Abstract
Background and Aims
Endoscopic biliary drainage (EBD) is essential for the management of malignant hilar biliary obstruction (MHBO). We prospectively evaluated the efficacy and safety of “inside-stent” therapy, where a plastic stent is placed above the sphincter of Oddi without endoscopic sphincterotomy, in patients with inoperable MHBO.
Methods
This study was a multicenter, single-blinded, randomized controlled trial at three centers. Patients with inoperable MHBO were enrolled in this study, and randomly assigned to receive an inside-stent or conventional-stent therapy. The primary endpoint was cumulative stent patency of the initial stent. The secondary endpoints were second stent patency, technical and clinical success rate, adverse events, re-intervention rate, and overall patient survival.
Results
Forty-three patients were randomly assigned to the inside-stent group (n = 21) or the conventional-stent group (n = 22). The median cumulative stent patency of the initial stent was 123 days in the inside-stent group and 51 days in the conventional-stent group (P = .031). For patients with the initial stent dysfunction in the conventional-stent group, the inside-stent was placed as a second stent, and its patency was significantly longer than that of the initial stent (P = .0001). The technical and clinical success rate, re-intervention rate, second stent patency, adverse events, and survival probability did not differ between the groups.
Conclusions
Inside-stent therapy appears to be useful not only as an initial stent but also as a second stent for patients with inoperable MHBO.
Trial registration number: UMIN000004587.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic biliary drainage (EBD) is an essential treatment for malignant hilar biliary obstruction (MHBO), and patient prognosis depends on its success. EBD for MHBO is usually performed with self-expandable metal stents (SEMSs) because of their longer patency compared with plastic stents (PSs) [1, 2]. Although biliary stents with longer patency are ideal, occlusion is associated with all stent types and re-intervention is often required. Once SEMSs for MHBO become occluded, endoscopic re-intervention may be difficult and time-consuming due to the complexity of procedure [3, 4]. Moreover, the patency of a second stent tends to be short, from 1–2 months [3, 5]. Considering the high probability of re-intervention, a PS is a reasonable choice for initial biliary drainage because it is easy to exchange after obstruction, with shorter procedure time and lower cost compared with SEMSs. To enhance the patency of PSs, we have been placing a stent above the sphincter of Oddi without endoscopic sphincterotomy (ES), which is referred to as an “inside-stent” [6]. Although we previously reported the effectiveness of inside-stents in patients with benign biliary stricture, that for patients with malignant biliary stricture is unknown [7].
In the present study, we prospectively evaluated the efficacy and safety of inside-stent therapy in patients with inoperable MHBO compared with conventional PS (conventional-stent) therapy.
Methods
Study design
This study was a prospective, randomized, multicenter trial performed at three tertiary-care referral centers (Kyoto University Hospital, Kitano Hospital, and Osaka Red Cross Hospital). The institutional ethics review boards of the participating hospitals each approved the study protocol, and written informed consent was obtained from all enrolled patients. The protocol was registered at the University Hospital Medical Information Network (UMIN000004587).
Patients
From July 2011 through July 2017, 43 patients with inoperable MHBO who met the eligibility criteria were enrolled in the study (Fig. 1). Inclusion criteria were as follows: pathology-confirmed malignant hilar biliary obstruction, inoperable, and resolution of obstructive jaundice achieved by a single or double endoscopic nasobiliary drainage (ENBD) tube. Exclusion criteria were as follows: Kranofsky performance status scale of less than 60% (estimated survival < 4 months), inaccessible papilla due to accompanying duodenal obstruction or altered anatomy, previous endoscopic sphincterotomy, jaundice not controlled even with double ENBD tubes placement, and an inability to provide informed consent. All the patients were pathologically confirmed as MHBO and considered inoperable based on imaging modalities such as multidetector-row computed tomography (CT), magnetic resonance imaging (MRI), and cholangiogram. After resolution of obstructive jaundice by ENBD, all patients who provided written informed consents were consecutively enrolled in the study. Computer-generated randomization assignments were obtained just before the stent placement in a 1:1 ratio to the inside-stent or conventional-stent group using the block randomization method (Fig. 1). The Web registration system was centralized and protected to ensure concealment. The patients were masked to treatment allocation, and clinical data before and after stent placement were collected from their medical records.
Endoscopic procedures
Endoscopic retrograde cholangiography (ERC) was performed with a side-viewing duodenoscope (TJF-260 V, Olympus Medical Systems Co. Ltd, Tokyo, Japan) in a standard manner under conscious sedation with diazepam and pethidine hydrochloride, and with continuous pulse oximetry to monitor oxygen saturation. After cannulation of the bile duct, cholangiography was performed and a single ENBD tube was inserted into the dominant biliary branch as an initial biliary drainage. While waiting for the pathology confirmation and the decision on operability, the resolution of jaundice was evaluated by blood tests. Cholangiography using an ENBD tube was necessary to evaluate the longitudinal spread of cancer, to assess the resectability, and to select a proper stent. In patients whose jaundice did not improve after the initial ENBD tube insertion, an additional ENBD tube was placed in another dominant biliary branch without performing ES.
Stent placement
After resolution of obstructive jaundice by single or double ENBD tubes, PS placement was performed. The assigned PS (inside-stent or conventional-stent) was deployed into the same biliary branch where the ENBD tubes had been placed. The PS used in this study was the standard type 7F PS made from polyurethane (Flexima, Boston Scientific, Natick, MA, USA). The length of the stent varied depending on the stricture length. The PS was inserted without performing ES. The inside-stent was modified from the conventional PS. The distal flap of the stent was removed to facilitate its insertion through the papilla of Vater, and a knotted nylon thread was attached to the distal side hole to permit easy removal of the stent (Fig. 2) [7]. Moreover, this stent was placed above the sphincter of Oddi (Supplementary Fig).
Follow-up
After placement of the PS, the patients were followed and underwent an interview, abdominal X-rays, and biochemical liver tests at least once a month. If there were any signs of stent dysfunction, ERC was performed, the PS was removed, and then an ENBD tube was inserted. The removed PS was cut open using a scalpel to evaluate the reason for the dysfunction. As for a second stent, an inside-stent was selected for all patients if at all possible after the resolution of jaundice or cholangitis by ENBD.
Definitions
Resolution of jaundice was defined as a reduction in the serum total bilirubin level to below 2.0 mg/dl and/or elevated biochemical liver function test values (total serum bilirubin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, and alkaline phosphatase) decreased by at least half. Dominant biliary branch was defined as the right anterior branch, the right posterior branch, or the left hepatic duct based on CT and MRI. Dominant biliary branch occupied by the main tumor and atrophic liver were avoided for effective biliary drainage. Technical success was defined as the successful deployment of the PS in the biliary branches inserted with ENBD. Clinical success was defined as the decrease in the total bilirubin level to 50% of the pretreatment value within 1 week or to 75% within 4 weeks. Stent dysfunction was defined as a composite endpoint of either occlusion or migration, which was accompanied with clinical symptoms, such as cholangitis and/or elevated liver function tests results, together with biliary dilatation on imaging studies. Stent migration was defined as the positional shift of PS confirmed by monthly abdominal X-rays. Stent patency was referred to the time from PS placement to stent dysfunction. Consensus guidelines were used for definitions, grading, and therapy for ERC-related adverse events [8]. Early adverse event was defined as any stent-related adverse event within 4 weeks, and late event was defined as one that occurred after 4 weeks.
Outcome measures
The primary endpoint was cumulative stent patency of the initial stent between the groups. The secondary endpoints were technical success rate, clinical success rate, median follow-up period, adverse events, re-intervention rate, cumulative patency of the second stent, overall patient survival between the groups, and comparison of stent patency between the initial and the second stent in the conventional-stent group.
Statistical analysis
Sample size calculation
At the time this study was planned, there were no reports about the patency of PS for MHBO. Therefore, we referred to the stent patency for malignant “distal” biliary obstruction (MDBO), which is reported as 120 days [9]. Placement of an inside-stent was estimated to improve stent patency for up to 240 days from our previous experience. To achieve a statistical power of 80% with the assumption of a 2-sided Type 1 error rate of 5%, a total of 40 patients per group was calculated. Assuming a dropout rate of 10% of enrolled patients, a sample size of 45 patients per group was required. The sample size was calculated by a statistics expert.
Outcome assessment
Intention-to-treat and modified intention-to-treat methods were used in this analysis. The intention-to-treat analysis was based on the original total cohort of enrolled patients. The baseline characteristics and technical success rate were evaluated by the intention-to-treat analysis. The modified intention-to-treat analysis was used for the subset of patients in whom stent deployment was successful. The rate of clinical success, re-intervention, adverse events, stent patency, and survival were evaluated by the modified intention-to-treat analysis because the study was based on technically successful placement of the stent. All continuous variables are expressed as median with range or percentage. The characteristics of the study groups were compared using t test or Mann–Whitney test for continuous variables and Fisher exact test for categorical variables, as appropriate. All p-values were two-sided, and a p-value less than 0.05 was considered statistically significant. Cumulative stent patency and survival were estimated by Kaplan–Meier analysis, and curves were compared by log-rank test. To estimate stent patency, patients with death or adverse events other than stent dysfunction were treated as censored cases at the time of death or PS removal, respectively. All statistical analyses were performed using JMP version 13.0 (SAS Institute, Cary, NC).
Results
Patients characteristics
During the study period, 95 patients with inoperable malignant hilar biliary stricture were assessed for the study eligibility. Fifty-five patients were eligible for the study, and informed consent was obtained from 43 patients (Fig. 1). Because the sample size did not reach the calculated target number of 90 during the study period, analyses were performed using the collected data. The demographic data are summarized in Table 1. Sex, age, Kranofsky performance status, total bilirubin level, and tumor etiology were not significantly different between the groups. The median follow-up duration was 289.5 days (range 69–1238 days) in the inside-stent group and 184.5 days (range 28–1244 days) in the conventional-stent group.
Technical and clinical success rates
The study outcomes are summarized in Table 2. Double ENBD tubes were needed to resolve jaundice in six patients in both the inside-stent group (28.6%) and the conventional-stent group (27,3%, P = 0.227). The technical success rate was 95.2% (20/21) in the inside-stent group and 100% (22/22) in the conventional-stent group (P = 0.488). In the inside-stent group, one patient (4.8%) with huge hepatocellular carcinoma underwent percutaneous drainage because the PS could not pass through the biliary stricture. In both groups, the clinical success rate in patients with technical success was 100%.
Stent patency and survival
At the time of the final analysis, one patient in the conventional-stent group was lost to follow-up after the second stent placement. Nineteen patients (95%) in the inside-stent group and 20 patients (90.9%) in the conventional-stent group died during the follow-up. The stent dysfunction occurred in 16 patients (80%) in the inside-stent group and19 patients (86.4%) in the conventional-stent group (Table 2). The cumulative stent patency was 123 days in the inside-stent group and 51 days in the conventional stent group (P = 0.031; Fig. 3). The survival probability did not differ significantly between the groups (P = 0.452; Fig. 4). Second stent placement was performed in all 16 patients with the initial stent dysfunction in the inside-stent group and in 18 of 19 patients in the conventional-stent group. One patient in the conventional-stent group died of fulminant cholangitis without undergoing endoscopic revision due to his poor condition. An inside-stent was selected as a second stent for 11 of 16 patients in the inside-stent group and 12 of 18 patients in the conventional-stent group. Either a SEMS or a PS as a conventional-stent was inserted in the remaining patients for some reasons, such as patient preference. The patency of the inside-stent as a second stent did not differ signifiantly between the groups (89 days vs. 90 days, respectively; P = 0.177; Fig. 5). On the other hand, in 12 patients with the inside-stent as a second stent after the conventional-stent dysfunction, the patency of the second stent lasted much longer than that of their initial stent (P = 0.0001; Fig. 6).
Adverse events
There were no serious stent-related adverse events including fatal cases within four weeks after stent placement. Cholangitis occurred in one patient in each group and no pancreatitis was observed. Early stent migrations requiring re-intervention occurred in two patients (10%: 2/20) in the inside-stent group and three patients (13.6%: 3/22) in the conventional-stent group. Early stent occlusion was found in one and six patients in the inside-stent group and the conventional-stent group, respectively. As for late adverse events, stent migration was observed in two patients in the inside-stent group at 87 days and 319 days. All stent migrations observed were distal-side dislocation. Stent occlusion was observed in 11 patients in the inside-stent group and 10 patients in the conventional-stent group.
Discussion
Biliary drainage for inoperable MHBO remains challenging due to its anatomical complexity compared with MDBO. A SEMS has been shown to be superior to a PS for inoperable MDBO in terms of stent patency and adverse events [9]; however, what is the optimal EBD for inoperable MHBO has yet to be determined. This is the first prospective randomized study suggesting that the inside-stent is effective for inoperable MHBO not only as an initial stent but also as a second stent in re-intervention.
The pros and cons of using a PS or a SEMS for inoperable MHBO have been debated; that is, a PS is easy to insert and exchange but its patency tends to be short, while a SEMS has a longer patency but re-intervention would be difficult [10,11,12,13,14,15,16,17]. Moreover, as for using a PS for inoperable MHBO, it remains controversial whether an inside-stent is more suitable than a conventional-stent [18, 19], though one retrospective study showed that the median duration of inside-stent patency was significantly longer than that of conventionally placed stents [19]. Thus, this multicenter prospective randomized study was conducted to investigate the benefits of using an inside-stent in patients with inoperable MHBO. As a result, this study revealed that the median stent patency was 123 days in the inside-stent group and 51 days in the conventional-stent group (P = 0.031). Notably, the patency of the inside-stent was comparable with the previously reported patency of SEMS (100–150 days) [12, 20], except for two reports showing the long-term patency of SEMS (252 days and 359 days) [2, 14]. On the other hand, the stent patency of 51 days in the conventional-stent group was not different from those from previous reports [12, 14, 15]. To date, there has been only one prospective randomized clinical trial comparing the usefulness of inside-stent with conventional-stent placement in malignant biliary obstructions, in which no significant difference in the stent patency was observed between the two procedures [18]. This result is inferred to be due to the high rate of stent migration. The migration rate of inside-stent in Odense’s report was 52.9%, whereas 20% in our study. This difference could be explained by the stent material: polytetrafluoroethylene and polyurethane were used in PSs of Odense’s trial and our study, respectively. The PSs made with polyurethane are very soft and pliable, and can easily adapt to the S shape of the bile duct. In fact, the frequency of stent migration is reported to be significantly lower in polyurethane stents than in polyethylene stents [21]. Consequently, the inside-stent was useful as an initial stent for inoperable MHBO compared with the conventional-stent.
Furthermore, re-intervention for an initial stent dysfunction remains another issue, but there is no research evaluating which type of stent is suitable as a second stent for inoperable MHBO patients with an initial stent clogging. Based on previous reports, a SEMS is also considered to be the best choice as a second stent following obstruction of the SEMS. However, the technical difficulty of SMES placement as a second stent and its patency have not been discussed in detail [5, 22]. Tomoda et al. developed a newly designed PS that was easy to insert into a SEMS and explored the utility of this stent, but the patency of the PS placed through the obstructed SEMS was by no means satisfactory (44 days) [3]. In our study, re-intervention was required in more than 80% of patients in both groups and the inside-stent was used as a second stent in most of these cases. The patency of the inside-stent as a second stent was 90 days regardless of the type of the initial stent, which was longer than a previously reported study [3]. Interestingly, even the inside-stent used as a second stent had much longer patency than the conventional-stent as an initial stent. This result implies that, in order to achieve preferable stent patency, it is important to avoid exposure to the duodenal contents by placing stents above the sphincter of Oddi, whether it is an initial stent or a second stent. Taken together, the inside-stent was useful for inoperable MHBO not only as an initial stent but also as a second stent. Indeed, the median survival time in the inside-stent group was 293 days and this is comparable to the longest median survival time from previous reports [2, 14, 15]. In patients with inoperable MHBO, both long-term stent patency and feasibility of re-intervention in case with stent dysfunction are essential in achieving a good prognosis. Thus, this study suggested that inside-stent placement was a rational choice of EBD for inoperable MHBO.
This study has some limitations. First, the number of patients did not reach the target sample size. However, we believed that it would be worthwhile to show the usefulness of inside-stent, even if only in small numbers. Further large-scale, multicenter studies are preferable to validate the favorable outcome of inside-stent placement against inoperable MHBO achieved in this study. Second, it has not been proven whether an inside-stent is optimal against inoperable MHBO, since this is not a direct comparison with a SEMS widely used for inoperable MHBO. Therefore, a comparative study is warranted to demonstrate the superiority of inside-stent against inoperable MHBO.
In conclusion, this study suggested that an inside-stent is useful in patients with inoperable MHBO compared with a conventional-stent and it may also be suitable as a second stent in cases with the initial stent dysfunction, which may contribute to improved prognosis in patient with inoperable MHBO.
Abbreviations
- EBD:
-
Endoscopic biliary drainage
- MHBO:
-
Malignant hilar biliary obstruction
- SEMS:
-
Self-expandable metal stent
- PS:
-
Plastic stent
- ENBD:
-
Endoscopic nasobiliary drainage
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- KPS:
-
Kranofsky performance status
- ERC:
-
Endoscopic retrograde cholangiography
- ES:
-
Endoscopic sphincterotomy
- MDBO:
-
Malignant “distal” biliary obstruction
References
De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, Persico G (2003) Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc 58:50–53
Lee TH, Kim TH, Moon JH, Lee SH, Choi HJ, Hwangbo Y, Hyun JJ, Choi JH, Jeong S, Kim JH, Park DH, Han JH, Park SH (2017) Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc 86:817–827
Tomoda T, Kato H, Kawamoto H, Muro S, Akimoto Y, Uchida D, Matsumoto K, Horiguchi S, Tsutsumi K, Okada H (2017) Usefulness of a newly designed plastic stent for endoscopic re-intervention in patients with malignant hilar biliary obstruction. Endoscopy 49:1087–1091
Lee TH, Moon JH, Kim JH, Park DH, Lee SS, Choi HJ, Cho YD, Park SH, Kim SJ (2013) Primary and revision efficacy of cross-wired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures. Endoscopy 45:106–113
Kim JY, Kang DH, Kim HW, Choi CW, Kim ID, Hwang JH, Kim DU, Eum JS, Bae YM (2009) Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video). Gastrointest Endosc 70:1109–1115
Uchida N, Tsutsui K, Ezaki T, Fukuma H, Kamata H, Kobara H, Matsuoka H, Kinekawa F, Aritomo Y, Yokoyama F, Kita Y, Masaki T, Ogawa M, Nakatsu T, Watanabe S, Kuriyama S (2005) Estimation of the stent placement above the intact sphincter of Oddi against malignant bile duct obstruction. J Gastroenterol 40:291–296
Kurita A, Kodama Y, Minami R, Sakuma Y, Kuriyama K, Tanabe W, Ohta Y, Maruno T, Shiokawa M, Sawai Y, Uza N, Yazumi S, Yoshizawa A, Uemoto S, Chiba T (2013) Endoscopic stent placement above the intact sphincter of Oddi for biliary strictures after living donor liver transplantation. J Gastroenterol 48:1097–1104
Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 71:446–454
Davids PHP, Groen AK, Rauws EAJ, Tytgat GNJ, Huibregtse K (1992) Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. The Lancet 340:1488–1492
Perdue DG, Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Overby CS, Ryan ME, Bochna GS, Snady HW, Moore, Group EOSE (2008) Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J Clin Gastroenterol 42:1040–1046
De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C (2001) Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc 53:547–553
Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P (2012) Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc 76:93–99
Dumonceau JM, Tringali A, Blero D, Deviere J, Laugiers R, Heresbach D, Costamagna G, European Society of Gastrointestinal E (2012) Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 44:277–298
Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H (2013) Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci 20:214–222
Fukasawa M, Takano S, Shindo H, Takahashi E, Sato T, Enomoto N (2017) Endoscopic biliary stenting for unresectable malignant hilar obstruction. Clin J Gastroenterol 10:485–490
Cheon YK, Oh HC, Cho YD, Lee TY, Shim CS (2012) New 10F soft and pliable polyurethane stents decrease the migration rate compared with conventional 10F polyethylene stents in hilar biliary obstruction: results of a pilot study. Gastrointest Endosc 75:790–797
Sawas T, Al Halabi S, Parsi MA, Vargo JJ (2015) Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc 82:256–267
Pedersen FM, Lassen AT, Schaffalitzky de Muckadell OB (1998) Randomized trial of stent placed above and across the sphincter of Oddi in malignant bile duct obstruction. Gastrointest Endosc 48:574–579
Inatomi O, Bamba S, Shioya M, Mochizuki Y, Ban H, Tsujikawa T, Saito Y, Andoh A, Fujiyama Y (2013) Threaded biliary inside stents are a safe and effective therapeutic option in cases of malignant hilar obstruction. BMC Gastroenterol 13:31
Almadi MA, Barkun A, Martel M (2017) Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol 112:260–273
van Berkel AM, Bruno MJ, Bergman JJ, van Deventer SJ, Tytgat GN, Huibregtse K (2003) A prospective randomized study of hydrophilic polymer-coated polyurethane versus polyethylene stents in distal malignant biliary obstruction. Endoscopy 35:478–482
Inoue T, Naitoh I, Okumura F, Ozeki T, Anbe K, Iwasaki H, Nishie H, Mizushima T, Sano H, Nakazawa T, Yoneda M, Joh T (2016) Reintervention for stent occlusion after bilateral self-expandable metallic stent placement for malignant hilar biliary obstruction. Dig Endosc 28:731–737
Author information
Authors and Affiliations
Contributions
Akira Kurita had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design Kurita, Kodama, Uza, Takemura, Yoshimura, Seno. Acquisition of data: Kurita, Uza, Kodama, Asada, Takemura, Yazumi, Seno. Analysis of data: Kurita, Yoshimura, Uza, Kodama. Interpretation of data: Kurita, Uza, Kodama, Yazumi, Seno. Drafting of the manuscript: Kurita, Kodama, Uza, Yazumi. Critical revision of the manuscript for important intellectual content: Kodama, Uza, Seno, Yazumi. Statistical analysis: Yoshimura.
Corresponding author
Ethics declarations
Disclosures
Akira Kurita, Norimitsu Uza, Kenichi Yoshimura, Masanori Asada, Yuzo Kodama, Shujiro Yazumi, and Hiroshi Seno have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
464_2021_8576_MOESM1_ESM.tif
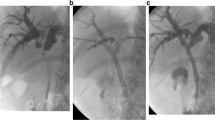
Supplementary file1 Supplementary Figure: Insertion of inside-stents. A Cholangiogram shows a biliary stricture around the hilar portion due to gallbladder cancer.B Placement of two inside-stents across the biliary strictures above the sphincter of Oddi. C An endoscopic view of the papilla of Vater after insertion of inside-stent. The inside-stent can be removed by pulling the nylon thread (TIF 3449 kb)
Rights and permissions
About this article
Cite this article
Kurita, A., Uza, N., Asada, M. et al. Stent placement above the sphincter of Oddi is a useful option for patients with inoperable malignant hilar biliary obstruction. Surg Endosc 36, 2869–2878 (2022). https://doi.org/10.1007/s00464-021-08576-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08576-x