Abstract
Introduction
This is the cumulative technical report on the operative procedures and limitations of fetoscopic bag insertion, intestinal bag placement, and bag fixation to the fetus in a series of pilot studies in an ovine model for prenatal treatment of gastroschisis.
Material and methods
In 24 German blackhead sheep, a surgically created gastroschisis was managed by fetoscopic placement of the extruded intestines into a bag. The bag was then fastened onto the fetal abdominal wall. Different materials (sterile gloves, latex condoms, laparosopic retrieval bags) and different fixation techniques (laparoscopic staplers, interrupted and continuous sutures) have been examined. The fetuses were retrieved and evaluated at the end of gestation.
Results
Uterine bag insertion was successful in 15 of 24 (62.5%) and intestinal bag placement in 10 of 15 available fetuses (66.6%). The main factor limiting fetoscopic procedures was chorioamniotic separation (CAS). Sterilized condoms provided the most appropriate type of bags and the V-Loc™ running suture, the most expedient type of fixation, which was achieved in 9 of the 10 fetuses (complete = 2, partially = 7) by using a three port access (5 mm and 2 × 3 mm). All bags were encountered completely or partially dislocated from the fetus at the end of gestation.
Conclusions
Fetoscopic intestinal bag placement and fixation in gastroschisis technically demanding. None of the evaluated techniques led to permanent anchorage of the bag to the fetus. The development of specially designed instruments, bags and fixation methods is required to optimize this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The hallmark of gastroschisis is intestinal damage due to the prenatally injured intestine and its consecutive long term morbidity and mortality [1, 2].
Prenatal primary repair or covering of gastroschisis by open fetal surgery led to a reduction of inflammatory peel formation, intestinal damage, as well as an improvement in Cajal pacemaker cell density in several animal models [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Fetoscopic primary repair has been unsuccessful in two reports, but the results of fetoscopic covering by intestinal bag placement were comparable to the open approach, although all bags dislocated during the course of gestation [3, 4, 6]. With similar results after fetoscopic spina bifida repair, where the adhesive failed to permanently seal the covering patch until birth, the optimal fetoscopic fixation technique withstanding the tension exhibited by the growing fetus has yet to be defined [17].
Therefore, we report our cumulative results on technical aspects and considerations of fetoscopic bag placement and fixation for gastroschisis in 24 fetuses. Of those, several animals were included in previous reports: First, fetoscopic creation and evaluation of a gastroschisis (n = 8). Second, 19 animals have been reported on a fetoscopic visual grading score, the extent of the defect and its pathological implication for embryogenesis and third, 21 animals have been reported regarding the effect of fetoscopic bag placement on peel formation, intestinal damage, and levels of interstitial cells of Cajal [3, 4, 18].
Methods
All experiments were approved by the Hamburg state administration for animal research (81/09 FETAL-GS and 75/10 FETAL-GS II) and conducted in accordance with the institutional animal care and use committee. The anesthetic and surgical procedures have been published elsewhere [3, 4, 18]. Fetoscopic bag placement and bag fixation was performed at a median gestational day 96 (Range 42) and median 19 (minimum six and maximum 43) days after creation of the gastroschisis. The procedure was divided into three steps, namely bag insertion, bag placement and bag fixation.
The types of bags examined were laparoscopic retrieval bags (Endopouch, Ethicon, Germany), sterile surgical latex gloves (SSG, Peha Taft, Hartmann, Germany), sterile examination copolymer gloves (SEG, Sentina, Lohmann & Rauscher, Austria), and sterilized condoms (Fromms, MAPA, Germany). The bags were chosen according to the estimated size of the eviscerated intestine during fetoscopic evaluation of the gastroschisis. As reported earlier, any type of self-expanding gastroschisis bag with a spring, as used in postnatal management of gastroschisis, was not applicable because of the small size of the abdominal defect and the compression of vessels of fetal circulation by the spring [4].
Fixation methods used were monofilament sutures (continuous or single knots, Prolene®, Ethicon, Germany), a running barbed suture (V-Loc™, Covidien, Germany), and endoscopic tackers (Helix Protack Titan 5 mm, Autosuture, Germany; PDS EasyTac®, PermaFix, Germany). Different bags and fixation techniques have been examined in the same fetus, allowing for a smaller number of animals (3Rs: Replacement, Reduction and Refinement) [19].
At the end of gestation, the fetus was delivered, euthanized, and inspected. The ewe was allowed to recover.
Results
The results are displayed in Table 1.
Bag insertion
Bag insertion was possible in 15/24 animals (62.5%). In eight cases, chorioamniotic membrane separation (CAS) made introduction of the bags impossible (33%) and an omental cyst led to cancelation of the fetoscopic procedure in one more.
The laparoscopic retrieval bag required a 10 mm port and insertion could be performed without additional instrumentation.
Applying gloves as bags, they were cut below the fingers and the corresponding end was tied with a 3/0 silk suture which was left with a length of 5 cm for better maneuverability. Their introduction required a 6 mm port, which resulted in increased CAS compared to 3 mm ports during creation of the gastroschisis.
Sterilized condoms were prepared in the same manner, resulting in a 3 cm long bag with the elastic condom ring at the open and a 5 cm silk suture for maneuvering at the tied end. The condoms were initially introduced through a 6 mm port, resulting in additional instrumentation with an increase of operating time similar to gloves. During the course of the experiments, a Floseal endoscopic applicator (Baxter, UK) loaded with the prepared condom was used. The applicator could be introduced through a 5 mm port resulting in easy and fast insertion of the bag into the amniotic cavity by simply pushing it inside with the device obturator. With this technique, bag insertion could be performed within a matter of seconds and improved the procedure as intra-amniotic instrumentation could be reduced.
Bag placement
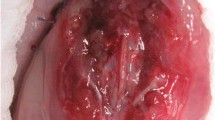
Intestinal bag placement was successful in 10 of the 15 fetuses after bag insertion (66.6%) and in 41.6% of all 24 available fetuses, respectively. Bag placement was unsuccessful with the laparoscopic retrieval bag, it was too big for instrumentation inside the uterus. Sterilized surgical gloves (SSG) could be easier manipulated by the elastic ring at their proximal end, resulting in bag placement in two of five cases (40%). The sterile examination glove (SEG) was maneuvered over the intestines in a similar fashion. Intestinal placement into the condoms was successful in seven of eight cases (88%): Their volume was similar to the eviscerated intestines and the proximal ring of the condom provided stability in the amniotic environment (Fig. 1).
Bag fixation
Bag fixation was possible in 9 of the 10 fetuses (90%, 37.5% of all available fetuses) with complete bag fixation in two (condoms) and partial fixation in seven fetuses (condoms and two sterile gloves). Complete fixation was achieved by using the V-Loc™ running suture applying a three port access (5 mm and 2 × 3 mm, Table 1). The running suture was faster and more convenient to perform, as knot tying was only required at the start and end of the suture, the V-Loc™ was placed in the same manner, due to its configuration knot tying was not necessary (Fig. 2).
Stapling was expected to be a time saving alternative, but the applied staplers could not reliably penetrate the bag or fix the bag to the fetal abdominal wall (Fig. 3).
Anchoring the bag to the abdomen of the fetus was technically challenging due to restrictions of operative space, the fluid filled uterus and CAS. Additionally, instrumentation and underwater knot tying were impeded on the contralateral side of the eviscerated intestine as the fetus is floating freely and can not be positioned and held in place without the use of additional ports.
Chorioamniotic separation (CAS) was obstructing the fetoscopic procedures in nearly all cases although mostly to a lesser extent. Membrane detachment occurred even by using a 24G needle but was more pronounced by increasing size of the introduced ports and impaired vision with the need to abandon the fetoscopic approach in extreme cases (Fig. 4A–C).
Fetal and bag evaluation
None of the examined techniques resulted in a reliable and continuous fixation of the bag as all were found partially or completely dislocated from the fetus at the end of gestation. The sutures could still be found attached to the bags and appeared to be torn or grown out of the fetal skin (Fig. 5).
Although being covered only temporarily, fetal bag placement of the gastroschisis lead to improved bowel histology and interstitial cell (Cajal, ICC) levels in the experimental fetuses, as reported earlier by our group [3].
Discussion
We were able to demonstrate successful percutaneous fetoscopic bag insertion, intestinal placement, and bag fixation in a fetal sheep model of gastroschisis by employing condoms through an applicator system for insertion and a continuous running stitch with a barbed suture.
The procedures were complicated by chorioamniotic separation (CAS) thus increasing the time for surgery, necessitating repositioning of the ports and leading to complications or abandonment of the fetoscopic procedures. However, one may argue that, due to their loose attachments in sheep, the membranes may not pose such a great obstacle in human pregnancies [4, 6, 18]. Furthermore, the fetoscopic intervention was performed on a previously operated uterus (for creation of the gastroschisis), thereby increasing the risk of complications by repetitive surgery.
The procedures were performed in the amniotic fluid. Working in this environment requires different techniques and handling on a freely floating fetus, that is associated with a different learning curve compared to conventional laparoscopic surgery [20]. Although fixation by sutures appears favorable, fetoscopic suturing for gastroschisis is technically interfered by the abovementioned reasons. Some authors suggested the use of PACI (partial amniotic gas (CO2) insufflation) for amniodistension and better instrumentation of the fetus. Previously discounted based on some data in sheep, there is growing evidence that warmed, humidified carbon dioxide under low pressure does not have the earlier reported deleterious effects and may become the preferred method compared to amniofusion [21, 22].
The age of the fetuses at intervention with a range from 78 to 120 gestational days may act as a confounder in this report, as the fetus and its intestine grows rapidly during this period. One part of the included studies was the evaluation of the effect of amniotic fluid exposure on intestinal damage, for which the time of intervention had to be between seven and 42 days [18]. Thus, the size of the eviscerated intestines and amniotic cavity could not be standardized for this study.
Strengths
This is the first report of successful completely percutaneous fetoscopic bag insertion, placement, and fixation for gastroschisis and its technical aspects. The strengths of this study, compared to other ovine models of gastroschisis, is the amount of animals (24), the complete percutaneous access to the fetus and the different bags and techniques used for fixation, resulting in a broad experience with the distinctive techniques. Furthermore, this report on fetoscopic fixation techniques can be applied to other fetoscopic procedures, as covering spina bifida, as well.
Limitations
Although we were able to demonstrate successful fetoscopic bag placement and fixation, our studies have several limitations:
First of all, permanent fixation of the bags onto the fetal abdomen was unsuccessful, all bags dislocated at least partially from the fetus. It appears that the growing fetus and its intestines exhibit pressure onto the suture lines which result in their detachment from the fetus, while still being fixed to the bag. Alternative fixation techniques might be more efficient. Biomimetic glues have been evaluated in fetoscopy to seal port defects and, therefore, prevent amniotic fluid leakage and iatrogenic preterm premature rupture of membranes, which affects 10–47% of patients after fetoscopic procedures [23]. Glues tested were a multiphase adhesive modeled after the sandcastle worm (Phragmatopoma californica) and mussel derived bioadhesives [24, 25]. The results were promising for underwater (intra-amniotic) application and tensile strength. But a surgical adhesive (Bioglue®) used to fasten patches onto the fetus in fetoscopic spina bifida repair did not prevent them to become entirely detached at birth in 85% of reported cases [17]. It, therefore, appears that the available glues will not withstand the tension of the growing fetus in relation to the stiff membrane of the patch and—in gastroschisis cases—the pressure exhibited onto the bag by the growing intestine. An alternative might be to induce the ingrowth of the cover into the fetal skin, therefore, increasing the durability of fixation. This method was examined by Fontecha et al. in a sheep model of myelomeningocele: A silastic patch (SUMSA, Madrid, Spain) plus Marlex mesh (BARD, NJ, USA) was placed over the myelomeningocele and fixed with CoSeal bioadhesive (CoSeal Surgical Sealant; Baxter Healthcare Corporation, Deerfield, IL) to the adjacent skin. Their results demonstrated a certain amount of dermal growth over the Marlex mesh [26, 27]. A probable ingrowth of adhesive mesh might also be induced by laser or electric coagulation of the fetal skin to the mesh or membrane. A prototype of a fetoscopic instrument combining a self expandable bag with better instrumentation that can be easily manipulated and held onto the fetus until the process of fixation is finished is suggested in Fig. 6.
Drawing of a prototype instrument for fetoscopic bag placement and fixation. A reversed umbrella shaped bag is loaded on self-expandable wires and fitted into a 3 mm (or smaller) diameter tube. The tube can be brought into the amnitic cavity by puncture, the bag is pushed into the cavity and automatically expanded once leaving the tube. While still being fitted onto the tube. it can be easily maneuvered over the intestine and once fixed onto the fetus, for example by adhesive glue or laser activated coagulation, it can be detached from the wire and the tube and wire extracted from the amniotic cavity
Another probable drawback of this report is the fetal mortality of 33%. We attribute this to the experimental character of our pilot studies: different access-sites, ports, bags and fixation techniques were evaluated with operative times up to 450 min. [10] But although being pilot studies, mortality in our series is comparable to similar studies of open or hybrid fetal surgery for gastroschisis [5, 6, 8, 10, 11].
Another limitation is the small number of fetuses in each group, precluding statistical analysis for the determination of the best technique. But the animal model and the fetoscopic procedures had to be developed from scratch [4, 18]. No bags or instruments were available which were particularly designed for this procedure and had, therefore, to be used off-label and custom made. Different combinations of bags and fixation devices as well as ports and their placement, bag insertion, intrauterine manipulation, and sutures had to be evaluated. Thus, for each of these specific combinations, only small numbers of fetuses were available.
And, of course, the techniques utilized herein would in general not be applicable to the human fetus—multiple large trocars would carry unacceptable risks of membrane disruption, amniotic fluid leak, and premature delivery. Recently, Belfort developed a hybrid fetoscopic technique including chorioamniotic plication and PACI, which was able to significantly reduce the incident of chorioamniotic separation [28, 29]. With this approach, a hybrid fetoscopic intervention with acceptable morbidity might become applicable [28,29,30].
One further limitation is the current lack of prenatal diagnostic markers for complex gastroschisis and the yet unconfirmed hypothesis of the effect of prenatal therapy on the reduction of complex cases. Generally believed to be a “benign” congenital malformation, we were able to demonstrate that the subgroup of patients with complex gastroschisis (CGS) suffers from a mortality of up to 17% and a relevant long term morbidity [1]. Those complex cases might be the primary target for prenatal intervention, although the direct effect of prenatal coverage of gastroschisis on the incidence of complex gastroschisis has not been demonstrated yet. Whether the inflammatory peel of the intestines leads to necrosis, perforation or intestinal atresia (three of the four criterions for complex gastroschisis) is unclear. Nevertheless, there is good evidence that the inflammatory changes of the intestine seen in gastroschisis are a result of prolonged amniotic fluid exposure, which we have also demonstrated in fetuses included in this report, and we and others have demonstrated that in utero covering of gastroschisis reduces the inflammatory damage and improves ICC levels [3, 12, 31]. From a pathophysiological point of view, intestinal inflammatory damage leading to necrosis and perforation appears consistent. It, therefore, seems plausible, that prenatal coverage of gastroschisis will prevent at least the abovementioned fraction of cases with complex gastroschisis. Nonetheless, another argument for in utero surgery for fetal gastroschisis is the demonstrated improvement of ICC levels, leading to the reduction of postnatal intestinal dysmotility which is often the main cause for prolonged postoperative hospital stay in affected newborns [12, 32, 33].
As mentioned above, prenatal identification of complex cases is difficult. There is no current sonographic or blood marker available for the reliable prenatal identification of complex cases, although it appears that measurement of intra-abdominal bowel dilatation at 20–22 or at 30–32 weeks' gestation may be useful in the prediction of complex gastroschisis [34,35,36,37,38,39,40].
With the continuing progress in prenatal diagnostics, one may argue that prenatal identification of complex gastroschisis will be just a matter of time. Then, being able to offer a prenatal management will be the next logical step.
Nevertheless, in any case, our data are too preliminary to discuss any human application of fetoscopic bag placement in patients with gastroschisis.
Conclusion
This report is the cumulative technical paper on the experimental development of a probable technique for fetal surgery for gastroschisis. We were able to demonstrate that fetoscopic bag placement and its fixation to the fetus is possible but technically challenging by restrictions of port placement, chorioamniotic separation, limited operative space and bag dislocation during the course of gestation. Best results were obtained with condoms as bags and a running V-Loc™ suture. The development of specially designed instruments, as proposed in this report, bags and fixation methods are required to optimize and minimalize this approach.
References
Bergholz R, Boettcher M, Reinshagen K, Wenke K (2014) Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality-A systematic review and meta-analysis. J Pediatr Surg 49:1527–1532. https://doi.org/10.1016/j.jpedsurg.2014.08.001
Marven S, Owen A (2008) Contemporary postnatal surgical management strategies for congenital abdominal wall defects. Semin Pediatr Surg 17:222–235. https://doi.org/10.1053/j.sempedsurg.2008.07.002
Krebs T, Boettcher M, Schäfer H, Eschenburg G, Wenke K, Appl B, Roth B, Andreas T, Schmitz C, Fahje R, Jacobsen B, Tiemann B, Reinshagen K, Hecher K, Bergholz R (2014) Gut inflammation and expression of ICC in a fetal lamb model of fetoscopic intervention for gastroschisis. Surg Endosc. https://doi.org/10.1007/s00464-014-3494-x
Bergholz R, Krebs T, Wenke K, Andreas T, Tiemann B, Paetzel J, Jacobsen B, Fahje R, Schmitz C, Mann O, Roth B, Appl B, Hecher K (2012) Fetoscopic management of gastroschisis in a lamb model. Surg Endosc 26:1412–1416. https://doi.org/10.1007/s00464-011-2048-8
Guys JM, Esposito C, Simeoni J, D’Ercole C, Paut O, Bouzid A, Boubli L (2002) An experimental model of gastroschisis using fetoendoscopy: preliminary results and technical considerations. Surg Endosc 16:317–319. https://doi.org/10.1007/s00464-001-9033-6
Kohl T, Tchatcheva K, Stressig R, Gembruch U, Kahl P (2009) Is there a therapeutic role for fetoscopic surgery in the prenatal treatment of gastroschisis? A feasibility study in sheep. Surg Endosc 23:1499–1505. https://doi.org/10.1007/s00464-009-0394-6
Kahl P, Buettner R, Tchatcheva K, Stressig R, Gembruch U, Kohl T (2012) Macroscopic and histopathologic findings in a laparoschisis model in fetal sheep: comparisons with gastroschisis in human fetuses and implications for prenatal interventions. Arch Gynecol Obstet 285:15–19. https://doi.org/10.1007/s00404-011-1890-1
Langer JC, Bell JG, Castillo RO, Crombleholme TM, Longaker MT, Duncan BW, Bradley SM, Finkbeiner WE, Verrier ED, Harrison MR (1990) Etiology of intestinal damage in gastroschisis, II. Timing and reversibility of histological changes, mucosal function, and contractility. J Pediatr Surg 25:1122–1126
Roelofs LAJ, Eggink AJ, Hulsbergen-van de Kaa CA, van den Berg PP, van Kuppevelt TH, van Moerkerk HTB, Crevels AJ, Lotgering FK, Feitz WFJ, Wijnen RMH (2008) Fetal abdominal wall repair with a collagen biomatrix in an experimental sheep model for gastroschisis. Tissue Eng 14:2033–2040. https://doi.org/10.1089/ten.tea.2007.0191
Roelofs LAJ, Geutjes PJ, Hulsbergen-van de Kaa CA, Eggink AJ, van Kuppevelt TH, Daamen WF, Crevels AJ, van den Berg PP, Feitz WFJ, Wijnen RMH (2013) Prenatal coverage of experimental gastroschisis with a collagen scaffold to protect the bowel. J Pediatr Surg 48:516–524. https://doi.org/10.1016/j.jpedsurg.2012.07.056
Stephenson JT, Pichakron KO, Vu L, Jancelewicz T, Jamshidi R, Grayson JK, Nobuhara KK (2010) In utero repair of gastroschisis in the sheep (Ovis aries) model. J Pediatr Surg 45:65–69. https://doi.org/10.1016/j.jpedsurg.2009.10.012
Vargun R, Aktug T, Heper A, Bingol-kologlu M (2007) Effects of intrauterine treatment on interstitial cells of Cajal in gastroschisis. J Pediatr Surg 42:783–787. https://doi.org/10.1016/j.jpedsurg.2006.12.062
Gonçalves FLL, Bueno MP, Schmidt AF, Figueira RL, Sbragia L (2014) Treatment of bowel in experimental gastroschisis with a nitric oxide donor. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2014.09.025
de Lagausie P, Guibourdenche J, de Buis A, Peuchmaur M, Oury JF, Aigrain Y, Sibony O, Luton D (2002) Esophageal ligature in experimental gastroschisis. J Pediatr Surg 37:1160–1164
Till H, Muensterer O, Mueller M, Klis V, Klotz S, Metzger R, Joppich I (2003) Intrauterine repair of gastroschisis in fetal rabbits. Fetal Diagn Ther 18:297–300
Gonçalves FLL, da Silva R, Schmidt AF, de Oliveira MG, Sbragia L (2010) Hydrogel protection: a novel approach to reduce bowel inflammation in experimental gastroschisis. Eur J Obstet Gynecol Reprod Biol 148:35–39. https://doi.org/10.1016/j.ejogrb.2009.10.009
Guilbaud L, Roux N, Friszer S, Garabedian C, Dhombres F, Bessières B, Fallet-Bianco C, Di Rocco F, Zerah M, Jouannic J-M (2017) Fetoscopic patch coverage of experimental myelomenigocele using a two-port access in fetal sheep. Childs Nerv Syst ChNS 33:1177–1184. https://doi.org/10.1007/s00381-017-3461-7
Bergholz R, Krebs T, Wenke K, Boettcher M, Andreas T, Tiemann B, Jacobsen B, Fahje R, Schmitz C, Roth B, Appl B, Reinshagen K, Hecher K (2013) Abdominal wall incision with or without exteriorization of bowel: results from a fetal lamb model for the embryogenesis of gastroschisis. Fetal Diagn Ther 33:55–60. https://doi.org/10.1159/000342421
Hughes M, Health JBS of P the principles of humane experimental technique. In: Johns Hopkins Bloom. Sch. Public Health. https://altweb.jhsph.edu/pubs/books/humane_exp/het-toc. Accessed 12 Feb 2019
Mietzsch S, Boettcher J, Yang S, Chantereau P, Romero P, Bergholz R, Reinshagen K, Boettcher M (2016) Training significantly improves fetoscopy performance: a pilot randomized controlled trial. Eur J Pediatr Surg 26:436–442. https://doi.org/10.1055/s-0035-1564712
Baschat AA, Ahn ES, Murphy J, Miller JL (2018) Fetal blood-gas values during fetoscopic myelomeningocele repair performed under carbon dioxide insufflation. Ultrasound Obstet Gynecol 52:400–402. https://doi.org/10.1002/uog.19083
Kohl T (2016) Impact of partial amniotic carbon dioxide insufflation (PACI) on middle cerebral artery blood flow in mid-gestation human fetuses undergoing fetoscopic surgery for spina bifida aperta. Ultrasound Obstet Gynecol 47:521–522. https://doi.org/10.1002/uog.15761
Mann LK, Papanna R, Moise KJ, Byrd RH, Popek EJ, Kaur S, Tseng SCG, Stewart RJ (2012) Fetal membrane patch and biomimetic adhesive coacervates as a sealant for fetoscopic defects. Acta Biomater 8:2160–2165. https://doi.org/10.1016/j.actbio.2012.02.014
Haller CM, Buerzle W, Brubaker CE, Messersmith PB, Mazza E, Ochsenbein-Koelble N, Zimmermann R, Ehrbar M (2011) Mussel-mimetic tissue adhesive for fetal membrane repair: a standardized ex vivo evaluation using elastomeric membranes. Prenat Diagn 31:654–660. https://doi.org/10.1002/pd.2712
Kivelio A, Dekoninck P, Perrini M, Brubaker CE, Messersmith PB, Mazza E, Deprest J, Zimmermann R, Ehrbar M, Ochsenbein-Koelble N (2013) Mussel mimetic tissue adhesive for fetal membrane repair: initial in vivo investigation in rabbits. Eur J Obstet Gynecol Reprod Biol. https://doi.org/10.1016/j.ejogrb.2013.09.003
Fontecha CG, Peiro JL, Sevilla JJ, Aguirre M, Soldado F, Fresno L, Fonseca C, Chacaltana A, Martinez V (2011) Fetoscopic coverage of experimental myelomeningocele in sheep using a patch with surgical sealant. Eur J Obstet Gynecol Reprod Biol 156:171–176. https://doi.org/10.1016/j.ejogrb.2010.12.046
Fontecha CG, Peiro JL, Aguirre M, Soldado F, Añor S, Fresno L, Martinez-Ibañez V (2009) Inert patch with bioadhesive for gentle foetal surgery of myelomeningocele in a sheep model. Eur J Obstet Gynecol Reprod Biol 146:174–179. https://doi.org/10.1016/j.ejogrb.2009.06.022
Belfort MA, Whitehead WE, Shamshirsaz AA, Bateni ZH, Olutoye OO, Olutoye OA, Mann DG, Espinoza J, Williams E, Lee TC, Keswani SG, Ayres N, Cassady CI, Mehollin-Ray AR, Sanz Cortes M, Carreras E, Peiro JL, Ruano R, Cass DL (2017) Fetoscopic open neural tube defect repair: development and refinement of a two-port, carbon dioxide insufflation technique. Obstet Gynecol 129:734–743. https://doi.org/10.1097/AOG.0000000000001941
Belfort M, Deprest J, Hecher K (2016) Current controversies in prenatal diagnosis 1: in utero therapy for spina bifida is ready for endoscopic repair. Prenat Diagn 36:1161–1166. https://doi.org/10.1002/pd.4972
Belfort MA, Whitehead WE, Bednov A, Shamshirsaz AA (2017) Low-fidelity simulator for the standardized training of fetoscopic meningomyelocele repair. Obstet Gynecol. https://doi.org/10.1097/AOG.0000000000002406
Midrio P, Faussone-Pellegrini MS, Vannucchi MG, Flake AW (2004) Gastroschisis in the rat model is associated with a delayed maturation of intestinal pacemaker cells and smooth muscle cells. J Pediatr Surg 39:1541–1547. https://doi.org/10.1016/j.jpedsurg.2004.06.017
Midrio P, Vannucchi MG, Pieri L, Alaggio R, Faussone-Pellegrini MS (2008) Delayed development of interstitial cells of Cajal in the ileum of a human case of gastroschisis. J Cell Mol Med 12:471–478. https://doi.org/10.1111/j.1582-4934.2008.00277.x
Auber F, Danzer E, Noché-Monnery M-E, Sarnacki S, Trugnan G, Boudjemaa S, Audry G (2013) Enteric nervous system impairment in gastroschisis. Eur J Pediatr Surg 23:29–38. https://doi.org/10.1055/s-0032-1326955
Nagy B (2019) Cell-free nucleic acids in prenatal diagnosis and pregnancy-associated diseases. EJIFCC 30:215–223
Hijkoop A, Lap CCMM, Aliasi M, Mulder EJH, Kramer WLM, Brouwers HAA, van Baren R, Pajkrt E, van Kaam AH, Bilardo CM, Pistorius LR, Visser GHA, Wijnen RMH, Tibboel D, Manten GTR, Cohen-Overbeek TE (2019) Using three-dimensional ultrasound in predicting complex gastroschisis: a longitudinal, prospective, multicenter cohort study. Prenat Diagn 39:1204–1212. https://doi.org/10.1002/pd.5568
Dewberry LC, Hilton SA, Zaretsky MV, Behrendt N, Galan HL, Marwan AI, Liechty KW (2019) Examination of prenatal sonographic findings: intra-abdominal bowel dilation predicts poor gastroschisis outcomes. Fetal Diagn Ther. https://doi.org/10.1159/000501592
Oakes MC, Porto M, Chung JH (2018) Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg 27:289–299. https://doi.org/10.1053/j.sempedsurg.2018.08.006
Robertson JA, Kimble RM, Stockton K, Sekar R (2017) Antenatal ultrasound features in fetuses with gastroschisis and its prediction in neonatal outcome. Aust N Z J Obstet Gynaecol 57:52–56. https://doi.org/10.1111/ajo.12565
Andrade WS, Brizot ML, Rodrigues AS, Tannuri AC, Krebs VL, Nishie EN, Francisco RPV, Zugaib M (2018) Sonographic markers in the prediction of fetal complex gastroschisis. Fetal Diagn Ther 43:45–52. https://doi.org/10.1159/000464245
Andrade WS, Brizot ML, Francisco RPV, Tannuri AC, Syngelaki A, Akolekar R, Nicolaides KH (2019) Fetal intra-abdominal bowel dilation in prediction of complex gastroschisis. Ultrasound Obstet Gynecol 54:376–380. https://doi.org/10.1002/uog.20367
Acknowledgements
Robert Bergholz and Thomas Krebs contributed equally to this manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Robert Bergholz and Thomas Krebs were promoted by a grant of the foundation “Hamburg macht Kinder gesund e.V. Robert Bergholz and Thomas Krebs received funding by Karl Storz Company GmbH, Tuttlingen, Germany, in form of materials (Storz Camera Telepack, Storz 3- and 5-mm laparoscopic instruments). Birte Cremieux, Carla Georgi, Felipe Fromm, Michael Boettcher, Thomas Andreas, Bastian Tiemann, Katharina Wenke, Konrad Reinshagen and Kurt Hecher have no conflict of interest of financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bergholz, R., Krebs, T., Cremieux, B. et al. Fetoscopic techniques for prenatal covering of gastroschisis in an ovine model are technically demanding and do not lead to permanent anchoring on the fetus until the end of gestation. Surg Endosc 35, 745–753 (2021). https://doi.org/10.1007/s00464-020-07441-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07441-7