentary) and biliopancreatic limb
A Roux limb of 130-cm or of 160-cm if the patient had a BMI > 50 was associated with higher adverse outcomes compared to a Roux limb that was 100-cm (coefficient = 2.346, p-value = 0.025). A 150-cm biliopancreatic limb in diabetic patients, 125-cm in nondiabetic patients, and 50 to 75-cm in any patient were associated with higher rates of bleeding (coefficient = 3.460, p-value = 0.020) compared to a 50-cm biliopancreatic limb. Having a 150-cm biliopancreatic limb that could be increased up to 100-cm to avoid tension was associated with higher total adverse outcomes (coefficient = 1.581, p-value = 0.001), bleeding (coefficient = 2.260, p-value = 0.041), and readmission (coefficient = 0.974, p-value = 0.035). Currently, there are no standardized lengths of the Roux and biliopancreatic limbs, despite the many studies that have attempted to maximize weight loss while minimizing protein malnutrition. Wang et al. found that a common channel less than 200-cm led to severe protein malnutrition, and Mahawar et al. echoed a similar message when recommending that the combined length of the Roux and biliopancreatic limbs should be 100–200 cm in length [19, 20]. Another study by Kamocka et al. suggested that long versus short biliopancreatic limbs did not have significant differences in terms of weight loss [21]. However, Kwon et al. found that a longer biliopancreatic limb correlated with greater remission rates of diabetes, while the length of the alimentary limb did not have an effect [22]. Our study had a short follow-up at 30 days, and therefore our study may not capture complications related to chronic protein malnutrition.
Pre-op UGI series or EGD before bariatric surgery
In our group, all ten surgeons in our group reported that they did not routinely use UGI series, and therefore subgroup analysis could not be performed. We found that routine preoperative EGD had fewer adverse outcomes and fewer bleeding events. The utility of routine preoperative UGI series and EGD is still a topic of debate. Previous studies recommend completing a pre-operative UGI series or EGD to rule out pathology that would change or dictate surgical management, including but not limited to Barrett’s esophagus, H. pylori ulcers, gastritis, or malignancy [23,24,25]. In a systematic review by Ansari et al., 16% of patients had changes in their surgical plan due to the findings in their preoperative EGD [26]. On the other hand, preoperative UGI series and EGD can be uncomfortable and costly, adding to the number of barriers to care that already exist for the bariatric patient [27].
In summary, all roads might not lead to Rome. There were some considerable differences in outcomes with regard to technique. It was not feasible to test for mortality given the low incidence. This unique study, however, shows that there may be some interesting differences in technique that may impact adverse outcomes: (1) The learning curve and surgeon ability must be taken into account to achieve parity between robotic and laparoscopic approaches. (2) Routinely performing the JJ anastomosis first may benefit patient outcomes and should be studied further. (3) Additional studies on flat positioning may be needed to understand the effect on patient outcomes. (4) Further studies are needed to evaluate whether or not a routine division of mesentery leads to better patient outcomes. (5) The use of staplers with very low or very high heights may result in more adverse outcomes. (6) Routine closure of the jejunojejunostomy mesenteric defect should be considered with its risks. (7) Hand-sewing the common enterotomy may prevent complications. Further prospective studies for the aforementioned are required to better understand their true impact on outcomes. There is a lack of consensus on many of these techniques for RYGB, and surgeon experience and preferences in technique may play a role in outcomes.
Limitations
This study was limited by the inherent nature of its retrospective and univariate statistical design. Several issues prevented multivariate analysis. First, there are 711 observations for 14 variables, the majority of the variables have homogeneous responses or are highly correlated, and thus require a much larger sample size to detect a difference in a multivariable regression model. The sample size of surgeons was small, and the follow-up time frame of 30 days was relatively short. Due to the low number of observations, the study treated each technique as independent, not accounting for interaction. Therefore, surgical techniques may be indistinguishable from individual surgeon skills and may lead to confounding bias. Furthermore, the data obtained through MBSAQIP complication data were aggregated to the individual surgeon, and therefore we had a lack of information on patient demographics, which has been demonstrated in other studies to contribute to outcomes.
Future improvements would be to increase the study sample size to include more observations and include patient-specific data regarding complications. Future directions include accruing additional data using the same model, addition of more Delphi consensus techniques, multivariate regression models and prospective studies, and inclusion of antecolic versus retrocolic Roux limbs, one anastomosis gastric bypass, single anastomosis duodeno-ileal bypass, and other variations of bypass.
References
Madan AK, Lo Menzo E, Dhawan N, Tichansky DS (2009) Internal hernias and nonclosure of mesenteric defects during laparoscopic Roux-en-Y gastric bypass. Obes Surg 19(5):549–552. https://doi.org/10.1007/s11695-008-9722-5
Dang JT, Deprato A, Verhoeff K et al (2022) Variation of laparoscopic Roux-en-Y gastric bypass techniques: a survey of 518 bariatric surgeons. Obes Surg 32(7):2357–2365. https://doi.org/10.1007/s11695-022-06087-9
Kaijser MA, van Ramshorst GH, Emous M, Veeger NJGM, van Wagensveld BA, Pierie JEN (2018) A delphi consensus of the crucial steps in gastric bypass and sleeve gastrectomy procedures in the Netherlands. Obes Surg 28(9):2634–2643. https://doi.org/10.1007/s11695-018-3219-7
Economopoulos KP, Theocharidis V, McKenzie TJ, Sergentanis TN, Psaltopoulou T (2015) Robotic vs. laparoscopic roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg 25(11):2180–2189. https://doi.org/10.1007/s11695-015-1870-9
Wang L, Yao L, Yan P et al (2018) Robotic versus laparoscopic Roux-en-Y gastric bypass for morbid obesity: a systematic review and meta-analysis. Obes Surg 28(11):3691–3700. https://doi.org/10.1007/s11695-018-3458-7
Artuso D, Wayne M, Cassaro S, Cerabona T, Teixeira J, Grossi R (2005) Hemodynamic changes during laparoscopic gastric bypass procedures. Arch Surg 140(3):289–292. https://doi.org/10.1001/archsurg.140.3.289
Backman O, Freedman J, Marsk R, Nilsson H (2019) Laparoscopic Roux-en-Y gastric bypass without division of the mesentery reduces the risk of postoperative complications. Surg Endosc 33(9):2858–2863. https://doi.org/10.1007/s00464-018-6581-6
Gutierrez M, Ditto R, Roy S (2019) Systematic review of operative outcomes of robotic surgical procedures performed with endoscopic linear staplers or robotic staplers. J Robot Surg 13(1):9–21. https://doi.org/10.1007/s11701-018-0822-5
Ghosh SK, Roy S, Chekan E, Fegelman EJ (2016) A narrative of intraoperative staple line leaks and bleeds during bariatric surgery. Obes Surg 26(7):1601–1606. https://doi.org/10.1007/s11695-016-2177-1
Lundvall E, Ottosson J, Stenberg E (2019) The influence of staple height on postoperative complication rates after laparoscopic gastric bypass surgery using linear staplers. Surg Obes Relat Dis 15(3):404–408. https://doi.org/10.1016/j.soard.2019.01.017
Magouliotis DE, Tzovaras G, Tasiopoulou VS, Christodoulidis G, Zacharoulis D (2020) Closure of mesenteric defects in laparoscopic gastric bypass: a meta-analysis. Obes Surg 30(5):1935–1943. https://doi.org/10.1007/s11695-020-04418-2
Hajibandeh S et al (2020) Closure versus non-closure of mesenteric defects in laparoscopic Roux-en-Y gastric bypass: a systematic review and meta-analysis. Surg Endosc. https://doi.org/10.1007/s00464-020-07544-1
Amanda D, Elin P, Eva N, Stenberg E (2022) The influence of mesenteric defects closure on the use of computed tomography for abdominal pain 5 years after laparoscopic gastric bypass-a post hoc analysis of a randomized clinical trial. Obes Surg 32(2):266–272. https://doi.org/10.1007/s11695-021-05778-z
Kristensen SD, Floyd AK, Naver L, Jess P (2015) Does the closure of mesenteric defects during laparoscopic gastric bypass surgery cause complications? Surg Obes Relat Dis 11(2):459–464. https://doi.org/10.1016/j.soard.2014.10.013
Kristensen SD, Gormsen J, Naver L, Helgstrand F, Floyd AK (2021) Randomized clinical trial on closure versus non-closure of mesenteric defects during laparoscopic gastric bypass surgery. Br J Surg 108(2):145–151. https://doi.org/10.1093/bjs/znaa055
Hedberg S, Thorell A, Engström M, Stenberg E, Olbers T (2022) Surgical technique in constructing the jejunojejunostomy and the risk of small bowel obstruction after Roux-en-Y gastric bypass. Surg Obes Relat Dis 18(9):1151–1159. https://doi.org/10.1016/j.soard.2022.05.020
Munier P, Alratrout H, Siciliano I, Keller P (2018) Bidirectional jejunojejunal anastomosis prevents early small bowel obstruction due to the kinking after closure of the mesenteric defect in the laparoscopic Roux-en-Y gastric bypass. Obes Surg 28(7):1838–1844. https://doi.org/10.1007/s11695-017-3094-7
Jiang H, Lin L, Jiang X, Qiao H (2016) Meta-analysis of hand-sewn versus mechanical gastrojejunal anastomosis during laparoscopic Roux-en-Y gastric bypass for morbid obesity. Int J Surg 32:150–157. https://doi.org/10.1016/j.ijsu.2016.04.024
Wang A, Poliakin L, Sundaresan N et al (2022) The role of total alimentary limb length in Roux-en-Y gastric bypass: a systematic review. Surg Obes Relat Dis 18(4):555–563. https://doi.org/10.1016/j.soard.2021.08.022
Mahawar KK, Kumar P, Parmar C et al (2016) Small bowel limb lengths and Roux-en-Y gastric bypass: a systematic review. Obes Surg 26(3):660–671. https://doi.org/10.1007/s11695-016-2050-2
Kamocka A, Chidambaram S, Erridge S, Vithlani G, Miras AD, Purkayastha S (2022) Length of biliopancreatic limb in Roux-en-Y gastric bypass and its impact on post-operative outcomes in metabolic and obesity surgery-systematic review and meta-analysis. Int J Obes (Lond) 46(11):1983–1991. https://doi.org/10.1038/s41366-022-01186-0
Kwon Y, Lee S, Kim D et al (2022) Biliopancreatic limb length as a potential key factor in superior glycemic outcomes after Roux-en-Y gastric bypass in patients with type 2 diabetes: a meta-analysis. Diabetes Care 45(12):3091–3100. https://doi.org/10.2337/dc22-0835
Wolter S, Duprée A, Miro J et al (2017) Upper gastrointestinal endoscopy prior to bariatric surgery-mandatory or expendable? An analysis of 801 cases. Obes Surg 27(8):1938–1943. https://doi.org/10.1007/s11695-017-2622-9
Chang VC, Pan P, Shah SK et al (2020) Routine preoperative endoscopy in patients undergoing bariatric surgery. Surg Obes Relat Dis 16(6):745–750. https://doi.org/10.1016/j.soard.2020.02.002
Schigt A, Coblijn U, Lagarde S, Kuiken S, Scholten P, van Wagensveld B (2014) Is esophagogastroduodenoscopy before Roux-en-Y gastric bypass or sleeve gastrectomy mandatory? Surg Obes Relat Dis 10(3):411–416. https://doi.org/10.1016/j.soard.2014.01.015
El Ansari W, El-Menyar A, Sathian B, Al-Thani H, Al-Kuwari M, Al-Ansari A (2020) Is routine preoperative esophagogastroduodenoscopy prior to bariatric surgery mandatory? Systematic review and meta-analysis of 10,685 patients. Obes Surg 30(8):3073–3083. https://doi.org/10.1007/s11695-020-04672-4
Mani VR, Kalabin A, Nwakanama C, Suman P, Ahmed L (2019) Preoperative versus intraoperative diagnosis of hiatal hernia in bariatric population. Surg Obes Relat Dis 15(11):1949–1955. https://doi.org/10.1016/j.soard.2019.08.553
Acknowledgements
Susanna Yun B.F.A. for surgical illustrations pertaining to related presentations.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Drs. Alexander Hien Vu, M.D., Jessica Chiang, M.D., Yunzhi Qian M.P.H., Nilufar Tursunova M.D., Jaein Nha B.A., George Ferzli, M.D. have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Appendix 1. A search strategy was constructed for technique with gastric bypass for the purpose of identifying a similar study design to this current study: (“Gastric Bypass”[MeSH]) AND (technique[Title]) as well as “MBSAQIP” AND (“surgical technique” OR “surgeon technique”). No filters were applied. Results were screened manually.
Appendix 2. A search strategy was constructed for surgical approach with gastric bypass: (“Robotic Surgical Procedures”[MeSH]) AND (“Laparoscopy”[MeSH]) AND (“Gastric Bypass”[MeSH]). The following filters were applied: “ Filters applied: Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Systematic Review.). Results were screened manually.
Appendix 3. A search strategy was constructed for performing gastrojejunostomy or jejunostomy first/before with gastric bypass: (“Gastric Bypass”[MeSH]) AND (gastrojejunostomy versus jejunojejunostomy) AND (first or before). No filters were applied. No relevant results.
Appendix 4. A search strategy was constructed for gastric bypass and positioning: (“Gastric Bypass”[MeSH]) AND (“Patient Positioning”[MeSH] OR “table positioning” OR “operative positioning” OR “patient position” OR “patient positioning” OR “flat position” OR “flat positioning” OR “Head-Down Tilt”[MeSH] OR “reverse Trendelenburg position” OR “reverse Trendelenburg positioning”). Results were screened manually.
Appendix 5. A search strategy was constructed for division of mesentery with gastric bypass: (“Mesentery”[MeSH]) AND “division” AND (“Gastric Bypass”[MeSH]). No filters were applied. Results were screened manually.
Appendix 6. A search strategy was constructed for staplers with gastric bypass: ((“Surgical Staplers”[MeSH]) OR “Linear Stapler” OR “Staple”) AND (“Gastric Bypass”[MeSH]). The following filters were applied: Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, Systematic Review. Results were screened manually.
Appendix 7. A search strategy was constructed for bidirectional and unidirectional anastomosis with gastric bypass: (“Gastric Bypass”[MeSH]) AND (“bidirectional” OR “unidirectional”). No filters were applied. Results were screened manually.
Appendix 8. A search strategy was constructed for closure of enterotomy with gastric bypass: (“Gastric Bypass”[MeSH]) AND (“hand-sewn” OR “handsewn” OR “stapled” OR “stapler” OR “linear cutter” OR “triple stapling” OR “double stapling” OR “double stapled” OR “triple stapled” OR “enterotomy closure” OR “closure of enterotomy” OR “enterotomy”). The following filters were applied: Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Systematic Review. Results were screened manually.
Appendix 9. A search strategy was constructed for closure of the jejunojejunostomy mesenteric defect with gastric bypass: (“Gastric Bypass”[MeSH]) AND (“mesentery closure” OR “closure of mesentery” OR “close the mesentery” OR “mesenteric defect” OR “defect of mesentery” OR “mesentery defect”). The following filters were applied: Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, Systematic Review. Results were screened manually.
Appendix 10. A search strategy was constructed for length of roux and biliopancreatic limb with gastric bypass: (“Gastric Bypass”[MeSH]) AND ((“length” AND “roux”) OR (“distance” AND “roux”) OR (“length” AND “limb”) OR (“distance” AND “limb”) OR (“length” AND “biliopancreatic”) OR (“distance” AND “biliopancreatic”) OR (“length” AND “alimentary”) OR (“distance” AND “alimentary”)). The following filters were applied: Meta-Analysis, Systematic Review. Results were screened manually.
Appendix 11. A search strategy was constructed for routine preoperative upper GI series and preoperative EGD prior to gastric bypass: ((“Gastric Bypass”[MeSH]) OR : “sleeve gastrectomy”) AND ((“Endoscopy, Digestive System”[MeSH]) OR “EGD” OR “upper gastrointestinal series” OR “upper GI series”) AND ((“Preoperative Period”[MeSH]) OR (“Preoperative Care”[MeSH])). No filters were applied. Results were screened manually.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vu, A.H., Chiang, J., Qian, Y. et al. Do all roads lead to Rome?: A retrospective analysis on surgical technique in Roux-en-Y gastric bypass. Surg Endosc 37, 7254–7263 (2023). https://doi.org/10.1007/s00464-023-10257-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10257-w