Abstract
Background
The aim of the study is to analyze the feasibility, the safety and short- and medium-term survival of totally laparoscopic simultaneous resections (LSR) of colorectal cancer (CRC) and synchronous liver metastases (LM).
Methods
This is a retrospective study of a single-center series. Patients ASA IV, ECOG ≥ 2, major hepatectomies (≥ 3 segments), symptomatic CRC as well as low rectal tumors were excluded from indication. The difficulty level of all liver resections was classified as low or intermediate according to the Iwate Criteria. Dindo–Clavien classification for postoperative complications evaluation was used.
Results
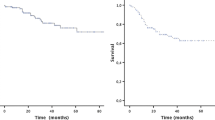
15 Patients with 21 liver lesions were included. Laparoscopic liver surgery was performed first in every case. Median size of the lesions was 20 mm (r 8–69). Major complications (Dindo–Clavien ≥ 3) occurred in 3 patients (20%); median hospital stay was 7 days (r 4–35), and only one patient (6.6%) was readmitted upon the first month from the surgery. 90-day mortality rate was 0%. After a median follow-up of 24 months (r 7–121), disease-free survival at 1, 2 and 3 years was 58%, 36% and 24%, respectively; overall survival at 1, 2 and 3 years was 92.3%.
Conclusions
In selected patients, LSR of CRC and LM is technically feasible and has an acceptable morbidity rate and mid-term survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Approximately 25% of colorectal cancers (CRC) show synchronous liver metastases (LM) at diagnosis. Resection of both tumor focus in combination with chemotherapy constitutes the best therapeutic option and offers 5-year survival rates of between 20 and 50% [1, 2]. Simultaneous resection (SR) is infrequent; however, in selected patients it has proven to be a feasible procedure with results comparable to those of two step surgery [3,4,5,6,7].
The use of laparoscopic resection techniques for colorectal tumors and liver lesions has become the norm in the last two decades [8,9,10,11]. Laparoscopic liver surgery appears to decrease intraoperative bleeding rates, the number of major complications and the length of hospital stay with comparable oncological outcomes [10, 12, 13]. In this context, some groups have presented their initial experiences in laparoscopic simultaneous resection (LSR) of the primary tumor and the LM [14,15,16], but the literature focusing on LSR is scant as shown in a recent review [17]. Reports present a small number of patients, usually between 7 and 50. Moreover, most of the LSR reports are usually highly selected patients with a median of 1–2 resected liver lesions of a median size of between 2 and 3 cm. Consequently, minor resections account for 75–100% of the reported laparoscopic liver resections. In the review, 30-day mortality was not consistently reported and when studied, short- and mid-term survival was comparable to that obtained after open resection [17]. Initial reports show that LSR in selected patients is feasible and safe.
We started the laparoscopic liver surgery program in 2007. In 2009 we carried out the first LSR: a left lateral sectionectomy (LLS) with concomitant oncologic sigmoidectomy. Since then, this surgical approach is indicated in our center for selected patients. Herein we present our experience with LSR of CRC and synchronous LM with the aim to demonstrate feasibility, emphasizing technical aspects and those related to candidate selection and short- and medium-term survival results.
Materials and methods
Study population and data collection
We carried out a retrospective analysis according to the PROCESS guidelines [18] based on a single-center prospective case series. LSR were performed between August 2009 and October 2019. Data were collected from the medical charts of the patients and informed written consent was signed in all cases. The study was approved by the Institutional Review Board at Cruces University Hospital.
In our protocol, patients with CRC and synchronous LM are evaluated by a Multidisciplinary Committee, involving hepato-pancreato-biliary (HPB) and colorectal surgeons, anesthetists, pathologists, gastroenterologists, and medical and radiation oncologists. Image techniques always include triple-contrast full-body computed tomography combined with Primovist® liver magnetic resonance imaging and/or contrast-enhanced liver ultrasound with SonoVue®. The combined laparoscopic approach is indicated in all patients with CRC and synchronous LM excluding those with a pre-anesthetic evaluation of ASA IV, poor performance status (ECOG ≥ 2), need for major hepatectomies (≥ 3 segments), highly symptomatic CRC (obstruction, perforation, bleeding) and/or low rectal tumors. Furthermore, since 2014 the preoperative difficulty of liver resection has been assessed using the Iwate Score Index [8] and every attempt is made to avoid procedures entailing a greater than intermediate risk. Prior to this date the difficulty of liver resections was assessed retrospectively.
Routinely, neoadjuvant treatment is applied in cases with locally advanced rectal carcinomas or those with high-risk liver disease [19]. After surgery, all patients are re-evaluated by a medical oncologist to determine if adjuvant therapy is indicated.
We evaluated the demographic aspects of the patients, the general characteristics of the CRC and the LM, the Iwate Score, and the intraoperative aspects and postoperative complications according to the Dindo–Clavien classification [20]. Estimated overall survival (OS) and disease-free survival (DFS) at 3 years were also calculated.
Description of the surgical technique
Once anesthetized, the patient is placed in the French position. Both HPB and colorectal surgeons jointly decide on the placement of the laparoscopic trocars, which generally do not usually exceed a total of 5. The intraabdominal pressure limit is set at 12 mmHg for both surgical procedures. The intervention begins initially with the liver procedure, with the patient in the anti-Trendelenburg position and working at low central venous pressure which contributes to reduce venous bleeding. Intraoperative ultrasonography (IUS) is performed systematically. The intermittent Pringle maneuver as described by Rotellar et al. [21] is performed selectively, with a maximum duration of 15 min and 5 min of release. For liver transection, harmonic shears or a bipolar vessel sealer are used along with an ultrasonic dissector in some cases, with a desirable resection margin of at least 1 cm. We follow a liver parenchyma preserving policy. Once the lesion is resected, it is placed in a retrieval bag. Subsequently, the colorectal resection is performed according to the classic oncological principles of high vessel ligation and medial to lateral dissection including the entire mesentery or mesorectal excision in cases of anterior resection of the rectum. Per protocol, no abdominal drainage is put in place for either the hepatic or colonic resections while it is always used after rectal surgery; however, the final decision always depends on the surgeon´s criteria. Finally, both specimens are extracted through the same incision used for the extraction of the colorectal specimen.
Statistical analysis
Quantitative variables were described using the median and range or the mean and standard deviation, while qualitative variables were described with percentages. Patients survival was estimated using the method of Kaplan–Meier. DFS was defined as survival from the date of the intervention to the date of recurrence. Patients were censored if death occurred from a cause other than tumor progression or if the patient was alive at last follow-up.
Statistical analysis was performed using R (version 4.0.1).
Results
A total of 17 SLR were carried out during the study period. One intervention was converted to laparotomy due to the finding of peritoneal carcinomatosis, and cytoreductive surgery was performed. Combined resection was completed laparoscopically in 16 patients. In one case the pathological result was liver adenoma and therefore this was also excluded. In sum, a total of 15 patients were finally included in the study.
The demographics and the general characteristics of the LM and the CRC are shown in Table 1. Median age was 63 years (range 41–79) and 9 patients (60%) were male. Nine patients were considered to be ASA II (60%) and 6 ASA III (40%). Four patients presented a performance status assessment of ECOG 1 (26.6%). Median CEA was 6.2 ng/mL (r 0.7–492). Regarding the distribution of the CRC, 10 tumors were located in the colon (66.6%) and 5 in the rectum (33.3%). Neoadjuvant chemotherapy due to locally advanced preoperative staging was indicated in the only patient with the middle rectum tumor and in one of the four patients with high rectal tumor.
In our patients, neoadjuvant treatment was not considered for the liver disease as all patients were considered at low-risk for recurrence [21]. In the preoperative assessment, 14 patients presented a solitary LM (93.3%) and 1 patient presented 3 lesions (6.6%). Using IUS, another four LM were found, one in the patient who previously had three metastases and three in a patient with a solitary LM, thus making a total of 21 lesions that were resected. The median size of the tumors was 20 mm (r 8–69). Based on the evaluation according to the Iwate Score, 5 patients (33.3%) scored ≥ 4 points (intermediate risk), and none fell into the advanced or expert risk groups (Table 2).
Surgical resections are presented in Table 3. They consisted of an LLS, an anatomic resection of liver segment-VI, and 13 non-anatomical resections. 11 of these 13 were single metastasectomies, and in the other two patients multiple metastasectomies were performed: one quadruple and one triple with which radiofrequency (RF) of a deep LM was associated. Intermittent Pringle clamping was practiced in 5 patients (33.3%), with an average time of 38.75 ± 12.5 min. The median operative time was 300 min (r 180–410). All resections were considered R0.
The perioperative and postoperative results are shown in Table 3. 3 Patients (20%) presented major complications (Dindo–Clavien ≥ III) within 90 days of surgery: (1) 1 perihepatic fluid collection which was drained percutaneously, (2) 1 biliary leak combined with pseudo-aneurysm of the hepatic artery in an hemophiliac patient in whom angio-embolization was initially performed and subsequent laparotomy was required to evacuate the hemoperitoneum, and (3) 1 anastomotic leak in a rectal tumor that had received neoadjuvant chemotherapy, in which resection of the anastomosis and terminal colostomy was performed. The median hospital stay was 7 days (r 4–35). The Enhanced Recovery After Surgery (ERAS) program was completed by 11 patients (73.3%). Only 1 patient (6.6%) was readmitted within 90 days of the surgery due to an intraabdominal fluid collection which required only medical treatment. 13 Patients (86.6%) received adjuvant chemotherapy, of whom nine completed the full course. One patient rejected this option, and another patient was not a good candidate due to his comorbidities.
After a median follow-up of 24 months (r 7–121), tumor progression was observed in 10 patients (66%), with a median time to recurrence of 14 months (r 2–33). Recurrence sites included two cases in the liver, two in the lung, four in the liver and lung, one in the mesenteric lymph nodes and one as generalized peritoneal carcinomatosis (Table 4). DFS at 1, 2 and 3 years was 58%, 36% and 24%, respectively (Fig. 1A). Of the 10 patients that presented tumor progression, 8 received surgical rescue with curative intent. Liver re-resection was accomplished in five patients, one of which consisted of a laparoscopic LLS, while the rest underwent surgery using an open technique. RF of a LM was also applied in one case, in combination with the liver resection. The other three interventions corresponded to two atypical lung resections and one lymph node resection at the origin of the inferior mesenteric vein. In a patient with small and diffuse pulmonary metastases, maintenance chemotherapy was indicated with persistent radiological stability. The patient who presented generalized peritoneal disease received chemotherapy and cytoreduction surgery with hyperthermic intraperitoneal chemotherapy (HIPEC).
Two deaths occurred during follow-up, none of which was related to malignant disease progression: the hemophiliac patient died at 5 months of a stroke, and the first patient of the series, who had surgery in August 2009, died more than 10 years later due to mesenteric ischemia. Both were disease-free. OS at 1, 2 and 3 years was 92.3% (Fig. 1B).
Discussion
Approximately 25% of the patients diagnosed with colorectal tumors present synchronous LM [1]. When possible, the classic approach has been resection of the primary tumor first; however, the inverse approach—the liver first—has been popularized in recent years for those patients whose liver disease is a more crucial factor for patient outcomes [22]. More recently, SR has shown comparable postoperative and oncological outcomes in selected patients [4,5,6,7]. In addition, SR may provide early access to adjuvant therapies, psychological benefit for the patients and a reduction in costs by avoiding a second procedure as long as a good selection of the patients is made and major complications with a subsequent extended hospital stay do not occur [9, 23]. In this sense SR should be discouraged when major hepatectomy or complex rectal surgery is needed because of a significant increase in postoperative morbidity in these cases [5, 24]. In 2015 an Expert Group on OncoSurgery management of Liver Metastases (EGOSLIM group) recommended the simultaneous approach in patients with colorectal tumors requiring limited hepatic resection [25].
Laparoscopic liver surgery has developed during the last two decades and currently almost all kinds of liver resections can be performed in experienced units [11]. With regard to colorectal LM, recent studies have demonstrated better results for the laparoscopic approach versus open surgery in terms of perioperative morbidity, transfusion requirements and hospital stay with comparable OS and DFS [12, 13]. Going one step further, a recent systematic review has analyzed four non-comparative studies of LSR and eight comparative studies of LSR versus open synchronous resections [17]. In this review by Moris et al., the results of LSR were comparable to open resections in terms of postoperative complications and both OS and DFS. In accordance with most reports, optimum patient selection for LSR is paramount to achieve good results. Minor liver resection comprised between 75 and 100% of the patients included in the different studies, with a median number of hepatic lesions between 1 and 2 with a median maximum size of 2.5 to 4 cm [17]. In addition, all liver resections were minor in a recent multicenter propensity score study of 61 patients with LSR, and a 69% rate of solitary lesions and a tumor size ≤ 3 cm in 78% of the patients [26]. In this study, outcomes after LSR were comparable to those obtained in a control group of laparoscopic CRC resection demonstrating that LSR can be safely performed without increasing the risk of postoperative morbidity compared to laparoscopic colorectal surgery alone.
In our center, we have followed a strict selection policy for LSR, avoiding complex liver or colorectal resections. This policy is similar to that we used for simultaneous open surgery prior to the laparoscopic era. All the patients in our series were treated with minor liver resections. Tumors were solitary in 87% of cases with a median size of 2 cm. According to the Iwate Scoring System, none of our patients scored more than 6 points and therefore all could be included in the low and intermediate levels of difficulty. Multiple tumors were treated with multiple metastasectomies and laparoscopic ablation following our policy of preferring parenchyma preserving procedures. Furthermore, rectal surgery comprised only one third of the colorectal procedures with no cases of low rectal tumor. In this scenario, no intervention was converted to open surgery and the overall complication rate was 26.6% with an incidence of Dindo–Clavien ≥ 3 complications of 20% and a zero 90-day mortality rate. These results are comparable to those reported by other authors. Van der Poel et al. reported a 15% incidence of Dindo–Clavien ≥ 3 complications and no 30-day mortality [26]. In the review by Moris et al., the overall complication rate after LSR was 7–35% with a rate of Dindo–Clavien ≥ 3 complications of 8.5–12.5% [17]. In most of the studies, no significant difference was seen in the complication rate when compared with open synchronous surgery.
Administration of neoadjuvant chemotherapy in patients with CR liver metastases is a matter of debate. While survival benefits have been observed in high-risk patients after neoadjuvant chemotherapy, upfront surgery is an accepted option in low-risk patients such as ours with metastases limited in number and size [27, 28]. On the other hand, two of our patients received neoadjuvant chemotherapy due to locally advanced preoperative staging of the rectal tumor. This circumstance makes our series heterogeneous relating oncological therapy and might impact survival; however, these patients were maintained in the study as our primary aim was to demonstrate feasibility emphasizing technical aspects and those related to candidate selection. Regarding adjuvant treatment, there is no strong evidence to support its use in patients with favorable oncological and surgical criteria after liver resection; however, adjuvant treatment is recommended in patients who have not received any previous chemotherapy. In our series, all patients were indicated to receive adjuvant chemotherapy.
DFS in our study was 58% and 24% at 1 and 3 years, respectively. We recognize that our DFS rate could be improvable; however, it is comparable to that obtained by other authors after LSR. Recently, Chen et al., in a single-center experience of 16 cases with LSR, reported an 85% OS at 3 years with a DFS of 56% and 35% at 1 and 3 years, respectively [29]. In another report, Xu et al. also showed a 36.6% 3-year DFS in a cohort of 20 LSR [30]. Overall, DFS rates after LSR compare well with those reported after metachronous surgery. De Jong et al. in a multi-institutional analysis of 1669 patients undergoing open surgery for colorectal LM with curative intent found a DFS rate of 69.2% and 37.7% at 1 and 3 years, respectively [31]. Moreover, a 3-year DFS of 30.4–40% has been reported after laparoscopic liver resection of metachronous colorectal metastases [12, 13]. The liver-first strategy is another therapeutic option to be considered in patients with synchronous colorectal liver metastases. This reverse approach initially proposed by Mentha et al. is currently indicated in patients with more oncologically advanced disease and a poorer prognosis, while combined resection for simultaneous primary colorectal tumor and liver metastases is recommended only for patients who require a limited hepatic resection [32, 33]. The liver-first approach achieves a 3-year DFS of 30–42.2% [34, 35]. Noteworthy, no difference in survival has been demonstrated in patients with synchronous colorectal metastases managed by either synchronous, liver-first or classical approach [36,37,38].
In contrast with the DFS, the 3-year OS of 92.3% observed in our study was remarkable compared with that already published for the combined open surgery and the liver-first strategy: 60–70% and 48–79%, respectively [35, 39, 40]. We believe that this high OS may be related to several specific factors: (1) the strict selection policy for combined surgery, (2) our policy in favor of parenchymal sparing techniques in liver surgery for LM and, (3) the aggressive attitude for salvage surgery of recurrent disease followed in our unit. In fact, 8 out of the 10 patients who developed recurrence in our series underwent surgery with curative intent.
Regarding the technical aspects, we prefer starting with the liver resection because this sequence allows us to use the incision performed during the colorectal surgery to extract both specimens. Of note, in our selected patients, surgical time is usually short for liver resections and the Pringle maneuver is always intermittent though randomly used, therefore reducing possible splenic congestion. In fact, the intermittent Pringle maneuver was used in only one third of the patients for a mean time of 38 min. Median total operative time was 300 min, which compares well to that reported in the literature for open synchronous resection [41]. We believe that good planning of the surgical procedure with the colorectal team in terms of trocar position and timing is essential to perform LSR.
We recognize some limitations to our study. It is retrospective and the number of patients is limited. Our study does not have a comparative group; however, this is so because all the potential candidates for simultaneous surgery are assessed to undergo laparoscopic surgery according to our selection criteria. Open resection is indicated only in those patients who do not fulfill those criteria and thus, both groups cannot be compared. In addition, median follow-up is only 2 years and this does not allow us to report long-term outcomes. Nevertheless, the literature relating to this novel surgical approach is scarce and most reports are limited, single-center, retrospective and non-comparative experiences, therefore, leading to limitations for the generalization of the procedure [27]. We believe that our experience may add to the current knowledge on LSR.
In summary, simultaneous resection of the primary colorectal tumor and synchronous liver metastases is feasible and safe in selected patients with postoperative morbidity levels and oncological outcomes comparable to those of open combined surgery. Prospective studies with larger series and longer follow-up are needed to confirm these initial findings. Data from international registries such as the European Registry of Minimally Invasive Surgery would be of help to achieve this goal.
References
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422
Weiser MR, Jarnagin WR, Saltz LB (2013) Colorectal cancer patients with oligometastatic liver disease: what is the optimal approach? Oncology 27:1074–1078
Elías D, Detroz B, Lasser P, Plaud B, Jerbi G (1995) Is simultaneous hepatectomy and intestinal anastomosis safe? Am J Surg 169(2):254–260
Martin R, Pay P, Fong Y, Grace A, Cohen A, DeMatteo R et al (2003) Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 197(2):233–241
Ono Y, Saiura A, Arita J, Takahashi Y, Takahashi M, Inoue Y (2017) Short-term outcomes after simultaneous colorectal and major hepatic resection for synchronous colorectal liver metastases. Dig Surg 34(6):447–454
de Haas RJ, Adam R, Wicherts DA, Azoulay D, Bismuth H, Vibert E et al (2010) Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg 97(8):1279–1289
Chen J, Li Q, Wang C, Zhu H, Shi Y, Zhao G (2011) Simultaneous vs. staged resection for synchronous colorectal liver metastases: a metaanalysis. Int J Colorectal Dis 26:191–199
Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y et al (2014) A novel difficulty scoring for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 21:745–753
Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW et al (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 267:199–207
Ciria R, Ocaña S, Gómez-Luque I, Cipriani F, Halls M, Fretland A et al (2020) A systematic review and meta-analysis comparing the short and long term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 34(1):349–360
Cherqui D (2016) Evolution of laparoscopic liver resection. Br J Surg 103(11):1405–1407
Cipriani F, Rawashdeh M, Stanton L, Armstrong T, Takhar A, Pearse NW et al (2016) Propensity score-based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg 103(11):1504–1512
Allard MA, Sa Cunha A, Gayet B, Adam R, Goere D, Bachelier P et al (2015) Early and long-term oncological outcomes after laparoscopic resection for colorectal liver metastases. Ann Surg 262:794–802
Hoekstra LT, Busch OR, Bemelman WA, van Gulik TM, Tanis PJ (2012) Initial experiences of simultaneous laparoscopic resection of colorectal cancer and liver metastases. HPB Surg. https://doi.org/10.1155/2012/893956
Ivanecz A, Krebs B, Stozer A, Jagric T, Plahuta I (2018) Simultaneous pure laparoscopic resection of primary colorectal cancer and synchronous liver metastases: a single institution experience with propensity score matching analysis. Radiol Oncol 52(1):42–53
Takorov I, Belev N, Lukanova T, Atanasov DG, Djurkov V et al (2016) Laparoscopic combined colorectal and liver resections for primary colorectal cancer with synchronous liver metastases. Ann Hepatobiliary Pancreat Surg 20:167–172
Moris D, Tsilimigras D, Machairas N, Merath K, Cerullo M, Hasemaki N et al (2019) Laparoscopic synchronous resection of colorectal cancer and liver metastases: a systematic review. J Surg Oncol 119(1):30–39
Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler A, Orgill DP, for the PROCESS Group (2018) The PROCESS 2018 Statement: Updating Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) Guidelines. Int J Surg 60:279–282
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Rotellar F, Pardo F, Bueno A, Martí-Cruchaga P, Zozaya G (2012) Extracorporeal tourniquet method for intermittent hepatic pedicle clamping during laparoscopic liver surgery: an easy, cheap, and effective technique. Langenbecks Arch Surg 397(3):481–485
Waisberg J, Ivankovics IG (2015) Liver-first approach of colorectal cancer with synchronous hepatic metastases: a reverse strategy. World J Hepatol 18(11):1444–1449
Ejaz A, Semenov E, Spolverato G, Kim Y, Tanner D, Hundt J et al (2014) Synchronous primary colorectal and liver metastasis: impact of operative approach on clinical outcomes and hospital charges. HPB (Oxf) 16:1117–1126
Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L et al (2007) Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 14(12):3481–3491
Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E et al (2015) Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 42:729–741
Van der Poel MJ, Tanis PJ, Marsman HA, Rijken AM, Gertsen EC, Ovaere S et al (2019) Laparoscopic combined resection of liver metastases and colorectal cancer: a multicenter, case-matched study using propensity scores. Surg Endosc 33:1124–1130
Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P et al (2009) Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 20:985–992
Vera R, González-Flores E, Rubio C et al (2020) Multidisciplinary management of liver metastases in patients with colorectal cancer: a consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin Transl Oncol 22:647–662
Chen YW, Huang MT, Chang TC (2019) Long term outcomes of simultaneous laparoscopic versus open resection for colorectal cancer with synchronous liver metastases. Asian J Surg 42:217–223
Xu X, Guo Y, Chen G, Li C, Wang H, Dong G (2019) Laparoscopic resections of colorectal cancer and synchronous liver metastases: a case controlled study. Minim Invasive Ther Allied Technol 27(4):2019–2216
De Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick R et al (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis. An international multi-institutional analysis of 1669 patients. Ann Surg 250:440–448
Mentha G, Majno P, Andres A, Rubbia-Brandt L, Morel P, Roth A (2006) Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 93(6):872–878
Welsh F, Chandrakumaran K, John T, Cresswell A, Ress M (2016) Propensity score-matched outcomes analysis of the liver-first approach for synchronous colorectal liver metastases. BJS 103:600–606
Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L et al (2012) A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases. Ann Surg 256:772–779
De Jong M, Beckers R, Van Woerden V, Sijmons J, Bemelmans M et al (2018) The liver-first approach for synchronous colorectal liver metastases: more than a decade of experience in a single centre. HPB 20:631–640
Kelly M, Spolverato G, Lê G, Mavros M, Doyle F, Pawlik T et al (2015) Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol 111(341):315
Sturesson C, Valdimarsson V, Blomstrand E, Eriksson S, Nilsson J, Syk I et al (2017) Liver-first strategy for synchronous colorectal liver metastases—an intention-to-treat analysis. HPB 19:52–58
Baltatzis M, Chan A, Jegatheeswaran S, Mason J, Siriwardena AK (2016) Colorectal cancer with synchronous hepatic metastases: systematic review of reports comparing synchronous surgery with sequential bowel-first or liver-first approaches. EJSO 42:159–165
Mayo S, Pulitano C, Marques H, Lamelas J, Wolfgang C, De Saussure W et al (2013) Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg 216(4):707–716
Brouquet A, Mortenson M, Vauthey J, Rodriguez-Bigas M, Overman M, Chang G et al (2010) Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 210(6):934–941
Yoshioka R, Hasegawa K, Mise Y, Oba M, Aoki T, Sakamoto Y et al (2014) Evaluation of the safety and efficacy of simultaneous resection of primary colorectal cancer and synchronous colorectal liver metastases. Surgery 155:478–485
Funding
No funding has been received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Arkaitz Perfecto, Mikel Gastaca, Mikel Prieto, Jorge Cervera, Patricia Ruiz, Alberto Ventoso, Ibone Palomares, José María García and Andrés Valdivieso have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perfecto, A., Gastaca, M., Prieto, M. et al. Totally laparoscopic simultaneous resection of colorectal cancer and synchronous liver metastases: a single-center case series. Surg Endosc 36, 980–987 (2022). https://doi.org/10.1007/s00464-021-08362-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08362-9