Abstract
Background
Patients requiring percutaneous endoscopic gastrostomy (PEG) for amyotrophic lateral sclerosis (ALS) related dysphagia represent a clinical challenge. Diminished pulmonary function and aspiration risks can lead to anesthesia-related complications, and gastric displacement from hemidiaphragm elevation may preclude safe gastric access. This study reports the efficacy and outcomes of a dedicated anesthesia/surgery management protocol for ALS patients undergoing PEG.
Methods
In 2013, a PEG placement protocol for ALS patients was developed emphasizing efficient pre-operative evaluation, rapidly metabolized anesthetic agents, and minimization of opioid use. Outcomes were analyzed retrospectively. Preoperative weight loss, pulmonary function tests, total analgesia, procedural time, and 90-day morbidity and mortality were recorded.
Results
From 2013–2019, 67 ALS patients (mean age 65.3 years, 52.2% female) received a PEG under the protocol. Mean percentage weight loss 6 months before PEG was 9.3 ± 5.1% with 38.8% of patients meeting criteria for severe malnutrition. Mean anesthesia time (propofol induction to anesthesia emergence) was 34.5 ± 10.8 min and mean operative time (endoscope insertion to dressing placement) was 16.4 ± 8.2 min. Regional anesthesia with liposomal bupivacaine was performed in 76.1%. All attempts at PEG placement were successful. With a mean follow-up of 6.1 ± 6.8 months, all PEGs were functional and there were no surgical site complications. Thirty-day readmission rate was 7.0% and 90-day mortality was 22.4% (46.7% occurring within 30 days). Mean time from surgery to death was 8.8 ± 7.8 months.
Conclusions
Protocols for optimizing PEG may help overcome challenges present in the ALS patient population. Despite patient comorbidities, protocol implementation and dedicated team members resulted in a high procedural success rate and low complication rate. Further study is warranted to optimize the timing of PEG placement in relation to ALS disease progression and determine the utility of regional anesthesia during PEG placement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with amyotrophic lateral sclerosis (ALS) and impaired oral intake have improved survival following percutaneous endoscopic gastrostomy (PEG) [1,2,3]. Despite this benefit, PEG placement in ALS patients carries significant risks. Their diminished pulmonary function and increased risk of aspiration can lead to anesthesia-related complications. The gastric displacement above the costal margin that results from bilateral hemidiaphragm elevation may also preclude safe endoscopic gastric access.
Recognizing the benefit from enteral access in ALS patients, efforts have focused on limiting the risk that these patients present. Existing studies have attempted to limit anesthesia related complications by avoiding intubation during these procedures using conscious sedation or even no sedation at all [4,5,6]. Other studies have compared methods of enteral access (typically PEG versus fluoroscopic-guided gastrostomy) with no consistent demonstration of a superior method and each method conferring a survival benefit after establishing enteral access [7].
These studies have focused primarily on outcomes after PEG – specifically the effect on survival benefit. In regards to procedural outcomes, a metanalysis including 6 studies with 322 PEG placement attempts in ALS patients reported pooled success rates ranging from 88.3–91.2% and the individual studies reporting success rates ranging from 55.0–93.6% [7]. Despite this high failure rate, there is limited literature available describing methods to improve procedural success rates and minimize complications. As a result, the aim of this study was to determine the efficacy and outcomes of a dedicated multispecialty management protocol for ALS patients undergoing PEG.
Materials and methods
Data sources
Following institutional review board approval, the institutional electronic medical record (EMR) was retrospectively reviewed to obtain all data. The EMR was queried for patients with both a Current Procedural Terminology (CPT) code for a PEG (43,246) and an International Classification of Disease (ICD) code for ALS (ICD-10 G12.21, ICD-9 335.20) at a single institution.
Population
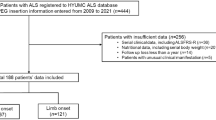
All patients undergoing PEG for ALS-related dysphagia from 2013–2019 were included. All procedures were performed by two-fellowship trained endoscopic surgeons and anesthesia was administered by two neuroanesthesiologists using the same treatment protocol at a single tertiary-care institution. Patients undergoing concomitant procedures in addition to PEG were excluded.
Procedure protocol
A PEG placement protocol for ALS patients was developed in 2013 by an ALS neurologist, a neuroanesthesiologist, and an endoscopic surgeon (protocol summary in Fig. 1). The protocol emphasized efficient pre-operative evaluation with pulmonary function tests and a detailed examination and documentation of bulbar functionality and respiratory adjuncts, adherence to the exclusive utilization of rapidly metabolized anesthetic agents, and the removal of opioids from the approach to perioperative analgesia. The protocol did not specify any cutoffs in pulmonary function as a contraindication to proceed with PEG placement. The protocol called for these dedicated team members to provide all care in the perioperative period. Partway through the case series, an additional endoscopic surgeon and a neuroanesthesiologist were added. Initially, a pharyngeal topical anesthetic was used, however this was discontinued due to concerns of potentiating aspiration risk as well as poor patient tolerance without apparent benefit to patients.
Upon correct identification of the patient and procedure and after obtaining informed consent by the surgical and anesthesia teams, the patient was brought to the operating theater. Standard monitors according to the American Society of Anesthesiologists guidelines were applied, including an automatic non-invasive blood pressure cuff, five lead electrocardiogram, and pulse oximetry. In patients with preoperative non-invasive ventilation (NIV), oxygenation and ventilation were achieved via the application of non-invasive nasal bi-level positive airway pressure. For all other patients, supplemental oxygen via nasal cannula or high flow nasal cannula was delivered with NIV initiated in patients that were unable to maintain adequate oxygenation and ventilation. The fraction of inspired oxygen and end tidal carbon dioxide were monitored via an anesthesia workstation. A propofol infusion was initiated and titrated to achieve deep sedation while maintaining spontaneous respirations. Initially, analgesia was accomplished by non-opioid analgesics such as intravenous (IV) acetaminophen and ultra-rapidly acting opioids such as remifentanil or alfentanil. As the case series progressed, regional anesthesia was introduced utilizing either a transversus abdominis plane (TAP) block, a rectus sheath block, or a combination using various types and concentrations of local anesthetics – this addition was intended to target postoperative pain. Currently, our institution utilizes the following regional anesthesia technique: a left sided rectus sheath block with 10 mL of 0.5% bupivacaine and a left sided subcostal TAP block with 20 mL of 1.3% liposomal bupivacaine (Exparel®). If no contraindications exist, other non-opioid analgesics are administered pre-operatively or during the immediate intra-operative period including 1000 mg of IV acetaminophen (Ofirmev®) and 800 mg of IV ibuprofen (Caldolor®). With the addition of regional anesthesia, opioids are avoided in the intra-operative period and used very sparingly in the post-operative period if required. Acetaminophen and ibuprofen are continued in the post-operative period.
In all procedures, a gastroscope is advanced through the oropharynx into the stomach which is inspected for pathologic findings. The stomach is then maximally insufflated, and the gastrostomy site is identified through transillumination of the abdominal wall and identification of one-to-one motion on finger palpation. In patients in which a gastrostomy site cannot be identified through these methods, intraoperative fluoroscopy is used to identify an appropriate gastrostomy location. Once the gastrostomy site is identified, a small (~ 8 mm) incision is made following sterile preparation of the skin. A 20 French gastrostomy tube is then placed using the Ponsky-pull technique. Gastropexy is then variably performed according to surgeon preference using endoscopic placement of gastrointestinal T-anchors. An abdominal binder is loosely placed after the gastrostomy dressing is applied to further secure the gastrostomy tube.
Outcomes
Outcomes were analyzed following implementation of the protocol through retrospective review of the medical record. Preoperative weight loss, pulmonary function tests, total analgesia, procedural time, and 90-day morbidity and mortality were recorded. Disease duration was defined as the time elapsed from symptom onset to PEG. Anesthesia time was defined as the time elapsed from propofol induction to anesthesia emergence. Operative time was defined as the time elapsed from endoscope insertion to dressing placement. Periprocedural analgesia was defined as all analgesia in the immediate preoperative, intraoperative, and postoperative (until discharge for outpatient procedures and until the end of postoperative day zero for inpatient procedures) periods. Moderate malnutrition was defined as 5–10% weight loss and severe malnutrition was defined as > 10% weight loss over the 6 months prior to PEG [8]. Postoperative mortality was determined through both review of the EMR and a search of publicly available obituaries.
Statistical analysis
Descriptive statistics were used to summarize the data and were performed using Microsoft Excel (version 1902, Microsoft Corporation, Redmond, Washington). For comparison of perioperative analgesia usage in patients before and after the protocol was modified to include regional anesthesia, continuous outcome variables were compared using two-sample t-tests and categorical outcome variables were compared using Chi squared tests with SAS (version 9.4, SAS Institute Inc., Cary, NC, USA) statistical software. Statistical significance level was predefined as p value < 0.05.
Results
A total of 67 patients with dysphagia secondary to ALS underwent PEG under the protocol. The average age was 65.3 years, and the majority were female (52.2%) and Caucasian (98.5%) with a mean body mass index of 24.9 kg/m2 (Table 1). Most (79.1%) had poor functional status with 79.1% of patients meeting criteria for either moderate or severe malnutrition with a mean preoperative weight loss of 9.3% in the 6 months prior to PEG. A small minority (6%) of patients had a history of prior failed gastrostomy placement prior to referral to our team.
On average, ALS disease onset was 1.6 years prior to PEG (Table 2). Preoperative revised ALS functional rating scale was 29.5 on average. Almost all patients (92.5%) had preoperative pulmonary function testing with a mean of 54.3% of predicted forced expiratory volume in 1 s (FEV1) and a mean of 53.4% of predicted forced vital capacity (FVC) resulting in a mean FEV1/FVC ratio of 0.81. Many of these patients (46.3%) were utilizing non-invasive ventilation (NIV) preoperatively.
After referral from neurology, patients underwent PEG at 15.2 days following referral on average (Table 3). Most procedures (86.6%) occurred in the outpatient setting. Mean anesthesia time was 34.5 min and mean operative time was 16.4 min. 67.2% of gastrostomies were placed in the left upper quadrant. Gastropexy with an anchor device was performed in 25.4%. The gastrostomy was secured with an abdominal binder loosely placed over the gastrostomy in 83.6% of patients. No patients were intubated and nearly all patients (96.8%) with preoperative NIV continued using this therapy during the procedure. Of the remaining 36 patients not utilizing NIV preoperatively, 9 (25.0%) required intraoperative NIV. No intraoperative complications were observed in this case series. All attempts at PEG placement were successful. Intraoperative fluoroscopy was used to guide PEG placement in 6.0% of patients.
When looking at perioperative analgesia, 76.1% of patients received regional anesthesia with TAP block being the most common modality (41.8%) followed by a combination of TAP block and rectus sheath block (33.3%; Table 4). Only 7.5% of patients received preoperative analgesics while 70.1% received intraoperative analgesics and 65.7% received postoperative analgesics. Two patients did not require any analgesics during the entire perioperative period. The mean morphine milligram equivalent administered was 1.9 with 83.5% of patients not requiring any opioid pain medications in the perioperative period.
Procedural outcomes of the protocol were then investigated (Table 5). The 30-day and 60-day readmission rates were 7.0% (2 acute respiratory failure, 1 postoperative pneumoperitoneum, 1 unknown) and 16.0% (2 acute respiratory failure, 1 postoperative pneumoperitoneum, 1 urinary tract infection, 1 community-acquired pneumonia, 1 dehydration, 1 parotid gland infection, 1 unknown) respectively. A single complication was observed in the 90-day postoperative period consisting of bleeding from the PEG site which was appropriately controlled with chemical cautery to the skin edge on an outpatient basis. With a mean follow-up of 6.1 months, all PEGs were functional and there were no surgical site complications. The 90-day mortality rate was 22.4% (46.7% occurring within 30 days) with no mortalities related to the PEG procedure. The mean time from PEG to death was 8.8 months. At the conclusion of study follow-up, 3 patients (4.5%) from this series were still living. All gastrostomy tubes were functioning at the time of death or last follow-up in this series.
Finally, causes of mortality were assessed. Of the 64 patients who died in this series, no documented cause of death was found in the EMR for 62 (96.9%) of which 33 patients (53.2%) had been on hospice care prior to death. Of the two remaining patients, both had acute hypercapnic respiratory failure as a documented cause of death occurring at 347- and 8-days post-procedure. For the patient who died at 8-days post-procedure, they presented to the emergency department (ED) in respiratory distress 36 h following an uncomplicated PEG placement under monitored anesthesia care. They were emergently intubated in the ED with a hospital course notable for placement of a chest tube for decompression of a small apical pneumothorax that became moderate sized on positive pressure ventilation. The patient ultimately died after failed attempts at extubation and was placed on hospice care prior to being terminally extubated.
Of note, after the first 15 patients, TAP blocks were added to the protocol with 94.2% of subsequent patients receiving a TAP block (73.1% overall). The method of regional anesthesia was further adjusted with the addition of rectus sheath blocks as a single therapy or combination therapy with TAP block after 33 patients with 67.6% of subsequent patients receiving a rectus sheath block (34.3% overall). Subsequent to the addition of TAP block, only 9.6% of patients required opioid analgesics in the perioperative period (compared to 40.0% of patients prior to addition of TAP blocks). After adding rectus sheath blocks to the protocol, only 5.6% of patients required opioid analgesics (compared to 27.3% prior to change).
The patients receiving regional anesthesia were compared to those who did not receive any regional anesthesia (Table 6). Patients in the regional anesthesia group received more overall acetaminophen (1277.9 vs. 678.1 mg, p = 0.0099). The regional anesthesia group was also more likely to not require opioid medications perioperatively (90.2% vs. 62.5%, p = 0.0091). The two groups were otherwise comparable in regard to perioperative analgesia usage.
Discussion
The implementation of this dedicated anesthesia and surgery protocol resulted in a high procedural success rate. Referral patterns were streamlined and resulted in minimal delay from identification of need for enteral feeding access to PEG placement. These procedures were completed in a primarily outpatient setting with the majority of the patients not requiring any perioperative opioid analgesics.
As noted previously, the reported procedural failure rate in the literature is approximately 10%. Reasons for failure cited in the literature include respiratory decompensation, patient intolerance to endoscopy, inability to perform transillumination, and laryngospasm [9, 10]. In this series, we were able to obtain 100% successful PEG placement in 67 consecutive ALS patients undergoing PEG. We believe the use of a dedicated team of endoscopists with the ability to use intraoperative fluoroscopy for further guidance in difficult cases is a reason for this success. In our series of patients, intraoperative fluoroscopy was utilized in 6% of patients and may explain in part the difference in success rates in our series and published data.
Notably, our study is limited by the lack of a historical control of ALS patients undergoing PEG placement prior to implementation of our protocol. We do not have patient data prior to 2013 to perform this analysis due to the establishment of the protocol coinciding with arrival of one of the study authors who initiated development of this protocol. Prior to this date, enteral access was achieved in this population through various providers of different specialties.
Of particular note in this case series is the change in referral and management patterns for ALS patients at our institution requiring enteral access. We believe this change to be related to the development of the protocol which was done in collaboration with one of the ALS neurologists at our institution. This collaboration led to institutional awareness of a team dedicated to PEG placement in ALS patients. Of the 4 patients with history of failed prior gastrostomy placement, all occurred during the first 10 consecutive patients to undergo PEG as part of this protocol. It would appear that prior failed attempts at gastrostomy placement via other methods without a PEG protocol specific to ALS patients prompted referral to our dedicated team at this institution. Currently at our institution, all PEG referrals in patients with ALS are routed to the dedicated surgery and anesthesia team detailed in the protocol.
Prior studies have suggested that earlier PEG placement in the ALS disease course results in better survival benefit following PEG [1, 3]. Many of these studies have suggested PEG placement when FVC was still ≥ 50% predicted and after weight loss exceeded 10% with more significant survival benefits in patients presenting with higher FVC [1, 3, 11]. In our series, only 9 patients met these criteria with an additional 15 presenting with more advanced disease demonstrated by FVC < 50% predicted with weight loss exceeding 10%. Attempts to identify patients who qualify for enteral feeding access via PEG earlier in the disease course would be well warranted in the further refinement of this protocol. However, these results would also suggest that a dedicated anesthesia and surgery protocol can result in high procedural success even in patients with advanced disease.
In this series, 58.2% survived greater than 6 months following PEG placement compared to reports of 75% survival following PEG at 6 months [12]. We believe this reflects the advanced disease of the patients in our series. The lower survival in this series may be reflective of the 22.4% of patients who underwent PEG placement with a predicted FVC lower than commonly accepted guidelines. Furthermore, ALS Functional Rating Scale (ALSFRS) scores less than 31 have been identified as a predictor of early death and FVC measurements of less than 85% have been estimated to increase the risk of death by 0.86 [13, 14]. In our patients, 46% had an ALSFRS score less than 31 and 84% had FVC measurements less than 85%. Additional refinement and implementation of this protocol is warranted to translate the high procedural success rate into patients earlier in the ALS disease course who may benefit for a longer period from enteral access. Such refinements must consider individualized quality-of-life decisions as some patients may defer PEG placement until dysphagia progression necessitates enteral access later in the disease course.
The high rate of postoperative mortality observed in this series is likely reflective of the natural progression of ALS. Due to the retrospective nature of this study, accurate determination of mortality causes was limited. Given the progression of neuromuscular respiratory failure inherent to ALS, we presume that the overwhelming majority of these patients died from respiratory failure secondary to ALS. Indeed, the two patients with cause of mortality described both had deaths attributed to respiratory failure with one of those mortalities occurring 8 days post-procedure as detailed in the results section. We believe PEG placement to be a reasonably safe procedure in this high-risk population with the appropriate precautions, particularly related to anesthesia delivery, as detailed in this study.
On our review of the literature, this would appear to be the first report of regional anesthesia in PEG. A small case series consisting of 5 patients has reported on the use of bilateral TAP block to avoid general anesthesia or sedation in open gastrostomy [15]. A similar case report reported minimal opioid use when a unilateral TAP block was used in open gastrostomy [16]. In another case series with 10 patients, bilateral thoracic paravertebral nerve blocks were used in percutaneous radiologic gastrostomy placement to again avoid general anesthesia or sedation [17]. In our series, regional anesthesia was used to minimize opioid usage and level of sedation. Future research efforts can investigate whether the success in these small case series can be translated into ALS patients undergoing PEG with further minimization and perhaps elimination of sedation from our protocol.
In conclusion, the use of a dedicated multispecialty protocol for ALS patients undergoing PEG may reduce failed attempts at gastric access, minimize perioperative morbidity and mortality, and highlight the use of regional anesthesia. Further efforts developing protocols for optimizing PEG may help overcome challenges present in the ALS patient population. Despite patient comorbidities, protocol implementation and the use of dedicated team members resulted in a high procedural success rate, low complication rate, and low MME requirement. Further study is warranted to optimize the timing of PEG placement in relation to ALS disease progression and determine the utility of regional anesthesia during PEG placement.
References
Miller RG et al (2009) Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 73(15):1218–1226
Cui F et al (2018) Therapeutic effects of percutaneous endoscopic gastrostomy on survival in patients with amyotrophic lateral sclerosis: a meta-analysis. PLoS ONE 13(2):e0192243
Bond L et al (2019) A comprehensive examination of percutaneous endoscopic gastrostomy and its association with amyotrophic lateral sclerosis patient outcomes. Brain Sci 9(9):223
Czell D et al (2013) Outcomes of percutaneous endoscopic gastrostomy tube insertion in respiratory impaired amyotrophic lateral sclerosis patients under noninvasive ventilation. Respir Care 58(5):838–844
Sato Y et al (2017) Safety of unsedated PEG placement using transoral ultrathin endoscopy in patients with amyotrophic lateral sclerosis. Nutr Neurosci 20(1):71–75
Thompson AG et al (2017) A risk stratifying tool to facilitate safe late-stage percutaneous endoscopic gastrostomy in ALS. Amyotroph Lateral Scler Frontotemporal Degener 18(3–4):243–248
Yang B, Shi X (2017) Percutaneous endoscopic gastrostomy versus fluoroscopic gastrostomy in amyotrophic lateral sclerosis (ALS) sufferers with nutritional impairment: A meta-analysis of current studies. Oncotarget 8(60):102244–102253
White JV et al (2012) Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 36(3):275–283
Carbó Perseguer J, Madejón Seiz A, Romero Portales M, et al (2019) Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis: Mortality and complications. Neurologia (Engl Ed) 34(9):582–588
Blondet A et al (2010) Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol 21(4):527–533
Bokuda K et al (2016) Predictive factors for prognosis following unsedated percutaneous endoscopic gastrostomy in ALS patients. Muscle Nerve 54(2):277–283
de Carvalho M, Gooch CL (2017) The yin and yang of gastrostomy in the management of ALS: friend or foe? Neurology 89:1435–1436
Wolf J et al (2014) Factors predicting one-year mortality in amyotrophic lateral sclerosis patients–data from a population-based registry. BMC Neurol 14:197
Su WM et al (2021) Predictors of survival in patients with amyotrophic lateral sclerosis: a large meta-analysis. EBioMedicine 74:103732
Hasan MS et al (2011) Open gastrostomy under ultrasound-guided bilateral oblique subcostal transversus abdominis plane block: a case series. Eur J Anaesthesiol 28(12):888–889
Lee AR, Choe YS (2015) Anesthesia experience for open gastrostomy with ultrasound-guided unilateral subcostal transversus abdominis plane block in a high risk elderly patient: a case report. Anesth Pain Med 5(4):e24890
Kalava A et al (2016) Bilateral thoracic paravertebral nerve blocks for placement of percutaneous radiologic gastrostomy in patients with amyotrophic lateral sclerosis: a case series. Rom J Anaesth Intensive Care. 23:149–153
Acknowledgements
The authors have no acknowledgements to make.
Funding
No internal or external financial support was used for this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Eric Pauli reports speaking and teaching honoraria Beckton, Dickinson and Company (BD), Medtronic, PLC, Ovesco Endoscopy AG and Boston Scientific Corp.; consultant fees from Boston Scientific Corp., Actuated Medical, Inc., Baxter International, Inc., Wells Fargo & Company, Cook Biotech, Inc., CMR Surgical, Neptune Medical, Surgimatix, Inc., Boehringer Laboratories, Inc., Allergan, PLC and Noah Medical; royalties from Springer and UpToDate, Inc.; financial interests in International Hernia Collaboration, Inc., Contamination Source Identification and Cranial Devices, Inc. Joshua Winder receives consultant fees from Boston Scientific Corp unrelated to this report. Vamsi Alli receives research support from Actuated Medical unrelated to this report. David Morrell, Marvin Chau, Edward Stredny, Elizabeth Sinz, Sprague Hazard and Zachary Simmons have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morrell, D.J., Chau, M.H., Winder, J.S. et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: outcomes of a dedicated anesthesia and surgery protocol. Surg Endosc 37, 4338–4344 (2023). https://doi.org/10.1007/s00464-023-09896-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-09896-w