Abstract
Background
Laparoscopic segment 7 segmentectomy and segment 6–7 bisegmentectomy are challenging resections because of the posterior position and the lack of landmarks. The anatomy of the right posterior Glissonean pedicle and the caudal view of laparoscopy make such resections suitable for the Glissonean pedicle-first approach.
Methods
The study population included all consecutive patients treated with laparoscopic liver resection from August 2019 to February 2020. The approach is based on the ultrasonographic identification of the right posterior or segmental pedicle from the dorsal side of the liver after complete mobilization. The pedicle of interest is isolated through mini-hepatotomy and clamped. The segment anatomy is defined by ischemia. The transection starts from the ventral side, close to the right hepatic vein that is exposed and followed craniocaudally.
Results
Ten patients underwent anatomical laparoscopic resection of right posterolateral segments. There were 7 colorectal liver metastases, 2 hepatocellular carcinoma, and 1 biliary cysto-adenoma. Five patients underwent Sg7 resection, one patient underwent a Sg7 subsegmentectomy, and 4 underwent Sg6-7 bisegmentectomy. The Glissonean pedicle-first approach was feasible in eight patients. The craniocaudal approach to the RHV was feasible in six patients, not indicated in three cases and was abandoned in one patient for technical difficulty. There was no operative morbidity or mortality. Median post-operative hospital stay was 5 days.
Conclusions
The Glissonean pedicle-first approach is safe and effective for laparoscopic anatomic resections of the right posterior sector. The craniocaudal approach to right hepatic vein from the ventral side is a convenient procedure to follow the segmental anatomy deep in the parenchyma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Segment 7 (Sg7) and right posterior sector (Sg6-7) resections have been considered challenging procedures since the inception of minimally invasive liver surgery (MILS) [1]. With the evolution of MILS technique and surgeons’ expertise, posterior segments are no longer “non-laparoscopic segments.” Nonetheless, Sg7 resection and right posterior sectionectomy still increase the score in the main difficulty scoring systems [2,3,4]. Furthermore, anatomical resections pose peculiar difficulties, because they require careful identification of the proper Glissonean pedicle to be dissected and hepatic veins to be exposed on the cut surface [5, 6]. The peculiar anatomy of the right posterior Glissonean pedicle, together with the caudal view provided by the laparoscopy [7], makes it suitable for the Glissonean pedicle-first approach. Here, we report our technique for laparoscopic Sg7 and Sg6-7 anatomic resections.
Materials and methods
Patients
The study population included all consecutive patients who underwent laparoscopic liver resection at our Institution, from August 2019, when we initiated the Glissonean pedicle-first approach, to February 2020. All resections were performed with totally laparoscopic technique. Indications to liver resection and type of approach were decided in a multidisciplinary meeting. The laparoscopic approach was considered for each case and was based on the number and position of the liver lesions and the possibility of obtaining a parenchyma-sparing resection when indicated. All patients signed an informed consent form for the surgical intervention and laparoscopic approach. Written consent and IRB approval were not needed for the concerned variation to the standard laparoscopic procedures.
Operative procedures
Surgical procedures were performed with the standard technique in use at our institution, which we have previously described [8, 9]. In detail, for resection of the right posterior segments, patients are placed supine, in mild reverse Trendelenburg position, with lower limbs apart and a 30° left rotation of the trunk. Before the study period, the patient’s right arm was suspended above the head, to facilitate the rotation of the trunk. However, this positioning limited the movements of the surgeon’s right hand while operating the subxiphoid trocar. Hence, the procedure was amended to modify the positioning of the patient’s arm, which is now resting along the body. This position allows wide exposure of the posterolateral part of the right costal margin and a convenient positioning of the rightmost trocar. Four 12-mm and one 5-mm trocars are placed along a curved line from the xiphoid to the edge of the costal margin (Fig. 1). Pneumoperitoneum is established by open technique through a 12-mm right pararectal trocar, 3–4 cm above the umbilicus and maintained at 12 mmHg. A 30° laparoscope is placed through the pararectal access. Three further 12-mm trocars are placed on the anterior axillary line, on the midline and just below the xiphoid process. One 5-mm trocar is placed in the rightmost position allowed under the right costal arch. This arrangement allows for three different trocar settings with the laparoscope in the middle and one operative instrument on either side. In the central setting, the laparoscope is in the pararectal port and the operative instruments in the ports at its left and right. For the lateral or medial setting, the laparoscope and operative instruments are switched, respectively, one position clockwise or counter-clockwise, allowing a lateral or medial view (Fig. 1). The surgeon stands between patient’s legs throughout the procedure. Laparoscopic ultrasonography (LUS) was performed using a BK Medical Pro Focus 2202 ultrasound system with a 4-way (12C4f) convex array flexible transducer (BK Medical Inc., Denmark) or a ProSound Alpha 7 ultrasound system with a 2-way (UST-5536-7.5) linear array flexible transducer (Hitachi Aloka Medical Ltd, Tokyo, Japan). The parenchymal transection was performed with an ultrasonic dissector (SonaStar Laparoscopic Probe MXA-L002; Misonix, Inc., Farmingdale, NY, USA) combined with a radiofrequency sealer-divider (LigaSure™, Covidien, Mansfield, MA). A tourniquet is placed round the liver pedicle for intracorporeal Pringle maneuver, if necessary. Starting with the central setting, the round and falciform ligament are cut and the bare area is dissected. The instruments are moved to the medial setting allowing for the further isolation of the space between the RHV and the common trunk and for the division of the right coronary ligament as far as possible beyond the RHV. Then the right liver is lifted and mobilized up to the caval plane, dividing short hepatic veins if necessary. With instruments in the lateral setting the hepato-caval ligament is dissected, the RHV is encircled and controlled with a tourniquet whenever possible. The liver is rotated to the left. LUS is performed on the dorsal side and the right posterior (G6-7), segment 6 (G6) and segment 7 (G7) Glissonean pedicles are scanned (Fig. 2). For a Sg7 resection (Vid. 1), the course of G7 is sketched onto the dorsal liver surface. The precise point where G7 will be divided, at appropriate distance from its origin with G6, from the right posterior portal branch is confirmed and marked. A LUS-guided mini-hepatotomy is performed with the ultrasonic dissector and the pedicle is soon identified thanks to its superficial course on the liver dorsal side (Fig. 3). The pedicle is clamped and its correct identification is confirmed by US color-doppler. The ischemic demarcation of Sg7 is outlined with the cautery on the liver surface up to the confluence of the RHV into the inferior vena cava (IVC). The parenchymal transection starts on the ventral side, close to the hepato-caval confluence to achieve early exposure the RHV. The transection then follows the RHV caudally, exposing it on the cut surface (Fig. 4), down to the border with Sg6, as defined by the ischemia. The craniocaudal dissection at the liver dome is facilitated by the medial setting of the trocars. The venous branches draining Sg7 in the RHV are isolated and divided at the confluence in the RHV.
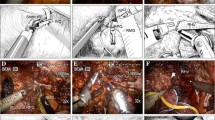
Sg7 segmentectomy: LUS exploration. Schematic representation (a) and intraoperative image (b) of the mobilized right liver, rotated to the left. The right posterior (G6-7), segment 6 (G6), and segment 7 (G7) Glissonean pedicles run close to the dorsal surface of the liver and can be scanned with laparoscopic ultrasonography (LUS) from the dorsal side. Right hepatic vein (RHV) is isolated and encircled with a tape (arrowhead). c Intraoperative LUS image: G6-7 and the bifurcation of G6 and G7 are visualized. Right anterior portal branch (G5-8) is visible behind G6-7. IVC Inferior vena cava
Sg7 segmentectomy: Glissonean pedicle-first approach. Schematic representation (a) and intraoperative image (b) of Sg7 Glissonean pedicle (G7) isolated through a mini-hepatotomy on the dorsal side. c Intraoperative image of the ischemic demarcation of Sg7 after G7clamping. RHV Right hepatic vein, G6-7 right posterior Glissonean pedicle, G6 Sg6 Glissonean pedicle
Sg7 segmentectomy: RHV craniocaudal approach. Schematic representation (a) and intraoperative image (b) of the craniocaudal approach to the right hepatic vein (RHV) from the ventral side. RHV is exposed starting from the hepato-caval confluence and followed caudally. A venous branch draining Sg7 (V7) is isolated and will be divided. c Sg7 resection is completed. RHV and a venous branch draining Sg6 (V6) are exposed on the cut surface. The stumps of G7 (white arrowhead) and Sg7 venous branches (black arrowheads) are visible on the cut surface
For anatomical Sg7 subsegmentectomy (Fig. 5), the Sg7 pedicles are scanned and sketched on the dorsal side. The pedicle of interest is isolated with the mini-hepatotomy and divided. In this case, the craniocaudal approach to the vein is not used, because part of Sg7 has to be spared thus the RHV need not be exposed.
Sg7 subsegmentectomy for a colorectal metastasis in the dorsal part of Sg7. a LUS performed from the dorsal side, scanning Sg7 Glissonean pedicle (G7) and its ventral (G7v) and dorsal (G7d) branches. b The course of G7, G7v, and G7d is sketched on the dorsal side with the cautery. Schematic representation (c) and intraoperative image (d) of G7d selectively isolated through a mini-hepatotomy on the dorsal side. G7d has been clamped, and the ischemic demarcation of the dorsal part of Sg7 has been marked with the cautery (arrowhead). The ventral part Sg7 is regularly vascularized
For Sg6-7 bisegmentectomy (Vid. 2), once the right liver is mobilized, the right posterior portal pedicle is identified with LUS, isolated and clamped with the technique described above. The RHV is dissected craniocaudally and exposed in its entire length on the Sg5-8 surface. The specimen is always placed in a plastic bag and removed through an additional incision. Routinely, no drains are placed.
Results
From August 2019 to February 2020, 10 patients underwent anatomical laparoscopic resection of right posterolateral segments at our Institution. Patients' characteristics are described in Table 1. Of the ten patients, eight were female and two were male. Seven patients had colorectal liver metastases (CRLM), two had hepatocellular carcinoma (HCC), and one had a biliary cysto-adenoma (BCA). Five patients underwent a Sg7 resection, in two of which the resection was partially extended to Sg6 and the dorsal part of Sg8. One patient underwent a Sg7 subsegmentectomy. Four patients underwent Sg6-7 bisegmentectomy, in one case extended to the dorsal part of Sg8 and in two cases to part of Sg5 and Sg8. The latter two cases required the resection of the RHV. Additional liver resections for multiple CRLM were performed in four patients. The Glissonean pedicle-first approach was feasible in eight patients. In one patient, an extrahepatic, intra-Glissonean approach was deliberately chosen for a Sg6-7 bisegmentectomy due to the peculiar presentation of colorectal metastases with endobiliary tumor growth, therefore allowing to cut the right posterior bile duct as distally as possible. In one case, the Glissonean pedicle-first approach was prevented by a large inferior right hepatic vein running in front of G6-7 on the dorsal liver surface. The craniocaudal approach to the RHV was feasible in six patients. It was not indicated for the Sg7 subsegmentectomy and for the two extended Sg6-7 bisegmentectomies with RHV resection. It was abandoned in one patient for technical difficulty, namely a prominent liver dome that hindered the approach. Median operative time was 337.5 min (range 256–484) and median blood loss was 250 ml (range 100–700 ml). There was no perioperative tansfusion nor conversion to open surgery. There was no operative morbidity or mortality. Median post-operative hospital stay was 5 days (range 4–10).
Discussion
Anatomical resection is generally required for hepatocellular carcinoma because of the oncological benefit associated [10]. Nonetheless, segmentectomies and subsegmentectomies are essential tools of the parenchyma-sparing strategy for colorectal liver metastases [11]. Anatomical segmentectomies are challenging due to the lack of clear separation of segments in the liver. Several methods have been proposed for the intraoperative identification of segmental anatomy, such as dye stain portal injection [12] or ultrasound-guided pedicle compression [13]. However, these techniques are difficult or not reproducible at all in laparoscopy. Our standard technique for laparoscopic liver resections [8, 9] based on LUS guidance allows all kinds of resections, including Sg7 segmentectomy [14]. In LUS-guided Sg7 segmentectomy, all the landmarks pertinent to the segment to be resected are identified: the RHV, the right posterior portal branch, G6, and G7. In the LUS-guided intrahepatic ventral approach as well, the correct identification and mapping of both pedicles on the dorsal side is required, in order to carry the parenchymal transection in the right direction and to reach G7 deep in the parenchyma at the desired point for the section, below the RHV. Nonetheless, a segmentectomy thus performed relies on anatomical boundaries identified by LUS, and not on actual segmental vascularization and represents the best approximation of an anatomical resection. The unique anatomy of the right posterior Glissonean pedicle and its tertiary-level branches makes it amenable for the pedicle-first approach. Those pedicles lie deep in the parenchyma but are quite superficial on the dorsal side, therefore accessible through a shallow hepatotomy after full mobilization and rotation of the right liver. LUS allows visualization the pedicle of interest, precise targeting of the desired point of section and exact guidance of the mini-hepatotomy to reach it. The pedicle can be clamped and its identification confirmed by US color-doppler. The resulting ischemia outlines the true anatomy of the segment and guides the resection. We used the pedicle-first approach successfully for Sg7 segmentectomies, Sg7 subsegmentectomy and for Sg6-7 bisegmentectomies. This approach could additionally be helpful for Sg6 segmentectomy or subsegmentectomy, although no patient required such resections in the study period. This approach was feasible in all cases except one, in which an interposed large right inferior hepatic vein proved to be an obstacle. In such cases, it may be necessary to abandon the Glissonean pedicle-first approach and perform an ultrasound-guided resection [14]. Another important feature of the procedure is the direct approach and exposure of the RHV as the first step of the parenchymal transection [15] followed by a craniocaudal parenchymal dissection with continuous exposure of the vein [16]. We performed the direct approach from the ventral side. This approach allowed for the immediate identification of the RHV that then was used as a guide for the resection deep in the parenchyma. Moreover, the venous branches draining the right posterior sector are isolated craniocaudally at the confluence in the RHV, and not spread apart with the specimen as usually happens during the conventional caudal approach, thus reducing the risk of split lesions and bleeding. We initiated the approach to the vein from the ventral side, taking advantage of the medial setting of the trocars, which allows the best visualization of the avascular space between the RHV and common trunk. The morphology of the liver dome can occasionally hinder the approach as happened in one patient with a prominent liver dome. Patient positioning and trocar disposition allowed for an optimal angle of incidence throughout all steps of the procedure, switching instruments medially or laterally, and intercostal trocars were never required. Operative time was acceptable, blood loss was limited with no major bleeding or need for intraoperative transfusion. The general good results of the minimally invasive approach were confirmed in this series, no patients suffered from complications with a short median hospital stay. In conclusion, the Glissonean pedicle-first approach is safe, feasible, and effective for laparoscopic anatomic resections of the right posterior sector. The craniocaudal approach to RHV from the ventral side is a convenient procedure to follow the segmental anatomy deep in the parenchyma.
References
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the Louisville Statement. Ann Surg 250:825–830
Hasegawa Y, Wakabayashi G, Nitta H, Takahara T, Katagiri H, Umemura A, Makabe K, Sasaki A (2017) A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc 31:5356–5363
Kawaguchi Y, Fuks D, Kokudo N, Gayet B (2018) Difficulty of laparoscopic liver resection: proposal for a new classification. Ann Surg 267:13–17
Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, D'Hondt M, Rotellar F, Dagher I, Aldrighetti L, Troisi RI, Edwin B, Abu Hilal M (2018) Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 105:1182–1191
Lin NC, Nitta H, Wakabayashi G (2013) Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 257:205–213
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2,804 patients. Ann Surg 250:831–841
Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, Scatton O (2015) A conceptual technique for laparoscopic right hepatectomy based on facts and oncologic principles: the caudal approach. Ann Surg 261:1226–1231
Ferrero A, Lo Tesoriere R, Russolillo N, Viganò L, Forchino F, Capussotti L (2015) Ultrasound-guided laparoscopic liver resections. Surg Endosc 29:1002–1005
Ferrero A, Lo Tesoriere R, Russolillo N (2019) Ultrasound liver map technique for laparoscopic liver resections. World J Surg 43:2607–2611
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242:252–259
Ferrero A, Viganò L, Lo Tesoriere R, Russolillo N, Sgotto E, Capussotti L (2009) Bisegmentectomies as alternative to right hepatectomy in the treatment of colorectal liver metastases. Hepatogastroenterology 56:1429–1435
Makuuchi M, Hasegawa H, Yamazaki S (1985) Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 161:346–350
Torzilli G, Procopio F, Cimino M, Del Fabbro D, Palmisano A, Donadon M, Montorsi M (2010) Anatomical segmental and subsegmental resection of the liver for hepatocellular carcinoma: a new approach by means of ultrasound-guided vessel compression. Ann Surg 251:229–235
Ferrero A, Russolillo N, Langella S, Forchino F, Stasi M, Fazio F, Lo Tesoriere R (2019) Ultrasound liver map technique for laparoscopic liver resections: perioperative outcomes are not impaired by technical complexity. Updates Surg 71:49–56
Honda G, Kurata M, Okuda Y, Kobayashi S, Sakamoto K, Takahashi K (2014) Totally laparoscopic anatomical hepatectomy exposing the major hepatic veins from the root side: a case of the right anterior sectorectomy (with video). J Gastrointest Surg 18:1379–1380
Okuda Y, Honda G, Kobayashi S, Sakamoto K, Homma Y, Honjo M, Doi M (2018) Intrahepatic glissonean pedicle approach to segment 7 from the dorsal side during laparoscopic anatomic hepatectomy of the cranial part of the right liver. J Am Coll Surg 226:e1–e6
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. A. Ferrero, R. Lo Tesoriere, F. Giovanardi, S. Langella, F. Forchino, and N. Russolillo have no conflicts of interest or financial ties to disclose concerning the funding of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 117317 kb)
Supplementary file2 (MP4 206548 kb)
Rights and permissions
About this article
Cite this article
Ferrero, A., Lo Tesoriere, R., Giovanardi, F. et al. Laparoscopic right posterior anatomic liver resections with Glissonean pedicle-first and venous craniocaudal approach. Surg Endosc 35, 449–455 (2021). https://doi.org/10.1007/s00464-020-07916-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07916-7