Abstract
Background
Laparoscopic-assisted thermal ablation has been used successfully to treat early hepatocellular carcinoma (HCC) tumors, defined as < 3 cm in diameter. This approach allows for ablation of tumors located in areas of the liver that are otherwise inaccessible for a percutaneous approach. Thermal ablation of exophytic tumors remains controversial due to a reported increased risk of tumor seeding of the abdominal cavity and incomplete ablation.
Methods
This cohort study consisted of 663 HCC tumors treated with thermal ablation at a single, quaternary academic medical center between 2/2001 and 1/2021. Post treatment, patients were followed at a defined interval schedule beginning at one month post treatment, then every 3 months for 2 years, every 6 months in year 3, followed by yearly studies. Patients’ medical records were reviewed for three years post ablation for evidence of complete ablation and intra-abdominal dissemination of disease.
Results
326 patient records met the inclusion criteria. Comparing the exophytic and non-exophytic groups, there were statistically significant differences in etiology of liver disease (p = 0.048) and TNM stage (p = 0.03), as well as a higher rate of incomplete ablation in the non-exophytic group (10.2% vs 3.3%; p = 0.045). Otherwise, there were no statistically significant differences in baseline characteristics, tumor characteristics, or use of thermal ablation technology. Rates of intra-abdominal dissemination of HCC were low in both groups: 1.1% (n = 1) in the exophytic group and 1.7% (n = 4) in the non-exophytic group. There was no significant difference in intra-abdominal dissemination of HCC between the groups (p > 0.99, RR = 0.66; 95% CI 0.07–5.79). Additionally, no differences were seen in dissemination between microwave ablation and radiofrequency ablation (p > 0.99).
Conclusion
This study demonstrates that laparoscopic-assisted thermal ablation of small, exophytic tumors is safe and does not increase the risk for disseminated intra-abdominal HCC disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Liver cancer is the fourth most frequent cause of cancer-related deaths worldwide and the second most lethal tumor with a five-year survival rate of 18% [1, 2]. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and often (> 80%) occurs in the setting of underlying liver disease [3]. In the United States, the incidence of liver cancer, predominantly HCC, has increased by 43% between 2000 and 2016, largely driven by the increased incidence of non-alcoholic fatty liver disease (NAFLD) secondary to obesity and metabolic syndrome [1, 4, 5]. This trend is expected to continue, with the incidence of NAFLD-related HCC projected to increase by 122% in the United States by 2030 [6].
Treatment for HCC is based on tumor staging [7, 8]. The Barcelona Clinic Liver Cancer algorithm is one of the most widely used set of staging guidelines and categorizes patients into one of five stages based on number and size of lesions, liver function, and the presence of macrovascular or extrahepatic spread. Very early-stage (BCLC 0) is defined as a solitary nodule less than or equal to 2 cm. Early-stage (BCLC A) is defined as a solitary nodule greater than 2 cm or 2 to 3 nodules all less than or equal to 3 cm. Liver function is preserved and there is no evidence of extrahepatic spread or macrovascular invasion from the tumor in BCLC stages 0 and A [1, 9].
Surgery is the optimal treatment for early HCC, either by tumor resection or liver transplant. Unfortunately, less than 30% of patients are candidates to undergo resection or transplant often due to the size and location of the tumor(s) or underlying comorbidities, including liver disease [4, 10, 11]. For these patients, local therapy is the best treatment option [12, 13]. Thermal ablation, a local organ-directed therapy, has been shown to be effective and safe at treating small (< 3 cm), early-stage HCC. However, there continues to be debate about the safety of using thermal ablation on exophytic HCC tumors. The prevailing opinion is that subcapsular and exophytic tumors are contraindications to thermal ablation because of a reported increased risk of abdominal tumor seeding, tumor recurrence, and incomplete ablation [14, 15]. The objective of this study is to evaluate the safety and efficacy of laparoscopic-assisted thermal ablation to treat exophytic HCC tumors in patients with cirrhosis.
Materials and methods
Study design, setting, and population
This is a single-center, retrospective cohort study of adult patients (age ≥ 18 years old) with underlying liver disease who were treated with thermal ablation for HCC tumors at a single, quaternary academic medical center between 2/2001 and 1/2021. Each tumor/operation is considered independently. Inclusion criteria were radiographic evidence of HCC, a single HCC nodule < 5 cm or up to three nodules each less than 3 cm, and thermal ablation completed via a laparoscopic approach. This study followed STROBE guidelines [16].
All cases were deemed unsuitable for liver resection due to the severity of portal hypertension, degree of liver dysfunction, or associated medical comorbidities. The study was approved by the Institutional Review Board. Exclusion criteria were vascular invasion, distant metastases, missing preoperative imaging, or lack of follow-up imaging at least 12 months post ablation. Additionally, cases in which ablation could not be safely performed or in which no tumor was identified intraoperatively were excluded from this study.
Preoperative workup
Medical records of all patients were reviewed. An exophytic lesion is defined as a tumor that extends beyond the hepatic capsule. Non-exophytic lesions describe all other intraparenchymal HCC tumors. Patients were identified as having exophytic lesions either via operative notes or preoperative imaging reviewed by the authors. MELD-Na score was calculated for every patient based on their most recent labs prior to ablation [17]. Tumor diameter was calculated via imaging and confirmed intraoperatively. If multiple lesions were ablated during a single operation, the diameter of the largest lesion was used.
Surgical procedure
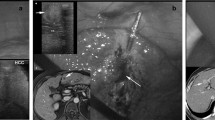
All procedures were laparoscopic and performed by a single surgeon experienced in minimally invasive hepatic surgery. All tumors were localized with intraoperative ultrasound and compared with preoperative magnetic resonance imaging (MRI) or computed tomography (CT) scan to confirm location. Lesion number and diameter noted in preoperative imaging were confirmed intraoperatively. Radiofrequency was used for the initial cases and the ablation was performed as per the manufacturer’s instructions (Boston Scientific Corporation, Natick, MA). Figure 1 demonstrates a typical example of an exophytic tumor treated in this study. Radiofrequency cases were performed between 2/2001 and 6/2007.
Microwave ablation procedures were performed with two different systems beginning in 7/2007. The initial system involved a single or double antenna for the ablation with a power of 45 watts (W) for 8–10 min using a 915-Hz microwave generator (NeuWave System, Ethicon, Raritan, NJ) as per established recommendations. Subsequent microwave ablations were performed with the 2.45 GHz, 13 G microwave antenna (Emprint SX, Medtronic, Boulder, CO). The generator is set between 50 and 100 watts (W) and treatment occurs for 5–10 min based on the projected size of the ablation zone. Ideal ablations create a 0.5–1.0 cm margin of treated tissue around the tumor.
The standard follow-up schedule includes an initial study at one month post treatment, then every three months for two years, and then every six months in year three, followed by yearly studies. Follow-up duration was calculated from date of thermal ablation to date of most recent abdominal imagining (CT and/or MRI). Records were reviewed up to 36 months post ablation.
Post-ablation medical records and imaging were reviewed for each patient to assess for complete ablation, local recurrence, and dissemination of HCC to extrahepatic sites. Complete ablation was defined as radiographic evidence (CT and/or MRI) of hepatic necrosis at the ablation site without evidence of persistent tumor within the first three months after ablation [18]. Incomplete ablation is defined as viable tumor present in any portion of the treated lesion on post-ablation imaging within 12 months of the ablation [19]. Abdominal dissemination of HCC was defined as radiographic evidence of HCC within the peritoneal cavity during the follow-up period [20].
Statistical analyses were performed with Stata 14 (Stata Corporation, College Station, TX). Chi X2, Fisher exact tests, and risk ratios were used to evaluate categorical variables when appropriate. Levene’s tests were run for primary and subgroup analyses when appropriate. Shapiro–Wilk tests were used to test for normality. Welch’s t-tests were used to evaluate continuous variables when appropriate. Multivariable logistic regressions were used to determine influence of year of ablation, diameter of lesion, and segmental location of tumor on dissemination and incomplete ablation events. All p values were two-tailed and p values < 0.05 are considered significant. We reported cumulative incidence as percentages with 95% confidence intervals. Data were analyzed from March to July 2021.
Results
We reviewed 663 ablation records from 451 unique patients. Each record corresponded to a single procedure during which an average of 1.24 tumors were ablated. Of these records, 89 used an approach other than laparoscopic-assisted and were excluded leaving 574 records. Of these, an additional 56 complete records could not be located via the EMR and were excluded. Of the 519 remaining records, 6 tumors were identified on preoperative imagining but either were not located intraoperatively or could not be safely ablated, leaving 512 records for analysis. 186 records were excluded because follow-up imaging was less than 12 months from the date of the ablation (Fig. 2). Among the remaining 326 records, demographic data and MELD scores were available for all patients, as well as the size and number of lesions for all tumors. The included records span from 2/2001 to 6/2020.
There are no significant differences in demographic baseline characteristics between patients in the exophytic group compared to those in the non-exophytic group (Table 1). The preoperative MELD score was 10 in the exophytic group and 10 in the non-exophytic group (p = 0.90 95% CI − 0.95 to 1.09), lesion diameter was 2.68 cm in exophytic vs 2.47 cm in non-exophytic (p = 0.07 95% CI − 0.02 to 0.43), and the number of lesions ablated per case was 1.27 vs 1.20 (p = 0.32, 95% CI − 0.06 to 0.19) (Table 2). There were statistically significant differences between underlying causes of liver disease (p = 0.048) (Table 1) and TNM staging (p = 0.03) (Table 2) between exophytic and non-exophytic groups.
The 326 ablation events included in the original analysis were performed on 285 unique patients. We performed a sub-analysis limited to one ablation record from each of the 285 unique patients, and this shows no significant differences in TNM staging, underlying cause of liver disease, or rates of incomplete ablation between the exophytic and non-exophytic groups. The mean number of lesions ablated per case was 1.29 for the exophytic group and 1.21 for the non-exophytic group (p = 0.24; 95% CI − 0.05–0.21) (Table 3). Eliminating the inclusion of duplicate patients reduces the probability of overweighting certain demographic features in this study.
The number of events of intra-abdominal dissemination of HCC tumors post ablation is very low, 1.5% (n = 1) in the exophytic group and 1.7% (n = 4) in the non-exophytic group. Fisher’s exact test showed no significant difference in dissemination between the two groups (p = > 0.99) with a risk ratio of 0.66 (95% CI 0.07–5.79) (Table 4). There are fewer incomplete ablations in the exophytic group (n = 3) compared to the non-exophytic groups (n = 24) (3.3% vs 10.2%, respectively; p = 0.045) (Table 5).
Subgroup analysis of the method of ablation (RFA vs MWA) does not show any significant difference in the percentage of tumors that were exophytic vs non-exophytic (p = 0.43) (Table S1), and there is no difference in dissemination rates based on the ablation method used in the procedure (p = > 0.99) (Table 6). Similarly, there is no correlation between diameter of lesion and dissemination (p = 0.68, 95% CI -0.78–1.20) or year of ablation and dissemination (p = 0.49, 95% CI -0.21–0.10) (Table 7).
Multivariable logistic regression analysis was used to identify risk factors for incomplete ablation in this study. Lesion diameter positively correlates with incomplete ablations (p = 0.04; 95% CI 0.03–0.89), while the year of ablation does not correlate (p = 0.45; 95% CI -0.05–0.11) (Table 8). When analyzing for a pattern of incomplete ablations, there is no significant temporal trend, but slight spikes surrounding the implementation of new ablation technologies (2001 for RFA and 2009 for MWA) are present (Figure S1).
Throughout the study period, more ablations per year occurred on lesions located in right-sided hepatic segments (defined as segments V-VIII) compared to left-sided hepatic segments (defined as segments I-IV). The percentage of right-sided hepatic lesions compared to left-sided hepatic lesions was relatively constant throughout the study period (Figure S2). Likewise, the segmental location of the first lesion ablated per event does not show a significant difference over the study period (p = 0.48) (Table S2). Lastly, the size of the tumor did not correlate with the year of ablation (p = 0.13; 95% CI -0.03–0.01).
Discussion
The various modalities to treat HCC have expanded over the past two decades and continue to evolve with the adoption of new techniques and technologies that are safe and efficacious. Therapies include liver transplant, resection, chemoembolization, radiotherapy, and thermal ablation. The choice of which therapy to use is based on several factors, including tumor stage and location, as well as patient comorbidities. For small, early-stage tumors, local ablation methods such as thermal ablation are effective alternatives to hepatic transplant or resection as they can successfully eradicate the tumor without injuring the remaining liver.
Ablation can be accomplished either through a percutaneous-only or laparoscopic-assisted approach with similar outcomes from either modality [21]. A major advantage of laparoscopy is the ability to ablate tumors that cannot be safely treated by a percutaneous approach (e.g., superior–posterior tumors or those near perihepatic viscera), allowing for ablation of more tumors. Exophytic tumors are common, with the frequency of these tumors ranging from 16 to 52% of all HCC tumors in previous studies [22]. Traditionally, ablation of exophytic and subcapsular tumors have been considered contraindications to thermal ablation because of the reported increased risk of tumor seeding, tumor recurrence, and incomplete ablation [14, 15]. Several early studies reported rates of tumor seeding of 2.7% to 12.5% [15, 23, 24] after percutaneous ablation, with exophytic lesions being a major risk factor for intra-abdominal spread [25, 26]. Given this reported risk, many societal guidelines, including the 2012 EASL Clinical Practice Guidelines for HCC, stated the use of thermal ablation be avoided in exophytic tumors[27]. Equipment and technical advances (e.g., coagulation of the needle tract, etc.), have led to a decrease in reported tumor seeding events leading to a change in the EASL guidelines between 2012 and 2018 suggesting that percutaneous thermal ablation could be considered for exophytic tumors in experienced centers [7]. Two small studies reported an overall seeding rate of < 2% secondary to percutaneous ablations [18, 28]. Other studies show no significant difference in outcomes when comparing ablation of subcapsular with intraparenchymal tumors [25, 29].
This single-center large cohort study demonstrates no increased risk of intra-abdominal dissemination of HCC in small exophytic HCC tumors treated with laparoscopic-assisted thermal ablation. While overall rates of incomplete ablations in the current study are comparable to previous studies [28, 30, 31], in this study, we report a three times more likely risk of incomplete ablation of non-exophytic lesions versus exophytic lesions. We believe this is because the exophytic lesions are more clearly identified using a combination of direct visualization with simultaneous ultrasound and it allows the surgeon to see visual changes on the surface of the treated lesion. For those lesions that are non-exophytic, the surgeon is wholly dependent on initial targeting with ultrasound and there is no real-time confirmation of the tissue that has been treated.
This study covers two distinct periods based on the ablation technology used (RFA from 2001 to 2007 and MWA from 2008 to 2021). At the introduction of each ablation technology, there was a small, not statistically significant, spike in incomplete ablations suggesting operator expertise plays a minor role. A risk factor for incomplete ablations noted in this study is lesion diameter, with increasing tumor size correlating with increased risk of incomplete ablation, this is consistent with previous studies [30, 32, 33]
While tumor location did not vary significantly over the study period, there were more lesions ablated in segments VI-VIII. Throughout the course of the study, the surgical team used an in-line approach with the ultrasound to guide targeting. Two augmented navigation technologies were trialed throughout the years and these innovations allow the surgeon to take an out-of-plane approach to treat the tumor. These findings support an increased use of laparoscopic-assisted thermal ablation for eligible exophytic tumors in patients who are not candidates for hepatic resection or liver transplant.
There are several limitations in the current study. The study period spans > 15 years, so it reflects several technological changes, which could have an impact on overall results. Additionally, a subset of patients did not have surveillance imaging beyond six months after their ablation either because they underwent liver transplantation, died secondary to other causes, or they were lost to follow-up. We believe it is unlikely that any of them died secondary to disseminated disease as these patients would have been referred to our center by the medical providers in the community. As these procedures were done at a single center by one surgeon experienced in minimally invasive hepatic surgery, this potentially limits the generalizability of this approach as ablation of exophytic HCC tumors by less experienced surgeons might carry an increased risk of intrabdominal HCC dissemination.
Conclusion
Laparoscopic-assisted thermal ablation is an effective therapy to eradicate early-stage HCC tumors with less morbidity than liver resection and has the added benefit of accessing difficult tumors that are not amenable to percutaneous-only approaches. This study supports laparoscopic-assisted thermal ablation as a safe and efficacious treatment for non-exophytic as well as exophytic tumors, thereby expanding the number of tumors that can be treated by minimally invasive methods.
References
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380:1450–1462
Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Murray CJL (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 5:1749–1768
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16:589–604
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314
Wong RJ, Cheung R, Ahmed A (2014) Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 59:2188–2195
Huang DQ, El-Serag HB, Loomba R (2021) Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 18:223–238
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G (2016) Hepatocellular carcinoma Nat Rev Dis Primers 2:16018
Cillo U, Noaro G, Vitale A, Neri D, D’Amico F, Gringeri E, Farinati F, Vincenzi V, Vigo M, Zanus G (2014) Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford) 16:979–986
Fong Y, Sun RL, Jarnagin W, Blumgart LH (1999) An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 229:790–799
Xuan D, Wen W, Xu D, Jin T (2021) Survival comparison between radiofrequency ablation and surgical resection for patients with small hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 100:e24585
Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA (2019) Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist 24:e990–e1005
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–451
Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, Solé M, Rodés J, Bruix J (2001) Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 33:1124–1129
Ghaferi AA, Schwartz TA, Pawlik TM (2021) STROBE reporting guidelines for observational studies. JAMA Surg 156:577–578
Sacleux SC, Samuel D (2019) A critical review of MELD as a reliable tool for transplant prioritization. Semin Liver Dis 39:403–413
Worakitsitisatorn A, Lu DS, Lee MW, Asvadi NH, Moshksar A, Yuen AD, McWilliams J, Raman SS (2020) Percutaneous thermal ablation of subcapsular hepatocellular carcinomas: influence of tumor-surface contact and protrusion on therapeutic efficacy and safety. Eur Radiol 30:1813–1821
Filippousis P, Sotiropoulou E, Manataki A, Konstantinopoulos O, Thanos L (2011) Radiofrequency ablation of subcapsular hepatocellular carcinoma: single center experience. Eur J Radiol 77:299–304
Ganne-Carrié N, Nault J-C, Ziol M, N’Kontchou G, Nahon P, Grando V, Bourcier V, Barge S, Beaugrand M, Trinchet J-C, Seror O (2014) Predicting recurrence following radiofrequency percutaneous ablation for hepatocellular carcinoma. Hepat Oncol 1:395–408
Santambrogio R, Barabino M, D’Alessandro V, Galfrascoli E, Zappa MA, Piccolo G, Zuin M, Opocher E (2020) Laparoscopic thermoablation for hepatocellular carcinoma in patients with liver cirrhosis: an effective procedure for tricky tumors. Med Oncol 37:32
Mogahed MM, Zytoon AA, Eysa B, Manaa M, Abdellatif W (2020) Outcome of laparoscopic assisted percutaneous microwave ablation for exophytic versus non-exophytic hepatocellular carcinoma. J Gastrointest Cancer 52:892–898
Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF (2008) Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 57:1592–1596
Latteri F, Sandonato L, Di Marco V, Parisi P, Cabibbo G, Lombardo G, Galia M, Midiri M, Latteri MA, Craxì A (2008) Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis 40:684–689
Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, Paik YH, Kim MJ, Ahn JH (2016) Long-term Therapeutic Outcomes of Radiofrequency Ablation for Subcapsular versus Nonsubcapsular Hepatocellular Carcinoma: A Propensity Score Matched Study. Radiology 280:300–312
Livraghi T, Lazzaroni S, Meloni F, Solbiati L (2005) Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg 92:856–858
(2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer 48:599–641
Sparchez Z, Mocan T, Radu P, Mocan LP, Sparchez M, Leucuta DC, Al Hajjar N (2018) Prognostic factors after percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma. Impact of incomplete ablation on recurrence and overall survival rates. J Gastrointestin Liver Dis 27:399–407
Francica G, Meloni MF, de Sio I, Smolock AR, Brace CL, Iadevaia MD, Santambrogio R, Sironi S, Scaglione M, Lee FT Jr (2016) Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: Safety and efficacy. Eur J Radiol 85:739–743
Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT (2008) Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol 15:782–790
Ayav A, Germain A, Marchal F, Tierris I, Laurent V, Bazin C, Yuan Y, Robert L, Brunaud L, Bresler L (2010) Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg 200:435–439
Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, Xu ZF, Liu GJ (2005) Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol 60:1018–1025
Vilana R, Bruix J, Bru C, Ayuso C, Solé M, Rodés J (1992) Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology 16:353–357
Funding
Dr. Gerber had a simulation grant sponsored by Medtronic, Inc. and received consulting fees from Medtronic in the past 5 years. He has no active consulting agreement or active financial ties to disclose.
Author information
Authors and Affiliations
Contributions
DA Gerber made substantial contributions to the conception, design, acquisition, analysis, and interpretation of data in this work. IC Garbarine made substantial contributions to the analysis and interpretation of data in this work. Both authors made substantial contributions to drafting and revising this work. Both authors have approved the final version of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosures
Mr. Garbarine has no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Garbarine, I.C., Gerber, D.A. Risk of intra-abdominal seeding after laparoscopic-assisted thermal ablation of exophytic hepatocellular carcinoma tumors. Surg Endosc 36, 7569–7576 (2022). https://doi.org/10.1007/s00464-022-09192-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09192-z