Abstract
Background
The good short-term and oncological outcomes of robot-assisted radical esophagectomy have been demonstrated, although its impact on long-term health-related quality of life (HRQoL) remains to be investigated. This study aimed to assess long-term HRQoL in patients after robot-assisted radical transmediastinal esophagectomy (TME), which is characterized as non-transthoracic esophagectomy comprising a robotic transhiatal approach and a video-assisted cervical approach, and transthoracic esophagectomy (TTE).
Methods
The European Organization for Research and Treatment of Cancer generic and disease-specific modules (QLQ-C30 and QLQ-OES18), nutritional status and body composition data were prospectively collected in patients undergoing TME or TTE before and at 3, 6, 12, 18, and 24 months after surgery. The results of long-term (≥ 2 years) survivors without recurrence were compared between the two groups.
Results
A total of 37 patients (TME; n = 18, TTE; n = 19) were included for analysis. Longitudinal survey of function scales revealed scores of physical, role, social, and emotional function to be significantly better in the TME group than in the TTE group at many points postoperatively. Markedly, the symptoms of general pain, esophageal pain, and dry mouth greatly worsened after surgery in the TTE group, but did not deteriorate in the TME group. In contrast, symptoms relating to eating difficulties, body composition data, and nutritional status did not differ between the groups over time. At 24 months after surgery, TME provided significantly higher scores of global QOL (P = 0.01) and emotional function (P = 0.01) and also resulted in significantly fewer problems of fatigue (P = 0.04), general pain (P = 0.04), insomnia (P = 0.02), and dry mouth (P = 0.03), as compared to TTE.
Conclusion
This study indicates that TME can provide better long-term HRQoL outcomes than TTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal carcinoma (EC) is the sixth most common cause of death from cancer worldwide [1] despite improvements in multimodal treatment strategies [2]. The principal means to treat EC is a surgical resection, although esophagectomy is a highly extensive procedure [2] that leads to considerable deterioration in health-related quality of life (HRQoL) [3,4,5].
In general, HRQoL of patients undergoing esophagectomy drastically deteriorates postoperatively [4, 6], beginning to improve within 6–12 months of surgery [7] and recovering to levels comparable to preoperative levels 3 years postsurgery [4, 6]. Although a majority of long-term survivors after esophagectomy have relatively good HRQoL [5], some patients do not fully regain HRQoL between 6 months and 5 years after surgery [5], suffering from persisting symptoms [8, 9]. Some studies suggested that poor HRQoL after esophagectomy was associated with poor long-term outcomes [10, 11], which necessitates useful perioperative strategies to improve long-term HRQoL. Past studies have identified some surgical factors in relation to postoperative HRQoL [12,13,14,15]. Among others, minimally invasive surgery (MIE) reportedly reduces surgical stress, resulting in enhanced postoperative recovery of HRQoL [16,17,18].
Recently, robot-assisted minimally invasive esophagectomy (RAMIE) has been developed, and results from several cohort studies suggested RAMIE to be safe and feasible with good oncological outcomes [19, 20]. Employing the innovative surgical technique, our institution lately developed “robot-assisted transmediastinal esophagectomy (TME)”, or “non-transthoracic esophagectomy”, with radical mediastinal lymphadenectomy combining a robotic transhiatal approach and a video-assisted cervical approach [21]. We have previously reported our experience with TME, demonstrating that it can be safely performed with equivalent oncological radicality [22]. In addition, our recent cross-sectional study indicated that TME provided better postoperative HRQoL than transthoracic esophagectomy (TTE) [23]. However, long-term HRQoL outcomes remain to be investigated in patients undergoing TME.
Herein, using well-validated HRQoL modules, we conducted a longitudinal survey to compare long-term HRQoL outcomes, body composition, and nutritional status of patients undergoing TME (a robotic transhiatal approach) with those of patients undergoing TTE (an open transthoracic approach).
Patients and methods
Patients
Between April 2015 and January 2017, a total of 110 patients with pathologically confirmed EC underwent potentially curative esophagectomy at the University of Tokyo Hospital. All these patients were preoperatively staged using esophagogastroduodenoscopy and computed tomography. The choice of treatment strategy was argued at a biweekly multidisciplinary cancer board. Based on our previous clinical study verifying the safety and utility of TME [22], TME was generally employed for patients with cT1-3N0-1 disease according to the 7th edition of the TNM classification [24] during the study period. Additionally, patients who received prior chemotherapy or radiotherapy to the operative field and those aged over 80 years were not eligible for TME [22]. The prospective survey of HRQoL scores, body composition data and nutritional status was performed in 56 patients after excluding patients undergoing salvage surgery (n = 14), those receiving two-stage operations (n = 11), those having synchronous multiple malignancies (n = 11), very elderly patients (age > 80; n = 7), those undergoing transhiatal esophagectomy (n = 5) and patients who did not consent to participate in the survey (n = 6). Among the 56 patients included in the prospective survey, long-term survivors without recurrence within 2 years after surgery were analyzed. This prospective study was approved by the local ethics committee of the Faculty of Medicine at the University of Tokyo (UMIN ID: 000017565). Written informed consent was obtained from all patients. Additionally, written informed consent to undergo robot-assisted surgery without receiving financial support from the national health insurance system was required for TME.
Surgical treatment and postoperative management
Transmediastinal esophagectomy, or TME, with three-field lymphadenectomy was performed using a robotic surgical system, da Vinci S (Intuitive Surgical, Sunnyvale, CA, USA), as described in previous studies [21, 22]. In the first phase, two surgical teams simultaneously performed (1) cervical lymph node (LN) dissection followed by upper mediastinal LN dissection under mediastinoscopy guidance and (2) abdominal and inferior mediastinal LN dissection via a laparoscopic approach. In the second phase, da Vinci S was brought into perform the remaining mediastinal LN dissection, mainly middle mediastinal LN dissection, via a transhiatal approach. The third phase included the harvest of surgical specimens, construction of gastric tube conduit and reconstruction with cervical anastomosis. Our standard TTE procedures comprised subtotal esophagectomy with mediastinal lymphadenectomy via right thoracotomy, upper abdominal lymphadenectomy and reconstruction with intrathoracic anastomosis using a gastric tube. Adjuvant chemotherapy was basically employed for patients with pT2-4N+ disease, if they were tolerable based on their physical status.
Data collection
The prospectively collected clinical information included patient and tumor characteristics, surgical details and other treatment variables, postoperative complications, and written HRQoL questionnaire responses collected at the time of admission for surgery and at 3, 6, 12, 18, and 24 months after surgery. The data of body composition and nutritional status (albumin and prealbumin) was obtained in the same schedule. The 7th edition of the TNM classification [24] was used to describe staging of the tumors. The Charlson comorbidity index (CCI) [25] was used to assess the frailty of the patients at the time of surgery. The Clavien–Dindo scale [26] was used to grade all postoperative morbidities.
Health-related quality of life
HRQoL was measured using well-established questionnaires developed by the European Organization for Research and Treatment of Cancer (EORTC) [27]. EORTC QLQ-C30 includes one global QOL scale, five functional scales (physical, role, emotional, cognitive, and social functioning), three general symptom scales (fatigue, pain, nausea, and vomiting) and 6 single items measuring symptoms common among cancer patients (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial impact) [27]. Esophageal cancer-related symptoms were estimated using an esophageal site-specific module (EORTC QLQ-OES18) [28], which comprises 4 symptom scales (dysphagia, eating, reflux, and esophageal pain) and 6 single items (trouble swallowing saliva, choking when swallowing, dry mouth, taste problems, coughing, and speech problems). Each item in both questionnaires had a response on a four-point Likert scale: ‘not at all’, ‘a little’, ‘quite a bit’ and ‘very much’, except for the items in the global quality-of-life scale, which includes 7-point items ranging from “very poor” to “excellent”. Higher scores correspond to better HRQoL in the function scales and the global quality-of-life scale, whereas higher scores for symptom scales and single items represent more problems. Missing responses were handled as recommended in the EORTC scoring manual.
Bioelectrical impedance analysis
Body composition was measured by bioelectrical impedance analysis using an Inbody 770 machine (Biospace, Tokyo, Japan) [29]. Using the manufacturer’s algorithm, various parameters, including body weight, body mass index, skeletal muscle mass, and body fat mass, were calculated separately.
Statistical analysis
Categorical variables were expressed in numerical figures and percentages and compared using Fisher’s exact test or the χ2 test, as appropriate. Continuous variables were expressed as the median values (range) or the mean values (standard deviation (SD)) and compared using Student’s t test. Statistical analyses were carried out using JMP 13.0.0 (SAS Institute, Cary, NC).
Results
Patient characteristics
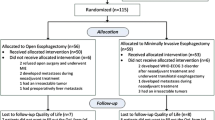
Among the 56 patients, 22 underwent TME, while the remaining 34 received TTE. Fifteen patients (27%) developing recurrence within 2 years of esophagectomy, 1 (2%) who died due to pneumonia at 18 months after surgery and 3 (5%) not returning the questionnaires were excluded. The remaining 37 (66%) patients (TME, n = 18; TTE, n = 19), who survived for at least 2 years after surgery without recurrence, were eligible for the analysis (Fig. 1). The clinicopathological features and surgical outcomes between the two groups are shown in Table 1. There were no significant differences in terms of demographic data, comorbidity, and postoperative complications. The TTE group included more patients with clinical stage 3 than the TME group, although it was not statistically significant (P = 0.06). Pathological tumor staging showed no significant difference between the two groups (P = 0.88). The number of retrieved LNs was not significantly different between the groups.
Longitudinal changes in global QOL and functional scales
Figure 2 shows the longitudinal changes in global QOL and functional scales determined by the QLQ-C30 questionnaire. Patients in both groups reported similar baseline scores for all functional scales, except that patients in the TME group had significantly higher global QOL scores preoperatively (P = 0.03, Fig. 2A). Global QOL, physical function, role function, and social function scores decreased at 3 months after surgery in both groups, but improved in the first year of follow-up, then stabilized at baseline levels (Fig. 2A–D). Compared with patients in the TTE group, those in the TME group recuperated the physical function scores more rapidly, resulting in having significantly higher scores at 3, 6, and 18 months postoperatively (Fig. 2D). Emotional function showed a consistent improvement after operation with significantly better scores in the TME group than in the TTE group at 6, 18, and 24 months after surgery (Fig. 2E), whereas cognitive function gradually declined over time in both groups (Fig. 2F).
Longitudinal changes in global QOL and functional scales. Global QOL score (A) and role (B), social (C), physical (D), emotional (E) and cognitive (F) functional scores were compared between patients undergoing TME (filled square) and TTE (open square) during 2 years after surgery. Means are presented. *P < 0.05; **P < 0.01, with Student’s t test
Longitudinal changes in symptoms and single items
Most symptoms and single items assessed by the QLQ-C30 and QLQ-OES18 questionnaires got drastically worse at 3 months after surgery, but improved in the first year of follow-up as described in Figs. 3 and 4. Patients in both groups exhibited similar preoperative scores for all symptom scales and single items. Mean general pain score showed an increase of 10 points postoperatively in the TTE group, but no deterioration in the TME group, leading to significantly better mean general pain scores in the TME group than in the TTE group throughout the period (Fig. 3A). Similarly, mean esophageal pain score did not get worse in the TME group postoperatively, yet greatly deteriorated by 15 points in the TTE group. Compared with patients undergoing TTE, those undergoing TME had significantly better mean esophageal pain scores at 3 and 18 months after surgery during the follow-up period (Fig. 3B). Additionally, patients in the TME group had fewer problems of fatigue (Fig. 3C), dyspnea (Fig. 3D), and insomnia (Fig. 3E),
Patients in the TME group experienced significantly less problems of dry mouth (at 12 and 24 months after surgery, Fig. 4A). Mean reflux scores were better in the TME group than in the TTE group, although the differences were not statistically significant (Fig. 4B). In contrast, the scores of nausea/vomiting, appetite loss and dysphagia showed no significant differences between the groups (Fig. 4C–E).
Longitudinal changes in body composition data and nutritional status
Body weight greatly declined after surgery (Fig. 5A). The mean (SD) percentage weight loss was 10% in the TTE group and 13% in the TME group at 6 months after surgery, without significant difference. Body mass index, body fat mass, and skeletal muscle mass followed a similar trend over time (Fig. 5B–D) without significant differences between the groups. Markedly, body fat mass decreased by half after surgery, which was more remarkable compared with the decrease in skeletal muscle mass. Albumin and prealbumin decreased after surgery, then gradually improved and returned to near baseline levels (Fig. 5E, F). Nutritional status did not differ significantly between the two groups.
Longitudinal changes in body composition data and nutritional status. No statistically significant differences were observed over time between the TME group (filled square) and TTE group (open square) in terms of weight loss (A), body mass index (B), body fat mass (C), skeletal muscle mass (D), albumin (E), and prealbumin (F)
HRQoL scores at 2 years after surgery
Many of the HRQoL scores at 24 months after surgery were better in the TME group than in the TTE group (Fig. 6). At 24 months after the operation, patients treated by TME had significantly higher global QOL scale (P = 0.01) and emotional function scale (P = 0.01) than those treated by TTE (Table 2). QLQ-C30 survey of general symptoms suggested that patients in the TTE group experienced more problems of fatigue (P = 0.04), general pain (P = 0.04), and insomnia (P = 0.02) than patients in the TME group did (Table 2). Regarding esophageal-specific HRQoL scores (QLQ-OES18), patients undergoing TTE experienced dry mouth more frequently than patients undergoing TME (P = 0.03, Table 2). Patients in the TTE group had higher mean score of esophageal pain than those in the TME group, but the difference was not statistically significant (P = 0.09, Table 2). As for other symptoms and single items, no significant differences were found between the two groups.
Discussion
Diagnostic and therapeutic advances have led to an increasing number of long-term survivors after curative resection for EC [30]. Even after surviving the refractory disease, many patients go through impaired HRQoL after conventional TTE [5, 9], which highlights the significance of useful strategies to maximize postoperative HRQoL while retaining oncological radicality. The present study revealed robot-assisted transmediastinal esophagectomy, or TME, to provide better HRQoL scores compared with conventional TTE. To our knowledge, this is the first prospective study to compare long-term postoperative HRQoL between robot-assisted esophagectomy and TTE [19].
Our TME procedure consists of a robotic transhiatal approach and a video-assisted cervical approach using mediastinoscopy [21]. We previously demonstrated the technique to be safely performed with sufficient lymphadenectomy [22]. Recently, the procedure has attracted attention as a new useful approach for patients with EC. In fact, the radical transmediastinal approach has been covered by insurance in Japan since April 2018 [31]. Furthermore, recent insurance coverage for robot-assisted gastrectomy [32] is prompting insurance application for robot-assisted esophagectomy. Additionally, Nakaguchi et al. lately proposed the utility of robot-assisted mediastinoscopy esophagectomy [33].
Some prior studies have revealed transhiatal esophagectomy (THE) [12, 13] and MIE [16, 18] to yield better postoperative HRQoL compared with TTE; however, THE has been employed less frequently for patients with esophageal squamous cell carcinoma because of its limited capacity for lymphadenectomy [31]. On the other hand, MIE, showing equal oncological outcomes to TTE [34], is becoming prevalent as a useful alternative to TTE [16, 18]. A recent prospective study suggested the good HRQoL scores following MIE to be mainly due to pain reduction [17], while Kauppila et al. revealed MIE to show no clinically relevant differences in most of the esophageal-specific HRQoL scores [14].
The present study highlighted several beneficial HRQoL-related aspects of TME. Firstly, patients treated by TME showed better functional scores at many points postoperatively compared with those receiving TTE. Patients in both groups experienced early deteriorations in physical, role, and social function scores, although patients undergoing TME achieved faster recovery than those in the TTE group. Additionally, emotional function, which improved after the surgery in both groups due to the removal of cancer [6], showed a more pronounced increase in the TME group.
Secondly, patients in the TME group suffered from fewer general and esophageal-specific symptoms as compared to those in the TTE group. In general, patients undergoing esophagectomy suffer from multiple concurrent long-persisting symptoms [8, 30, 35] caused by considerable anatomical and physiological changes. In the current study, general and esophageal pain did not worsen in the TME group in marked contrast to in the TTE group. Furthermore, TME provided faster recovery of dyspnea symptoms and significantly fewer problems of fatigue and insomnia at 24 months after the operation compared with TTE (Fig. 3). Given that symptoms of pain, fatigue, insomnia, and dyspnea are highly correlated with each other [36], the observed differences might be attributable to pain reduction. These results are in line with past studies showing the good HRQoL outcomes of MIE [17, 18] and short-term benefits of RAMIE [19].
Of note, patients in the TME group had significantly fewer problems of dry mouth (Fig. 3), which often persist for a long time after esophagectomy [5, 8]. Considering dry mouth is reportedly related to reflux [36], the significant better mean score of dry mouth in the TME group is partly due to the better scores of reflux in the TME group (Fig. 4). Our TME approach can prevent gastric conduit from negative intrathoracic pressure, possibly leading to better reflux scores in the TME group. This hypothesis is partly supported by a preclinical study [37] and past studies showing THE to yield better reflux scores than TTE [23, 38].
In contrast, symptoms in relation to eating problems, such as appetite loss, dysphagia, and nausea/vomiting [36], showed almost no differences between the groups (Fig. 4). The physiological changes caused by the removal of the gastric reservoir and vagotomy [9] often lead to long-lasting eating problems [30]. The obstinate problems are difficult to improve by surgical approaches [13, 17]. Rather, postoperative management, such as early oral feeding, can lead to a quicker recovery of bowel function and improve eating difficulties [39]. We also evaluated nutritional status and body composition data of the two groups, but detected no significant differences. This could be attributable to the aforementioned similarity between the groups in terms of eating problems.
Taken together, this prospective study raised the possibility that our TME procedure using a robotic surgical system is a rational surgical strategy because of its less deleterious impact on HRQoL. Robotic surgery can overcome the technical difficulties of MIE [20, 33] with magnified views and articulated forceps, allowing surgeons to perform safe and reproducible operations [32] with a short learning curve [20]. Additionally, the esophagus is regarded as an ideal organ for a robotic approach since it is anatomically located in a limited and narrow space surrounded by vital organs [40]. Furthermore, our TME procedure can avoid one-lung ventilation [21, 22] and can be performed on frail people, such as the elderly or patients with pulmonary comorbidities. Of course, robotic surgery still has to overcome several major problems, such as increased operative time, high cost [41], and high social burden [42]. Furthermore, it remains to be addressed whether robot-assisted TME is superior to MIE, which has yielded equivalent oncological outcomes [34] and better HRQoL outcomes [17] compared to conventional TTE. It appears to be difficult to show the benefits of robot-assisted TME over conventional MIE in a multicenter randomized trial since it would cost a lot and require a large number of patients, although it might be feasible and interesting to compare long-term HRQoL outcomes between TME and MIE.
Our study has some limitations. Firstly, the small patient number in both groups might have limited statistical power. Additionally, the sample size of long-term survivors was further limited due to the poor long-term prognosis of EC patients. Secondly, the backgrounds differed partly between the two groups because TME was generally employed for early EC patients that could afford to pay for the expensive procedure during the study period. Hence, this study potentially had a selection bias. Finally, this was a single-center study. It seems likely that a multi-center collaborative study with a large cohort could achieve more convincing results.
In conclusion, robot-assisted radical transmediastinal esophagectomy provides better HRQoL outcomes compared with conventional transthoracic esophagectomy. The new technique is a reasonable surgical approach for patients with EC.
Abbreviations
- CCI:
-
Charlson comorbidity index
- EC:
-
Esophageal carcinoma
- EORTC:
-
European Organization for Research and Treatment of Cancer
- HRQoL:
-
Health-related quality of life
- LN:
-
Lymph node
- MIE:
-
Minimally invasive surgery
- RAMIE:
-
Robot-assisted minimally invasive esophagectomy
- THE:
-
Transhiatal esophagectomy
- TME:
-
Transmediastinal esophagectomy
- TTE:
-
Transthoracic esophagectomy
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Lagergren J, Smyth E, Cunningham D, Lagergren P (2017) Oesophageal cancer. Lancet 390:2383–2396
Jacobs M, Macefield RC, Elbers RG, Sitnikova K, Korfage IJ, Smets EM, Henselmans I, van Berge Henegouwen MI, de Haes JC, Blazeby JM, Sprangers MA (2014) Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res 23:1097–1115
Blazeby JM, Farndon JR, Donovan J, Alderson D (2000) A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer 88:1781–1787
Djarv T, Lagergren J, Blazeby JM, Lagergren P (2008) Long-term health-related quality of life following surgery for oesophageal cancer. Br J Surg 95:1121–1126
Lagergren P, Avery KN, Hughes R, Barham CP, Alderson D, Falk SJ, Blazeby JM (2007) Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer 110:686–693
Parameswaran R, McNair A, Avery KN, Berrisford RG, Wajed SA, Sprangers MA, Blazeby JM (2008) The role of health-related quality of life outcomes in clinical decision making in surgery for esophageal cancer: a systematic review. Ann Surg Oncol 15:2372–2379
Daster S, Soysal SD, Stoll L, Peterli R, von Flue M, Ackermann C (2014) Long-term quality of life after Ivor Lewis esophagectomy for esophageal cancer. World J Surg 38:2345–2351
Schandl A, Lagergren J, Johar A, Lagergren P (2016) Health-related quality of life 10 years after oesophageal cancer surgery. Eur J Cancer 69:43–50
Djarv T, Lagergren P (2011) Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer 47:530–535
Djarv T, Metcalfe C, Avery KN, Lagergren P, Blazeby JM (2010) Prognostic value of changes in health-related quality of life scores during curative treatment for esophagogastric cancer. J Clin Oncol 28:1666–1670
de Boer AG, van Lanschot JJ, van Sandick JW, Hulscher JB, Stalmeier PF, de Haes JC, Tilanus HW, Obertop H, Sprangers MA (2004) Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol 22:4202–4208
Kauppila JH, Johar A, Gossage JA, Davies AR, Zylstra J, Lagergren J (2018) Health-related quality of life after open transhiatal and transthoracic oesophagectomy for cancer. Br J Surg 105:230–236
Kauppila JH, Xie S, Johar A, Markar SR, Lagergren P (2017) Meta-analysis of health-related quality of life after minimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg 104:1131–1140
Rutegard M, Lagergren J, Rouvelas I, Lindblad M, Blazeby JM, Lagergren P (2008) Population-based study of surgical factors in relation to health-related quality of life after oesophageal cancer resection. Br J Surg 95:592–601
Parameswaran R, Blazeby JM, Hughes R, Mitchell K, Berrisford RG, Wajed SA (2010) Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 97:525–531
Barbour AP, Cormack OMM, Baker PJ, Hirst J, Krause L, Brosda S, Thomas JM, Blazeby JM, Thomson IG, Gotley DC, Smithers BM (2017) Long-term health-related quality of life following esophagectomy: a nonrandomized comparison of thoracoscopically assisted and open surgery. Ann Surg 265:1158–1165
Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, Gisbertz SS, Biere SS, van der Peet DL, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ (2015) Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg 39:1986–1993
van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N, Mook S, Vleggaar FP, Borel Rinkes IHM, Ruurda JP, van Hillegersberg R (2019) Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 269:621–630
Seto Y, Mori K (2017) Robotic surgery for esophageal cancer: merits and demerits. Ann Gastroenterol Surg 1:193–198
Mori K, Yamagata Y, Wada I, Shimizu N, Nomura S, Seto Y (2013) Robotic-assisted totally transhiatal lymphadenectomy in the middle mediastinum for esophageal cancer. J Robot Surg 7:385–387
Mori K, Yamagata Y, Aikou S, Nishida M, Kiyokawa T, Yagi K, Yamashita H, Nomura S, Seto Y (2016) Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus 29:429–434
Yoshimura S, Mori K, Yamagata Y, Aikou S, Yagi K, Nishida M, Yamashita H, Nomura S, Seto Y (2018) Quality of life after robot-assisted transmediastinal radical surgery for esophageal cancer. Surg Endosc 32:2249–2254
Rice TW, Blackstone EH, Rusch VW (2010) 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 17:1721–1724
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezer O, Koller M, Arraras J, Bottomley A, Vickery CW, Etienne PL, Alderson D (2003) Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 39:1384–1394
Lee SY, Ahn S, Kim YJ, Ji MJ, Kim KM, Choi SH, Jang HC, Lim S (2018) Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance analyses for accuracy in measuring whole body muscle mass and appendicular skeletal muscle mass. Nutrients 10(6):738
Mantoan S, Cavallin F (2018) Long-term quality of life after esophagectomy with gastric pull-up. J Surg Oncol 117:970–976
Fujiwara H, Shiozaki A, Konishi H, Otsuji E (2019) Transmediastinal approach for esophageal cancer: a new trend toward radical surgery. Asian J Endosc Surg 12:30–36
Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, Ehara K, Obama K, Kuwabara S, Okabe H, Terashima M (2019) Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer 22:377–385
Nakauchi M, Uyama I, Suda K, Shibasaki S, Kikuchi K, Kadoya S, Ishida Y, Inaba K (2019) Robot-assisted mediastinoscopic esophagectomy for esophageal cancer: the first clinical series. Esophagus 16:85–92
Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, Rosman C, van Berge Henegouwen MI, Gisbertz SS, van der Peet DL (2017) Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 266:232–236
Derogar M, Lagergren P (2012) Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol 30:413–418
Wikman A, Johar A, Lagergren P (2014) Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer 120:286–293
Bemelman WA, Verburg J, Brummelkamp WH, Klopper PJ (1988) A physical model of the intrathoracic stomach. Am J Physiol 254:G168–G175
Akkerman RD, Haverkamp L, van Rossum PS, van Hillegersberg R, Ruurda JP (2015) Long-term quality of life after oesophagectomy with gastric conduit interposition for cancer. Eur J Cancer 51:1538–1545
Sun HB, Li Y, Liu XB, Zhang RX, Wang ZF, Lerut T, Liu CC, Fiorelli A, Chao YK, Molena D, Cerfolio RJ, Ozawa S, Chang AC (2018) Early oral feeding following McKeown minimally invasive esophagectomy: an open-label, randomized, controlled, noninferiority trial. Ann Surg 267:435–442
Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I (2016) Robotic surgery for upper gastrointestinal cancer: current status and future perspectives. Dig Endosc 28:701–713
Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH (2012) Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg 99:1554–1561
Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ (2016) Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg 263:103–109
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The contributions of the authors to this study are as follows: Kotaro Sugawara, Shuntaro Yoshimura, and Yasuyuki Seto are the authors mainly responsible for the study’s conception and design, acquisition of data, and analysis and interpretation of data. Koichi Yagi, Masato Nishida, Susumu Aikou, Yukinori Yamagata, Kazuhiko Mori and Hiroharu Yamashita contributed mainly to the drafting of the article and to revising it critically for important intellectual content. Drs. Kotaro Sugawara, Shuntaro Yoshimura, Koichi Yagi, Masato Nishida, Susumu Aikou, Yukinori Yamagata, Kazuhiko Mori, Hiroharu Yamashita, and Yasuyuki Seto have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugawara, K., Yoshimura, S., Yagi, K. et al. Long-term health-related quality of life following robot-assisted radical transmediastinal esophagectomy. Surg Endosc 34, 1602–1611 (2020). https://doi.org/10.1007/s00464-019-06923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06923-7