Abstract
Background and aims
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is frequently used for the preoperative histologic diagnosis of pancreatic cancer. However, debate continues regarding the clinical merits of preoperative EUS-FNA for the management of resectable pancreatic cancer. We aimed to evaluate the benefits and safety of preoperative EUS-FNA for resectable distal pancreatic cancer.
Methods
The medical records of 304 consecutive patients with suspected distal pancreatic cancer who underwent EUS-FNA were retrospectively reviewed to evaluate the clinical benefits of preoperative EUS-FNA. We also reviewed the medical records of 528 patients diagnosed with distal pancreatic cancer who underwent distal pancreatectomy with or without EUS-FNA. The recurrence rates and cancer-free survival periods of patients who did or did not undergo preoperative EUS-FNA were compared.
Results
The diagnostic accuracy of preoperative EUS-FNA was high (sensitivity, 87.5%; specificity, 100%; positive predictive value 100%; accuracy, 90.7%; negative predictive value, 73.8%). Among patients, 26.7% (79/304) avoided surgery based on the preoperative EUS-FNA findings. Of the 528 patients who underwent distal pancreatectomy, 193 patients received EUS-FNA and 335 did not. During follow-up (median 21.7 months), the recurrence rate was similar in the two groups (EUS-FNA, 72.7%; non-EUS-FNA, 75%; P = 0.58). The median cancer-free survival was also similar (P = 0.58); however, gastric wall recurrence was only encountered in the patients with EUS-FNA (n = 2).
Conclusion
Preoperative EUS-FNA is not associated with increased risks of cancer-specific or overall survival. However, clinicians must consider the potential risks of needle tract seeding, and care should be taken when selecting patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a minimally invasive procedure and allows tissue diagnosis of suspicious pancreatic lesions with high diagnostic accuracy [1]. In a recent meta-analysis of EUS-FNA for solid pancreatic cancer, the pooled sensitivity and specificity were 85% and 98%, respectively [2]. EUS-FNA is a relatively safe procedure with overall morbidity and mortality rates of 0.98% and 0.02%, respectively [2,3,4]. However, the debate regarding the clinical importance of preoperative tissue confirmation for the adequate management of suspected resectable pancreatic cancer continues [5]. The EUS-FNA results may not completely disregard the possibility of malignancy in patients with suspected pancreatic cancer at times, and there are concerns regarding tumor cell dissemination along the needle tract when EUS-FNA is performed [6, 7].
Theoretically, cancer seeding may occur more frequently in the body or tail of a lesion when the trans-gastric approach is used rather than in the head of a lesion when a trans-duodenal approach is adopted [8]. This is because the EUS-FNA tract of pancreatic head cancers is later resected en block with the pancreatic head during curative-intent surgery, whereas the EUS-FNA tracts to the body or tail lesions are present beyond the surgical resection margins [9, 10]. Therefore, the tumor seeding risk should be analyzed in patients with distal pancreatic cancer who have undergone preoperative EUS-FNA and subsequent curative resection. However, the majority of the previous studies have been conducted on pancreatic cancer cohorts that included pancreatic head cancer [3, 10, 11].
This study aimed to investigate the clinical benefits of preoperative EUS-FNA and potential adverse events after preoperative EUS-FNA in patients with suspected distal pancreatic cancer. We also analyzed the long-term outcomes of patients with pancreatic ductal adenocarcinoma who underwent preoperative EUS-FNA followed by distal pancreatectomy.
Patients and methods
Study design and patients
We retrospectively reviewed the medical records of consecutive patients with suspected distal pancreatic cancer between January 2007 and December 2017. The outcomes of preoperative EUS-FNA were evaluated for these patients. The inclusion criteria were (1) age > 18 years, (2) presence of resectable suspected pancreatic cancer, and (3) receipt of preoperative EUS-FNA. Resectable pancreatic cancer was defined as cancer without distant metastasis, without localized tumor expansion in the celiac axis or hepatic artery, and without invasion of the superior mesenteric vasculature. Patients were excluded if their suspicious lesions were located in the pancreas head or uncinate process or were of an unresectable stage.
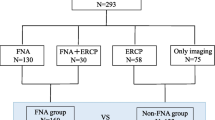
During the same period, we also reviewed the medical records of patients with distal pancreatic ductal adenocarcinoma who underwent distal pancreatectomy. The patients constituted two groups, namely, the EUS-FNA group (n = 193) wherein all patients underwent preoperative EUS-FNA and the non-EUS-FNA group (n = 335) wherein the patients did not receive EUS-FNA (Fig. 1). The long-term outcomes, including peritoneal recurrence or needle tract seeding, were assessed in both groups. The risk of recurrence after preoperative EUS-FNA was evaluated by comparing the recurrence-free survivals of the two groups. This study was approved by the institutional review board of the Asan Medical Center (approval no. 2018-0221).
Endoscopic procedures
All EUS procedures were performed using a linear array endoscope (GF-UCT260; Olympus Medical Systems, Tokyo, Japan) by 6 experienced endosonographers, who had each previously performed more than 1000 EUS examinations. The ultrasound images were analyzed using an ALOKA Prosound Alpha10 processor (ALOKA, Tokyo, Japan). Patients underwent EUS-FNA under conscious sedation with midazolam (3–5 mg i.v.) and pethidine (50 mg i.v.). EUS-FNA was performed by inserting a 19-, 22-, or a 25-gauge needle (Acquire, Boston Scientific Corporation, Natick, Mass, USA or Echotip, Cook Medical, Winston–Salem, NC, USA or Procore, Cook Medical, Winston–Salem, NC, USA) into the lesion in real-time under ultrasound guidance. The needle was moved back and forth > 10 times. The operators’ technique for specimen acquisition varied with each operator’s preference, including stylet slow-pull technique, standard suction, and number of to-and-fro movements with each needle pass.
Efficacy of preoperative EUS-FNA
The histopathologic findings of the resected specimens were used as the gold standard. When the EUS-FNA results were unsatisfactory or specimens were insufficient to achieve a specific diagnosis, the decision regarding repeated EUS-FNA or surgical resection without additional EUS-FNA depended upon the clinical findings associated with pancreatic cancer, i.e., new-onset diabetes or weight loss. In cases where surgical resection was not possible, the clinical course was followed by clinical and radiologic examinations, and pancreatic cancer was diagnosed if the lesion was aggravated.
Based on these results, we assessed the diagnostic sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of preoperative EUS-FNA for the diagnosis of pancreatic cancer in suspected lesions.
Early post-procedural adverse events
After EUS-FNA, physical and hematologic examinations, which included complete blood count, liver profile, and serum amylase and lipase levels, were performed, regardless of abdominal pain. Plain abdominal radiography was performed routinely. Post-procedural adverse events were classified as acute pancreatitis, bleeding, perforation, or infection.
Long-term clinical outcomes of EUS-FNA in patients with distal pancreatectomy
The long-term outcomes of EUS-FNA were investigated with respect to the effects of EUS-FNA on tumor recurrence and needle track seeding. These were analyzed by comparing with the outcomes of the patients who underwent curative surgery without preoperative EUS-FNA during the study period. Only long-term follow-up data from patients with pancreatic cancer were used. The patients visited our outpatient clinic 1 month postoperatively and were followed up for 3 months in the absence of a specific medical problems. Hematologic examinations, which included liver function tests, and imaging studies, such as computed tomography (CT) or endoscopic ultrasound, were performed at each follow-up visit. If no recurrence was encountered for 2 years, follow-up was performed every 6 months. Recurrence-free survival was calculated from the date of surgery to the date of recurrence of cancer in any organ.
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA). The sensitivity, specificity, PPV, NPV, and accuracy of preoperative EUS-FNA for the differentiation of pancreatic adenocarcinoma from suspected pancreatic malignant lesions were evaluated. The Kaplan–Meier method and the log-rank test were used to analyze overall survival and cancer-free survival. Univariate analyses were performed using the Cox proportional hazard model to identify the factors associated with overall survival and cancer-free survival. Continuous variables are presented as medians and 95% confidence intervals (CIs), and statistical significance were accepted for P values < 0.05.
Results
Patient demographic characteristics
The medical records of 2,824 patients with suspected pancreatic malignant lesions who underwent EUS-FNA were reviewed. Of these, 2,420 patients were excluded by applying the exclusion criteria. Finally, 304 patients with suspected pancreatic cancer located in the pancreatic body or tail were included (Fig. 1). The median patient age was 62 (range 30–88) years, and the male:female ratio was approximately 1.6:1.
Efficacy of preoperative EUS-FNA for pancreatic adenocarcinoma
Of the 304 patients (304 lesions) with resectable suspected pancreatic cancer lesions, 197 lesions were identified as pancreatic adenocarcinoma by EUS-FNA, and 193 patients underwent curative surgical resection; 4 patients refused to undergo surgery due to advanced age and/or comorbidities. The remaining 107 patients had negative EUS-FNA findings for pancreatic adenocarcinoma. Twenty-five of the 107 patients were diagnosed with another disease by EUS-FNA (neuroendocrine tumor (n = 9), autoimmune pancreatitis (n = 7), solid pseudopapillary tumor (n = 5), lymphoma (n = 2), chronic pancreatitis (n = 1), and tuberculosis (n = 1)). These diseases were treated conventionally, e.g., by surgical resection, steroid therapy, chemotherapy, or anti-tuberculosis medication, and observed closely. Most of these 25 patients responded to treatment and histologic diagnoses were confirmed by resection. However, one case of chronic pancreatitis diagnosed by EUS-FNA was changed to pancreatic ductal adenocarcinoma after resection. The other 82 cases with negative EUS-FNA findings for pancreatic adenocarcinoma were considered non-diagnostic specimens (n = 43) or negative for pancreatic cancer (n = 39) based on preoperative EUS-FNA. Twenty-five of the 82 patients underwent surgical resection based on the clinical features and radiologic findings compatible with pancreatic cancer, and 18 of these 25 were diagnosed with pancreatic adenocarcinoma; 4, neuroendocrine tumors; and 3, chronic pancreatitis. A total of 216 patients were diagnosed with pancreatic adenocarcinoma based on the histological findings of EUS-FNA or surgical specimens. The 57 patients not diagnosed histologically by EUS-FNA or surgical resection were placed under observation. Ten patients were clinically diagnosed with pancreatic cancer. A clinical diagnosis of pancreatic cancer was defined as the presence of a pancreatic mass suspected of malignancy based on the EUS findings of echogenicity and morphology or an increasing tumor size during the tracking period. Positron emission tomography–CT was performed for all 10 pancreatic masses, and positive findings were obtained for all. These patients did not survive > 18 months after pancreatic lesion detection. Finally, 225 patients were histologically or clinically diagnosed with pancreatic ductal adenocarcinoma. Of these, 197 were histologically confirmed by EUS-FNA, which indicated that preoperative EUS-FNA has a high sensitivity (87.5%) for differentiating between pancreatic adenocarcinoma and suspected pancreatic lesions. Seventy-nine patients diagnosed with other diseases, except for pancreatic cancer, with preoperative EUS-FNA avoided surgery. Consequently, the positive predictive value (PPV) of EUS-FNA was 100%; negative predictive value (NPV), 73.8%; specificity, 100%; and accuracy, 90.7%.
Post-procedural morbidity
Only 1 patient who underwent preoperative EUS-FNA developed post-procedural acute pancreatitis (defined as mild according to the consensus guidelines). The patient recovered uneventfully with conservative treatment. None of the patients experienced perforation, infection, or bleeding.
Long-term clinical outcomes of EUS-FNA
Between January 2007 and December 2017, 528 patients with distal pancreatic cancer underwent curative-intent distal pancreatectomy at the Asan Medical Center. The mean follow-up period was 34.1 months (95% CI, 31.7–36.4). The baseline characteristics of the patients are summarized in Table 1. These 528 patients constituted an EUS-FNA group (n = 193) and a non-EUS-FNA group (n = 335). A total of 232 patients were received gemcitabine chemotherapy (Days 1,8,15, 1000 mg/m2, for 6 cycles) and 70 patients were received concurrent chemotherapy (Days 1–5, Leucovorine 20 mg/m2 followed by fluorouracil 425 mg/m2, 6 cycles) and radiotherapy (50,4 GY) after surgical resection. The proportion of the patients were not different between groups (EUS-FNA, 63.7%; non-EUS-FNA, 53.4%; P = 0.07). Follow-up data were collected every 3–6 months postoperatively, and the final updates were obtained between April 2019 and July 2019.
The median overall survival was 28.9 months (95% CI, 23.1–34.8) in the EUS-FNA group and 25.1 months (95% CI, 21.9–28.3) in the non-EUS-FNA group (P = 0.1, Fig. 2). In the univariate Cox regression model, undergoing EUS-FNA was not significantly associated with unfavorable overall survival (hazard ratio (HR) 0.84; 95% CI, 0.68–1.03; P = 0.1, Table 2).
Overall survival periods after curative resection. The median overall survival was 35.4 months in the EUS-FNA group and 33.3 months in the non-EUS-FNA group (P = 0.58). There were no significant intergroup differences in the overall survival periods. EUS-FNA, endoscopic ultrasound-guided fine needle aspiration
Recurrence occurred in 373 (70.6%) of the 528 patients during follow-up. The most common location for recurrence was the liver (n = 111). The recurrence rates in the EUS-FNA and non–EUS-FNA groups were similar at 70.4% (136/193) and 70.7% (237/373), respectively (P = 0.51). Among them, 40 (20.7%) patients in the EUS-FNA group and 61 (18.2%) patients in the non-EUS-FNA group had peritoneal recurrence and showed no significant differences (P = 0.49). The median cancer-free survival was not significantly different between the groups (EUS-FNA group vs non–EUS-FNA group: 12.1 months (95% CI, 9.6–14.6) vs 12.4 months (95% CI, 10.5–14.4); P = 0.69, Fig. 3). In the univariate Cox regression model, none of the variables were associated with poorer cancer-free survival, and performing EUS-FNA was not an adverse prognostic factor (HR, 0.96; 95% CI 0.78–1.12; P = 0.69, Table 3). Two cases of gastric recurrence were encountered in the EUS-FNA group (Fig. 4). In these patients, no other metastasis except for gastric wall was found in one patient, but liver metastasis was observed together in another patient. On the other hand, no such recurrence occurred in the non-EUS-FNA group.
The representative images of gastric recurrence after EUS-FNA. A CT scan shows a low density mass at the pancreas (arrow). B EUS-FNA for the mass revealed adenocarcinoma. C CT scan, which was performed 11 months after distal pancreatectomy, shows a mass lesion at the stomach wall. D Esophagogastroduodenoscopy shows an ulcerofungating mass at the needle puncture site during EUS-FNA
Discussion
EUS-FNA is the preferred method for tissue acquisition in patients with pancreatic cancer because of the safety, relatively non-invasive nature, and high accuracy [12]. According to a recent meta-analysis performed with 4,984 patients, EUS-FNA has high sensitivity (85%) and specificity (98%) for diagnosing pancreatic malignancies [5]. In the present study, preoperative EUS-FNA also showed high sensitivity (87.5%), specificity (100%), and accuracy (90.7%) for the differentiation of pancreatic cancer from other pancreatic diseases. False-positive results for pancreatic cancer were not observed with preoperative EUS-FNA.
With regard to adverse event, EUS-FNA is considered as a safe procedure with major adverse event rate of ≤ 1%. The most reported adverse event was mild pancreatitis [1, 2]. In the current study, excellent safety characteristics were also observed. Only one documented procedure-related adverse event, mild pancreatitis, occurred among 304 patients who underwent preoperative EUS-FNA.
Based on its high discriminatory power for pancreatic cancer, and safety, we believe that EUS-FNA could be added to the preoperative examinations for suspected pancreatic cancer, especially when other diseases, such as lymphoma, autoimmune pancreatitis, and chronic pancreatitis, rather than pancreatic adenocarcinoma, are suspected.
However, our observations show that a negative preoperative EUS-FNA result does not entirely disregard the possibility of pancreatic cancer as the NPV of preoperative EUS-FNA was relatively lower (73.8%) than the other diagnostic values. An accurate differential diagnosis could not be achieved in 82 patients (non-diagnostic or negative for malignancy) with preoperative EUS-FNA; of these, 28 patients were finally diagnosed with pancreatic cancer by surgery or clinical observation. Similar or slightly lower NPV has been reported in previous studies which ranged from 55 to 66% [5, 13]. Given this low NPV of preoperative EUS-FNA, we believed that the negative results for pancreatic cancer in EUS-FNA may not be considered alone to determine subsequent management and recommend additional core samples using special needle that allows core samples to be collected by shearing tissue from the target lesion to overcome the limitation of low NPV of FNA [14]. According to a recently published prospective randomized study comparing FNA and fine needle biopsy (FNB), the technical success was not different between FNA and FNB on using needles for sampling solid lesions in 608 patients. The FNB needle had a significantly higher histological yield (77% vs 44%) and greater accuracy for malignancy diagnosis (87% vs 78%) and overall tissue classification based on the Bethesda cytopathology nomenclature system (82% vs 72%) [15]. Therefore, we believe that EUS-guided FNB should be favored over FNA when the results of FNA samples are non-diagnostic but clinically highly suspicious pancreatic cancer.
We found that EUS-FNA may be associated with needle tract seeding. Two (1.1%) cases of gastric wall recurrence were encountered in the EUS-FNA group, whereas no gastric wall recurrence occurred in the non-EUS-FNA group. However, our analysis of tumor recurrence rates, overall survival, and cancer-free survival periods did not reveal a significant intergroup difference between the EUS-FNA and non-EUS-FNA groups. Similar results were reported in previous studies. Ngamruengphong et al. [10] performed a retrospective study on 256 patients to evaluate whether preoperative EUS-FNA was associated with an increased risk of stomach or peritoneal recurrence and reported that the gastric or peritoneal recurrence rate was not significantly different (P = 0.21) between the EUS-FNA (n = 13; 7.5%) and non-EUS-FNA (n = 6; 2.1%) groups. Based on these results, some clinicians have concluded that EUS-FNA does not increase the risk of needle tract seeding or peritoneal dissemination [3, 16,17,18]. However, we believe that the reported results should be interpreted cautiously for the following reasons.
First, the number of patients in the previous studies and the present study might be not adequate to determine the actual frequency of tract seeding with accuracy. Given that the incidence of this complication in the current study was only 1.1%, a substantially larger-scale study will be essential to conclude that tumor seeding may occur along the needle tracts during EUS-FNA.
Second, pancreatic cancer is an aggressive malignancy with high relapse rates, even after curative resection, and it is challenging to differentiate local needle track seeding from local disease recurrence. Thus, there is a risk of underestimating the possibility of needle tract seeding, especially in cases involving distant metastasis. Therefore, we recommend that clinicians be aware of the possibility of needle tract seeding after EUS-FNA. Preoperative EUS-FNA should be implemented after multidisciplinary discussion regarding its requirement on a case-by-case basis to prevent overuse.
The present study has several limitations: First, it is limited by the single-center retrospective design, which introduces the potential of unmeasured confounding factors. Second, it is limited by an inadequate sample size. However, it is the largest study conducted to date to investigate the effects and safety of EUS-FNA for distal resectable pancreatic cancer treated by curative resection. Further large-scale investigation in a prospective setting is essential to confirm the outcomes and safety of preoperative EUS-FNA. Third, it may be difficult to determine whether recurrence was attributable to EUS-FNA or cancer progression, and the possibility of tumor recurrence from subclinical metastasis not detected at the time of surgery cannot be excluded.
In conclusion, preoperative EUS-FNA is useful and can be performed safely to differentially diagnose suspected resectable pancreatic masses. It does not negatively affect the overall survival, cancer-free survival, and recurrence rates. Therefore, EUS-FNA may be an appropriate diagnostic tool for identifying resectable distal pancreatic cancer. Moreover, preoperative EUS is expected to be able to provide important information for neo-adjuvant therapy in the patients with borderline stage of pancreatic cancer. However, clinicians must be aware of the potential risk of needle tract seeding also, and patients requiring EUS-FNA should be carefully selected after a multidisciplinary discussion.
References
Baek HW, Park MJ, Rhee YY, Lee KB, Kim MA, Park IA (2015) Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic lesions. J Pathol Transl Med 49:52–60
Chen G, Liu S, Zhao Y, Dai M, Zhang T (2013) Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology 13:298–304
Kim SH, Woo YS, Lee KH, Lee JK, Lee KT, Park JK, Kang SH, Kim JW, Park JK, Park SW (2018) Preoperative EUS-guided FNA: effects on peritoneal recurrence and survival in patients with pancreatic cancer. Gastrointest Endosc 88:926–934
Kudo T, Kawakami H, Kuwatani M, Eto K, Kawahata S, Abe Y, Onodera M, Ehira N, Yamato H, Haba S, Kawakubo K, Sakamoto N (2014) Influence of the safety and diagnostic accuracy of preoperative endoscopic ultrasound-guided fine-needle aspiration for resectable pancreatic cancer on clinical performance. World J Gastroenterol 20:3620–3627
Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ (2012) EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 75:319–331
Minaga K, Takenaka M, Katanuma A, Kitano M, Yamashita Y, Kamata K, Yamao K, Watanabe T, Maguchi H, Kudo M (2017) Needle tract seeding: an overlooked rare complication of endoscopic ultrasound-guided fine-needle aspiration. Oncology 93(Suppl 1):107–112
Chong A, Venugopal K, Segarajasingam D, Lisewski D (2011) Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc 74:933–935
Beane JD, House MG, Cote GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD (2011) Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery 150:844–853
Cosgrove ND, Yan L, Siddiqui A (2015) Preoperative endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic cancer in potentially resectable patients: Is this safe? Endosc Ultrasound 4:81–84
Ngamruengphong S, Swanson KM, Shah ND, Wallace MB (2015) Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut 64:1105–1110
Hartwig W, Schneider L, Diener MK, Bergmann F, Buchler MW, Werner J (2009) Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg 96:5–20
Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB (2005) Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc 61:854–861
Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L (2004) Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol 99:844–850
Levy MJ, Wiersema MJ (2005) EUS-guided Trucut biopsy. Gastrointest Endosc 62:417–426
van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, Van Der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque-Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ (2019) A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc 89:329–339
El Chafic AH, Dewitt J, Leblanc JK, El H II, Cote G, House MG, Sherman S, McHenry L, Pitt HA, Johnson C, Mohamadnejad M, Al-Haddad M (2013) Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy 45:883–889
Ikezawa K, Uehara H, Sakai A, Fukutake N, Imanaka K, Ohkawa K, Tanakura R, Ioka T, Tanaka S, Ishikawa O, Katayama K (2013) Risk of peritoneal carcinomatosis by endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. J Gastroenterol 48:966–972
Tsutsumi H, Hara K, Mizuno N, Hijioka S, Imaoka H, Tajika M, Tanaka T, Ishihara M, Yoshimura K, Shimizu Y, Niwa Y, Sasaki Y, Yamao K (2016) Clinical impact of preoperative endoscopic ultrasound-guided fine-needle aspiration for pancreatic ductal adenocarcinoma. Endosc Ultrasound 5:94–100
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. NRF-2017R1D1A1B04032097) and INHA University Grant.
Author information
Authors and Affiliations
Contributions
J-SP: conception and design, analysis and interpretation of the data, drafting of the article. JHL: drafting of the article and provision of study materials or patients. TJS: conception and design, critical revision of the article for important intellectual content, administration, and final approval of the article. JSL: collection and assembly of data and provision of study materials or patients. SJJ: analysis and interpretation of the data and administrative and technical support. DWO: collection and assembly of data and provision of study materials or patients. KBS, DWH, DHP, SSL, SCK, DWS, SKL, M-HK: critical revision of the article for important intellectual content and provision of study materials or patients.
Corresponding author
Ethics declarations
Disclosures
Jin-Seok Park, Jae Hoon Lee, Tae Jun Song, Joune Seup Lee, Seok Jung Jo, Dong Wook Oh, Ki Byung Song, Dae Wook Hwang, Do Hyun Park, Sang Soo Lee, Song Cheol Kim, Dong Wan Seo, Sung Koo Lee, and Myung-Hwan Kim have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, JS., Lee, J.H., Song, T.J. et al. The impact of preoperative EUS-FNA for distal resectable pancreatic cancer: Is it really effective enough to take risks?. Surg Endosc 36, 3192–3199 (2022). https://doi.org/10.1007/s00464-021-08627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08627-3