Abstract
Background
Peritoneal drainage has been used routinely after pancreaticoduodenectomy (PD) or distal pancreatectomy (DP). Our objective was to compare patients’ outcomes after PD or DP with or without peritoneal drainage.
Methods
We performed a systematic search using the following databases: PubMed, Embase, Web of Science, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov until 1 June 2019. We included trials comparing no peritoneal drainage versus drainage after PD and/or DP.
Results
Ten trials involving 2419 patients were eligible for inclusion. The meta-analysis showed a significantly lower rate of postoperative pancreatic fistula in the no-drain group (odds ratio [OR] 0.39; 95% confidence interval [CI] 0.29–0.51; p < 0.00001). However, there was no significant difference in the analysis of the subgroups, DP and DP + PD peritoneal drainage (p = 0.10, p = 0.19; respectively). The analysis of all studies showed no significant difference between groups regarding clinically related postoperative pancreatic fistula (OR 0.71; 95% CI 0.41–1.24; p = 0.23). Mortality was higher in the drain group in the PD + DP subgroup (OR 0.41; 95% CI 0.27–0.62; p < 0.0001). No significant differences were found regarding intra-abdominal abscess, delayed gastric emptying, biliary fistula, postoperative hemorrhage, or morbidity.
Conclusion
Our results showed comparable outcomes for PD and DP with or without drainage. However, we can draw no clear conclusions because of the study limitations. Further studies on this topic are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With advances in surgical technique and care, mortality and morbidity have decreased following pancreatic surgery, especially in high-volume pancreatic centers [1,2,3]. The two main procedures in pancreatic surgery are pancreaticoduodenectomy (PD) and distal pancreatectomy (DP). However, the incidence of postoperative complications with both procedures remains high. Prophylactic placement of intraperitoneal drains following PD and DP has been considered as a measure to reduce postoperative complications [4,5,6]. However, it is unclear whether routine drain placement is essential [7, 8], especially in DP. Prophylactic drainage may be placed in PD routinely; however, this concept is not accepted in DP during past decades. The operations have different complication profiles in PD and DP. Previous randomized controlled trials (RCTs) have demonstrated no significant difference in cholecystectomy, hepatectomy, gastrectomy, or colectomy with and without drainage [9,10,11,12], and several retrospective studies demonstrated that pancreatectomy without prophylactic drainage may be safe [7, 13, 14]. A randomized prospective trial performed by Conlon et al. found no significant difference between the drain and no-drain group for overall morbidity and mortality [4]. In the current study, we performed a meta-analysis comparing no-drain and drain in PD and DP.
Method
Search strategy
Two independent reviewers performed a systematic and electronic search of the PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov databases until 1 June 2019. The medical subject headings included, but were not limited to “drainage,” “drain,” “peritoneal drainage,” “Whipple,” “pancreaticoduodenectomy,” and “distal pancreatectomy.” The search was restricted to human patients and English language full-text articles. We also manually reviewed the references of the articles identified after the initial search.
Inclusion and exclusion criteria
This study was performed according to the PRISMA guidelines [15]. We performed a subgroup analysis of PD, DP, and PD combined with DP. Inclusion criteria were as follows: (1) studies comparing no drain versus a drain after PD and/or DP with the definition of POPF according to the ISGPF definition [16], and (2) the original article must have been published in English with full text. The exclusion criteria were as follows: (1) review articles, case reports, abstracts, editorials, and letters to the editor; (2) research involving central pancreatectomy or other pancreatic surgery; (3) repeat publication by the same author or agency; and (4) insufficient data on outcome measures.
Outcome measures
The primary outcome of this study was POPF and clinically related POPF (CR-POPF) defined using the ISGPF definition. The secondary outcomes were biliary fistula, delayed gastric emptying (DGE), intra-abdominal abscess, postoperative hemorrhage, postoperative radiological intervention, reoperation, morbidity, and mortality. The definition of DGE and postoperative hemorrhage were according to the ISGPF criteria [17], and we included all outcomes until the publication date.
Data extraction and quality assessment
The standardized selection form included the first author, year of publication, type of study, country in which the study was performed, and the sample size. Conflicts in data abstraction were resolved by consensus and by referring to the original article. We assessed the quality of the RCTs in accordance with the Cochrane Collaboration Handbook [18], and non-RCTs were assessed using the criteria of the Newcastle–Ottawa scale [19].
Statistical analysis
This meta-analysis was performed using Review Manager (RevMan) version 5.3 software (Cochrane Informatics and Knowledge Management Department, Nordic Cochrane Centre, Copenhagen, Denmark). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using fixed or random effects models. The I2 index was used as an indicator of between-study heterogeneity. We used a fixed effects model with I2 < 50%; otherwise, we used a random effects model. A two-tailed p value of < 0.05 was considered statistically significant. We assessed the potential for publication bias by visually inspecting a funnel plot asymmetry.
Results
Study selection and trial characteristics
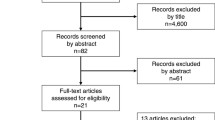
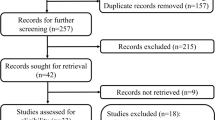
We identified 177 potentially eligible papers following the described search strategy; of these, we excluded 120 duplicate articles. The remaining 57 studies were retrieved based on their titles and abstracts, and an additional 47 citations were excluded for various reasons. Finally, ten studies involving 2419 participants were included in the current meta-analysis [7, 8, 13, 14, 20,21,22,23,24,25]. In this study, four trials were RCT and six were retrospective. A flowchart of the literature search process is shown in Fig. 1, and the characteristics of the included articles are presented in Table 1. PD was performed in six studies and DP was performed in two studies. Researchers in two studies performed PD + DP.
Outcome measures
POPF
Seven studies reported POPF data, and the meta-analysis showed a lower incidence of POPF in the no-drain group vs the no-drain group (OR 0.39; 95% CI 0.29–0.51; p < 0.00001) (Fig. 2A). Four studies reported POPF data in the subgroup undergoing PD, only one study provided data for the DP subgroup, and two studies provided data for the PD + DP. There was no significant difference between the drain and no-drain groups in the DP and PD + DP (OR 0.08; 95% CI 0.00–1.56; p = 0.10 and OR 0.35; 95% CI 0.07–1.68, respectively; p = 0.19) (Fig. 2B). However, the incidence of POPF in the PD subgroup receiving a lower incidence in the no-drain group (OR 0.42; 95% CI 0.29–0.62; p < 0.00001) (Fig. 2B).
CR-POPF
Nine trials provided data for the rate of CR-POPF, which was 9% (96/1058) in the no-drain group and 13% (135/1017) in the drain group. A pooled analysis showed no significant difference between the groups (OR 0.71; 95% CI 0.41–1.24; p = 0.23) (Fig. 3A), and a subgroup analysis showed no significant difference for any of the following three subgroups: PD, DP, and PD + DP (p = 0.25, p = 0.10, and p = 0.73, respectively) (Fig. 3B).
DGE
Four studies provided data for the incidence of DGE, which was 15% (51/336) for the no-drain group and 19% (93/476) for the drain group. A pooled analysis showed no significant difference between the two groups (OR 0.79; 95% CI 0.27–2.27; p = 0.66) (Fig. 4A). No studies provided data for DGE in the DP subgroup, and we found no significant difference between the no-drain and drain groups in the PD subgroup (OR 1.08; 95% CI 0.31–3.79; p = 0.90) (Fig. 4B). A study performed by Fisher et al. showed that the incidence of DGE was lower in the no-drain group (p = 0.03).
Biliary fistula
Biliary fistula was pooled in five studies, and we found no significant difference between the drain and no-drain groups (OR 0.89; 95% CI 0.32–2.47; p = 0.82) (Fig. 5A) and in the three-subgroup analysis (PD, DP, and PD + DP; p = 0.74, p = 0.51, and p = 0.50; respectively) (Fig. 5B).
Intra-abdominal abscess
Intra-abdominal abscess was reported in eight studies, and we found no significant difference between the drain and no-drain groups (OR 1.03; 95% CI 0.72–1.48; p = 0.86) (Fig. 6A). Similar results were also seen in the subgroup analysis for PD, DP, and PD + DP (p = 0.96, p = 0.78, and p = 0.95; respectively) (Fig. 6B).
Postoperative hemorrhage
Four studies provided data for postoperative hemorrhage, and we found no significant difference between the drain and no-drain groups (OR 0.79; 95% CI 0.41–1.54; p = 0.49) (Fig. 7A). No studies evaluating DP and PD + DP provided data for postoperative hemorrhage (Fig. 7B).
Postoperative radiological intervention
Nine studies provided data for postoperative radiological intervention, and we found no significant difference between the drain and no-drain groups (OR 0.90; 95% CI 0.65–1.23; p = 0.50) (Fig. 8A) or in the subgroup analysis (PD, DP, and PD + DP; p = 0.55, p = 0.53, and p = 0.91; respectively) (Fig. 8B).
Reoperation
Seven studies provided data for reoperation, and we found no significant difference between the drain and no-drain groups (OR 1.27; 95% CI 0.79–2.04; p = 0.33) (Fig. 9A). However, the rate of reoperation was higher in the no-drain group in the PD + DP subgroup (OR 5.21; 95% CI 1.34–20.24; p = 0.02) (Fig. 9B). We found no significant difference between the drain and no-drain groups for the remaining two subgroups (PD, DP; p = 0.93, p = 0.59; respectively) (Fig. 9B).
Morbidity
Seven studies provided morbidity data, and we found no significant difference between the drain and no-drain groups (OR 1.64; 95% CI 0.88–3.06; p = 0.12) (Fig. 10A) or in the subgroup analysis (PD, DP, and PD + DP; p = 0.16, p = 0.19, and p = 0.99; respectively) (Fig. 10B).
Mortality
We found no significant difference between the drain and no-drain groups regarding mortality, in the pooled analysis (OR 0.74; 95% CI 0.52–1.04; p = 0.09) (Fig. 11A). However, mortality was higher in the drain group in the PD + DP subgroup (OR; 0.41; 95% CI 0.27–0.62; p < 0.0001) (Fig. 11B). There was no significant difference for mortality in the PD and DP subgroups (p = 0.41 and p = 0.06, respectively) (Fig. 11B).
Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time. The omission of any study resulted in no significant difference, indicating that our results were statistically reliable.
Publication bias
Most graphical funnel plots of the parameters were symmetrical, and Egger’s test revealed no significant publication bias.
Discussion
This meta-analysis showed that there was no significant difference between the drain and no-drain groups regarding CR-POPF. However, the no-drain group had a lower incidence of POPF, and the subgroup analysis revealed a higher rate of POPF in the drain group in the subgroup undergoing PD + DP. No significant differences were found for intra-abdominal abscess, DGE, biliary fistula, postoperative hemorrhage, and morbidity.
Surgeons commonly place an intraperitoneal drainage during abdominal surgery, especially following pancreatic surgery; however, debate continues regarding whether peritoneal drainage is essential. Previous studies showed that omitting drainage may be safe after cholecystectomy, colorectomy, hepatectomy, and other abdominal surgeries [9,10,11,12]; however, few studies have investigated the safety of omitting postoperative drainage following PD and DP. The role of placement of abdominal drains remains pervasive in PD and DP. A study conducted by Van Buren et al. published in 2017 showed that it is safe for DP without intraperitoneal drainage [24]. Interestingly, previous study revealed that early removal of drains may be safe for DP [26].
POPF is considered to result in the highest morbidity after pancreatic surgery and can increase the incidence of intra-abdominal abscess, fluid accumulation, postoperative hemorrhage, and sepsis [27]. Several studies suggested that placing an abdominal drain intraoperatively allows the surgeon to detect POPF earlier and initiate treatment [25]. Our results revealed a higher incidence of POPF in the drain group, and we believe that POPF is more likely to be discovered with abdominal drainage. Previous studies have shown that closed suction drainage may damage tissues and increase the incidence of POPF [4, 28, 29]. However, interestingly, the incidence of POPF did not differ in our DP and PD + DP subgroups, which may be related to the small number of studies constituting these two subgroups. Using the ISGPF definition of POPF, our results showed no statistically significant difference in the incidence of CR-POPF between the drain and no-drain groups, similar to most previous studies. Additional radiological intervention was often considered for CR-POPF intra-abdominal abscess and fluid accumulation in previous studies; we saw no difference between the drain and no-drain groups regarding postoperative radiological intervention. A recent study published in 2011 reported that the rate of postoperative intervention was higher in the no-drain group [7]. A previous study performed by Mehta et al. reported that, compared with the no-drain group, the drain group experienced higher rates of CR-POPF [22]. Additionally, drain placement was associated with longer hospital stay, increased total complications, and infection complications [22]. A prospective study involving 104 consecutive patients by Kawai et al. concluded that early drain removal may reduce the risk of intra-abdominal infection and POPF [30]. Several studies have investigated the risk factors for POPF, namely small pancreatic duct diameter, soft pancreatic texture, and prolonged operative time. However, given the limitations in these studies, further study and higher-quality studies are required.
Despite developments in pancreatic surgery, mortality and morbidity remain high. Similar to a previous study by Huang et al. [31], we found no significant difference regarding the incidence of DGE, biliary fistula, postoperative hemorrhage, and reoperation between the drain and no-drain groups in all studies and the subgroup analysis. Several studies revealed that drain placement was associated with higher severe complications [32, 33]. After evaluating five studies totaling 1728 patients, Wang et al. showed that patients without prophylactic drainage after PD had significantly higher mortality [34]. However, given the study’s limitations, debate continues regarding whether omitting drainage is associated with lower morbidity. Previous studies also showed that drainage after pancreatic surgery may increase patients’ pain and length of hospital stay [22]. We found no significant difference between the drain and no-drain groups regarding mortality, in the pooled analysis, in our study; however, we found higher mortality in the PD + DP subgroup receiving drainage. Several factors may contribute to morbidity and mortality. Previous studies showed that pancreatic gland texture, pancreatic duct diameter, and other factors may be associated with high morbidity and mortality; however, the definition of morbidity and the study end points differed between studies, which contributed to heterogeneity.
There are several limitations in the current study. First, different definitions of postoperative complications were implied in the included studies. Second, most of the included studies had a small sample size and did not have data for all of the outcomes we evaluated. Third, POPF in the no-drain group may have been missed because of a lack of symptoms. Fourth, studies varied in the operative techniques, namely the use of pancreatic duct stenting, different methods of closure in DP, and different anastomosis methods in PD. Finally, of the included studies, only four were RCTs; the remaining were retrospective studies, and heterogeneity was present among these retrospective studies. Given the limitations of the current study, more large-scale high-quality RCTs are required.
Conclusion
Our results showed comparable outcomes following PD and DP with or without drainage. However, given our study’s limitations, we cannot provide a definitive conclusion. Further studies on this topic are recommended.
Data availability
All the data used in the study can be obtained from the original articles.
References
Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J (1993) One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 217:430–435 (discussion 435-438)
DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA (2006) Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 244:931–937 (discussion 937-939)
Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, Traverso LW (2007) Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg 11:1451–1458 (discussion 1459)
Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, Merchant N, Brennan MF (2001) Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg 234:487–493 (discussion 493-484)
Cullen JJ, Sarr MG, Ilstrup DM (1994) Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg 168:295–298
Yamaguchi M, Nakano H, Midorikawa T, Yoshizawa Y, Sanada Y, Kumada K (2003) Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepatogastroenterology 50:1155–1158
Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, Brunicardi FC (2011) Pancreatic resection without routine intraperitoneal drainage. HPB 13:503–510
Paulus EM, Zarzaur BL, Behrman SW (2012) Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary? Am J Surg 204:422–427
Shamim M (2013) Routine sub-hepatic drainage versus no drainage after laparoscopic cholecystectomy: open, randomized, clinical trial. Indian J Surg 75:22–27
Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, Poon RT, Wong J (2004) Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg 239:194–201
Kim J, Lee J, Hyung WJ, Cheong JH, Chen J, Choi SH, Noh SH (2004) Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg 8:727–732
Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, Pelissier E, Msika S, Flamant Y, French Association for Surgical Research (1999) Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery 125:529–535
Adham M, Chopin-Laly X, Lepilliez V, Gincul R, Valette PJ, Ponchon T (2013) Pancreatic resection: drain or no drain? Surgery 154:1069–1077
McMillan MT, Fisher WE, Van Buren G, McElhany A, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Velanovich V, Brown K, Morgan KA, Vollmer C (2015) The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. J Gastrointest Surg 19:21–30 (discussion 30-21)
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928–d5928
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Kunstman JW, Starker LF, Healy JM, Salem RR (2017) Pancreaticoduodenectomy can be performed safely with rare employment of surgical drains. Am Surg 83:265–273
Lim C, Dokmak S, Cauchy F, Aussilhou B, Belghiti J, Sauvanet A (2013) Selective policy of no drain after pancreaticoduodenectomy is a valid option in patients at low risk of pancreatic fistula: a case-control analysis. World J Surg 37:1021–1027
Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA (2013) Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. J Am Coll Surg 216:635–642 (discussion 642-634)
Van Buren G, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Vollmer C, Velanovich V, Riall T, Muscarella P, Trevino J, Nakeeb A, Schmidt CM, Behrns K, Ellison EC, Barakat O, Perry KA, Drebin J, House M, Abdel-Misih S, Silberfein EJ, Goldin S, Brown K, Mohammed S, Hodges SE, McElhany A, Issazadeh M, Jo E, Mo Q, Fisher WE (2014) A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg 259:605–612
Van Buren G, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG, Morgan KA, Hughes SJ, Karanicolas PJ, Allendorf JD, Vollmer CM Jr, Ly Q, Brown KM, Velanovich V, Winter JM, McElhany AL, Muscarella P 2nd, Schmidt CM, House MG, Dixon E, Dillhoff ME, Trevino JG, Hallet J, Coburn NSG, Nakeeb A, Behrns KE, Sasson AR, Ceppa EP, Abdel-Misih SRZ, Riall TS, Silberfein EJ, Ellison EC, Adams DB, Hsu C, Tran Cao HS, Mohammed S, Villafane-Ferriol N, Barakat O, Massarweh NN, Chai C, Mendez-Reyes JE, Fang A, Jo E, Mo Q, Fisher WE (2017) A prospective randomized multicenter trial of distal pancreatectomy with and without routine intraperitoneal drainage. Ann Surg 266:421–431
Witzigmann H, Diener MK, Kienkotter S, Rossion I, Bruckner T, Barbel W, Pridohl O, Radulova-Mauersberger O, Lauer H, Knebel P, Ulrich A, Strobel O, Hackert T, Buchler MW (2016) No need for routine drainage after pancreatic head resection: the dual-center, randomized, controlled PANDRA trial (ISRCTN04937707). Ann Surg 264:528–537
Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P (2010) Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 252:207–214
Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, Gumbs A, Pederzoli P (2004) Pancreatic fistula rate after pancreatic resection. Dig Surg 21:54–59
Schmidt CM, Choi J, Powell ES, Yiannoutsos CT, Zyromski NJ, Nakeeb A, Pitt HA, Wiebke EA, Madura JA, Lillemoe KD (2009) Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg 2009:404520
Grobmyer SR, Graham D, Brennan MF, Coit D (2002) High-pressure gradients generated by closed-suction surgical drainage systems. Surg Infect 3:245–249
Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H (2006) Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg 244:1–7
Huan L, Fei Q, Lin H, Wan L, Li Y (2017) Is peritoneal drainage essential after pancreatic surgery?: a meta-analysis and systematic review. Medicine 96:e9245
Kaminsky PM, Mezhir JJ (2013) Intraperitoneal drainage after pancreatic resection: a review of the evidence. J Surg Res 184:925–930
Zaghal A, Tamim H, Habib S, Jaafar R, Mukherji D, Khalife M, Mailhac A, Faraj W (2019) Drain or no drain following pancreaticoduodenectomy: the unsolved dilemma. Scand J Surg. SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society:1457496919840960
Wang YC, Szatmary P, Zhu JQ, Xiong JJ, Huang W, Gomatos I, Nunes QM, Sutton R, Liu XB (2015) Prophylactic intra-peritoneal drain placement following pancreaticoduodenectomy: a systematic review and meta-analysis. World J Gastroenterol 21:2510–2521
Acknowledgement
We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Yunxiao Lyu, Yunxiao Cheng, Bin Wang, Sicong Zhao, and Liang Chen have no conflicts of interest or financial ties to disclose.
Informed consent
IRB approval and informed consent were not needed for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lyu, Y., Cheng, Y., Wang, B. et al. Peritoneal drainage or no drainage after pancreaticoduodenectomy and/or distal pancreatectomy: a meta-analysis and systematic review. Surg Endosc 34, 4991–5005 (2020). https://doi.org/10.1007/s00464-019-07293-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07293-w