Abstract
Background and aims
Despite advances in pharmacological and endoscopic management of non-variceal upper gastrointestinal bleeding (NVUGIB), mortality is still relevant. TC-325 (Hemospray-Cook Medical) is a mineral powder with adsorptive properties, designed for endoscopic hemostasis. There are still no comparative trials studying this new hemostatic modality. The objective of this research was to compare the use of TC-325 (associated with epinephrine injection) with the combined technique of endoscopic clipping and epinephrine injection for the treatment of patients with NVUGIB.
Methods
We conducted a pilot randomized controlled trial with patients that presented NVUGIB with an actively bleeding lesion at the endoscopic evaluation. Patients were randomized either to the Hemospray or Hemoclip group. The randomization list was generated by a computer program and remained unknown throughout the entire trial. All patients underwent second-look endoscopy.
Results
Thirty-nine patients were enrolled. Peptic ulcer was the most frequent etiology. Primary hemostasis was achieved in all Hemospray cases and in 90% of Hemoclip group (p = 0.487). Five patients in Hemospray group underwent an additional hemostatic procedure during second-look endoscopy, while no patient in the Hemoclip group needed it (p = 0.04). Rebleeding, emergency surgery and mortality rates were similar in both groups. No toxicity, allergy events, or gastrointestinal obstruction signs were observed in Hemospray group.
Conclusions
TC-325 presents similar hemostatic results when compared with conventional dual therapy for patients with NVUGIB. Hemospray’s excellent primary hemostasis rate certifies it as a valuable tool in arduous situations of severe bleeding or difficult location site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/background
Non-variceal upper gastrointestinal bleeding (NVUGIB) is a critical condition, not only commonly found in emergency departments, but also among hospitalized patients. It represents a heterogeneous and wide range of causes, with peptic ulcer bleeding the most frequent [1, 2] and, therefore, the most studied [3, 4]. Despite the significant advances in pharmacological and endoscopic management of this situation over the past decades [5, 6], overall mortality is relatively unchanged [7]. The increased use of antithrombotic and antiaggregant therapies in an elderly population with multiple comorbidities is often cited as a plausible reason for this [8, 9]. It is also hypothesized that technical aspects such as difficult anatomic position (such as the posterior wall of the duodenal bulb and the lesser curvature of gastric body), intense or diffuse nature of the bleeding lesion, and endoscopist experience and skill may be the factors limiting a sharper drop of mortality rates [10]. In these contexts, the emerging technology of hemostatic powder may play an important role.
Hemospray (TC-325–Cook Medical Inc., Winston-Salem, North Carolina, USA) is a mineral powder with adsorptive properties, designed for the endoscopic therapy of gastrointestinal bleeding. This new agent has proven to enhance clot formation in vivo, and to shorten the coagulation time in vitro [11]. It achieves hemostasis by forming a stable mechanical barrier over the bleeding site, when the powder makes contact with water (from the actively bleeding lesion). As a noncontact technique, Hemospray may have an advantage on lesions with difficult anatomic access. Previous reports have shown its effectiveness on a massive hemorrhage situation [12] and on a challenging post-sphincterotomy bleeding [13]. Diffuse bleeding lesions, such as gastrointestinal malignancies, and patients receiving antithrombotic therapy may also benefit from this new technique [14, 15]. Finally, it is important to comment on the ease of application of this new hemostatic tool [10].
Despite the many theoretical advantages and some fair uncertainties towards the use of Hemospray, there are still no prospective comparative trials up until this date studying this new hemostatic modality. In addition, our facility required an evaluation of the clinical utility and safety of Hemospray before it could be purchased and made routinely available. Therefore, the objective of this research was to compare the use of Hemospray (associated with epinephrine injection) with the combined technique of endoscopic clipping and epinephrine injection for the treatment of patients with NVUGIB, in a randomized trial.
Methods
Trial design
This paper was written according to the CONSORT 2010 Statement guideline for reporting randomized clinical trials [16]. We conducted a pilot single-center randomized controlled trial with two parallel groups, in a 1:1 allocation ratio.
Participants
Initially, all patients admitted with a history of upper gastrointestinal bleeding or in-hospital patients with suspected upper gastrointestinal bleeding were eligible to enter the trial and were submitted to an endoscopic evaluation after clinical stabilization, with airways protection if judged necessary by the attending physician. Endoscopy was performed within a maximum of 6 h after hospital admission or recognition of bleeding symptoms for in-hospital patients.
We included all consecutive patients who consented to participate in this trial and presented with an active NVUGIB lesion at the endoscopic evaluation. The exclusion criteria were pregnancy and a previous history of endoscopic hemostatic treatment in the past 7 days. Prior to the endoscopic therapy, patients were randomized to the Hemospray group or to the Hemoclip group.
We collected demographic and clinical data, such as age, gender, smoking status, comorbidities, medications in use (especially antithrombotic or antiaggregant agents and nonsteroidal anti-inflammatory drugs), previous history of peptic ulcer, gastrointestinal bleeding or surgical procedures, and vital signs. Moreover, we collected all relevant information of the endoscopic findings, such as the etiology of the bleeding (peptic ulcer, esophageal tear, malignancy, post-polypectomy, post-sphincterotomy), its location, and the characteristic of the bleeding (spurting bleeding or oozing bleeding), besides the laboratory exams. We also stratified the risk of each patient according to the Rockall scale. Study data were collected and managed using REDCap (Research Electronic Data Capture), which is a secure, web-based application designed to support data capture for research studies [17]. Medical terminology used was according to SNOMED CT (Systematised Nomenclature of Medicine Clinical Terms) [18].
Hemostatic procedures
All hemostatic procedures were performed by a limited group of endoscopists who had experience with gastrointestinal bleeding management and were familiar with clip placement. This group did not have experience with Hemospray before the protocol, however; all endoscopists received proper orientation and training on how to use the Hemospray device. Additionally, nurses and fellows who assisted during the procedures were competently trained on the management of both devices.
Epinephrine injection was used in both groups. In the Hemospray group, epinephrine was used when possible, preferably after Hemospray therapy. In the Hemoclip group, the endoscopist could choose to use it tactically prior to the clip(s) application or as a complement treatment after the endoscopic clipping. Exceptionally, epinephrine injection was used around an adherent clot in a peptic ulcer base, previously to the clot removal; if relevant bleeding emerged after its removal, the patient was randomized to one of the groups and Hemospray therapy or clip application was performed.
The Hemospray device used in this trial comprised a 7Fr application catheter, a CO2 cartridge and a syringe containing 20 g of TC-325 powder. This new technique consists of delivering 1–1 s spray bursts to the bleeding site, under direct vision, through the catheter that should be positioned one to two centimeters distant to the bleeding point. The bursts should be repeated until a consistent mechanical barrier is formed, covering the bleeding lesion. Figure 1 shows a case of peptic ulcer spurting bleeding randomized to Hemospray group.
A case of peptic ulcer bleeding randomized to Hemospray group. A Peptic ulcer located in duodenal bulb, after clot removal. B After epinephrine injection, spurting bleeding emerged and the patient was randomized. C Primary hemostasis was achieved after Hemospray therapy. D Second-look endoscopy showed no signs of rebleeding
For the Hemoclip group, Resolution Clip (Boston Scientific Corp., Marlborough, Massachusetts, USA) was applied directly to the bleeding site, involving as much tissue around as possible, to achieve hemostasis, before or after epinephrine injection, as previously described. In Fig. 2, a patient with Mallory-Weiss tears is treated with epinephrine injection and metallic clip application.
All patients underwent second-look endoscopy approximately 24 h after the hemostatic procedure. If rebleeding was verified during this examination, a new hemostatic procedure was performed, and a different modality was used at the discretion of the endoscopist (usually, a combination of a thermal technique and a new epinephrine injection). Vital signs and hemoglobin levels were measured until hospital discharge. Red blood cell transfusion was performed if hemoglobin level dropped below 7 g/dL (the cut-off point was considered 8 g/dL in patients with coronary disease) and a new endoscopic examination was repeated whenever the attending physician judged necessary.
Outcomes
Primary outcome assessed was the achievement of hemostasis of the actively bleeding lesion. If no bleeding was observed from targeted site for 3 min after the hemostatic procedure, primary hemostasis was confirmed. In case of failure to achieve hemostasis, another hemostatic modality was chosen and attempted by the endoscopist, and angiographic procedure or emergency surgery was carried out if bleeding persisted.
Other outcomes measured were rebleeding, need for another hemostatic procedure during hospital stay, number of red blood cells packs needed for transfusion, emergency surgery or angiographic procedure rates, length of hospital stay, and mortality rate.
Rebleeding was defined as a drop of 2 g/dL or more of consecutive hemoglobin measurement or any bleeding observed during second-look endoscopy or another endoscopic examination performed during hospital stay. If a non-bleeding visible vessel was seen in the targeted site during second-look endoscopy, a new endoscopic hemostasis was performed, and this situation counted as a need for another hemostatic procedure but not as rebleeding, if not accompanied by any hemodynamic instability or drop of hemoglobin levels. In case of persistent bleeding or rebleeding after two attempts of different endoscopic approaches, angiographic procedure or emergency surgery was performed, and this decision was made after multidisciplinary discussion. Finally, after 48–72 h of stable vital signs and hemoglobin levels, hospital discharge was allowed.
Randomization and allocation concealment
Patients were randomized either to Hemospray group or to Hemoclip group in a 1:1 allocation ratio. The randomization sequence was generated by a computer randomization program (available at randomizer.org) and transferred into opaque, sealed and numbered envelopes. The group assignment sequence remained unknown to the authors throughout the entire trial. After the visual confirmation of an actively bleeding non-variceal lesion, the next envelope in sequence was open and the patient randomized to the designated treatment group.
Hemospray devices were provided specifically for this trial as an experimental technique, before this new tool was authorized for clinical usage in the country. The trial was initiated after the approval of our institution ethics committee.
Statistical analysis
Statistical analysis was performed using the SSPS 17.0 software. Qualitative variables were expressed as minimum and maximum values or mean and standard deviations (± SD). Event frequency and event rates were used for qualitative variables. Student’s t test was used for the comparison between means and medians of the groups. Whenever a normally distribution pattern was rejected, nonparametric Mann–Whitney test was applied. To test for the homogeneity of the sample, Chi-square test or Fisher exact test were used. A 5% significance level (p value) was used for the tests.
Results
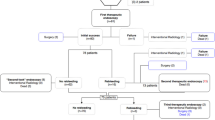
Thirty-nine patients were enrolled from July 2015 to July 2017. Patient number 4 was randomized twice within the first week of hemostatic therapy (protocol deviation). He was twice allocated for the Hemospray group due to different bleeding causes; however, only the first episode was considered for the statistical analyses. No additional subject was enrolled to preserve randomization and allocation concealment.
Most patients were male (66.7%) with a mean age of 56.8 ± 15.7 years. The groups were similar regarding sex, age, Charlson’s comorbidity score, hemodynamic status at presentation, Rockall score, hemoglobin (at admission), and INR (at admission), as shown in Table 1.
The majority of patients presented with oozing bleeding (35/39–89.7%). The most common bleeding site was the duodenum (18/39–46.2%) and peptic ulcer was the most frequent etiology (17/39–43.6%). Table 2 outlines the endoscopic findings. Primary hemostasis was achieved in all Hemospray cases and in 90% of the Hemoclip group (p = 0.487).
Twenty patients required red blood cells transfusions (52.6%), 9 (47.4%) from the Hemospray group and 11 (57.9%) from the Hemoclip group (p = 0.516). There was no difference between groups concerning rebleeding, need for ventilatory support, or use of vasoactive drugs within the first 24 h.
During second-look endoscopy, we found two patients (11.1%) from the Hemospray group with active bleeding, and three others (16.7%) with a non-bleeding visible vessel. Therefore, 5 patients (27.8%) required additional hemostatic procedure during the second-look endoscopy and were successfully treated. In the Hemoclip group, none presented active bleeding or high-risk findings (p = 0.04).
At 1-week follow-up, five patients presented a new hematemesis episode, three from the Hemospray and two from the Hemoclip group (p = 0.642). Besides them, one patient from the Hemoclip group presented with a small hemoglobin drop and the attending team requested another endoscopic examination that found a non-bleeding visible vessel, which required an additional prophylactic hemostasis. Therefore, six patients needed additional procedures within the first week of follow-up, three from each group. There were three deaths in the Hemospray and two in the Hemoclip group within the first week, but only three were directly related to the hypovolemic shock (two Hemospray and one hemoclip) with no statistical difference. Accordingly, the need for surgery was also similar between groups (1 Hemospray vs 0 Hemoclip, p = 0.45).
After 1 week, the patients were followed until hospital discharge or death. In total, eight patients presented with rebleeding, five from the hemostatic powder group and three from the Hemoclip group (p = 0.57) and there were eight deaths (4 Hemospray vs 4 Hemoclip, p = 0.46). Only the three aforementioned casualties were ultimately related to the UGIB episode, while the other deaths were mostly caused by infectious conditions. Moreover, the need for surgical procedure, units of packed red blood cell packs needed, and length of stay were also similar between groups. Table 3 shows the main hemostatic outcomes.
Discussion
To our knowledge, this research represents the first randomized controlled trial using Hemospray for the treatment of patients with NVUGIB. A pilot study was recently published, including patients with signs of recent bleeding [19]; however, we do not consider it appropriate to use hemostatic powder in non-actively bleeding situations, since it needs water from the bleeding site to trigger its hemostatic function. It is important to emphasize that this new technique was applied as the first-line therapy in the emergency setting of an actively bleeding lesion for all patients in our study. Moreover, as all consecutive patients were eligible and the inclusion criteria were very broad, many different etiologies were involved, eliminating the risk of selection bias, and generating a real-life scenario with some very critical patients.
The limited number of patients included in this trial (which was justified by the experimental character of the study, since the trial began before Hemospray was authorized for clinical usage in the country) may have driven away some more pertinent conclusions. However, the results of the study and some of the authors’ impressions should be discussed. Initially, the hemostatic powder’s primary hemostasis rate of 100% must be highlighted, especially considering the distressing conditions in which the actively bleeding situation may occur, such as location of the bleeding site and difficulty of a clear endoscopic view. Recently published retrospective analyses also show great rates of immediate hemostasis after the use of hemostatic powder in difficult scenarios [14, 20, 21]. Therefore, we believe that Hemospray will play a vital role in those critically unstable conditions, in which fast and precise hemostasis is needed.
The comparison between Hemospray group and the conventional dual-therapy group (combination of Hemoclip and epinephrine injection) showed no statistical differences except for one outcome, the requirement for an additional endoscopic hemostatic therapy during second-look endoscopy. A relevant point that must be discussed regarding this difference is that only a minority of patients who underwent a new hemostatic therapy had signs of rebleeding. Most patients presented a non-bleeding visible vessel and it was opted for a prophylactic therapy; however, it remains unclear if this adjuvant treatment was really necessary. Other outcomes assessed, including rebleeding rate, need for emergency surgery, or mortality revealed no significant differences. The rebleeding rate from Hemospray group in this study was similar to other published data on early experience with this new hemostatic tool, indicating that this therapy may not be definitive in some situations [10, 22, 23].
In our study, there were no device-related problems concerning the use of the hemostatic powder. Some episodes of catheter blockage had occurred; however, the Hemospray kit contains an extra catheter in the event the first becomes obstructed. Moreover, consistent hemostasis was always achieved before any malfunction of the second catheter or the emptying of the syringe containing TC-325 powder, even with the 7-French catheter. To avoid obstruction of the catheter with Hemospray powder, we recommend clearing the working channel with air before using the device and to minimize aspiration during the hemostatic procedure. As for the safety of Hemospray application, no systemic toxicity, allergy events, or gastrointestinal obstruction signs were reported, and the same safety profile was verified in children [24]. There was one dialytic patient admitted in the emergency department with refractory hemodynamic instability, who presented a severe spurting bleeding laceration located in distal esophagus, interpreted as a Mallory-Weiss tear. A few minutes after Hemospray application, he showed signs of abdominal distension, and the attending physician opted for a decompressive nasogastric tube, which led to an early rebleeding (probably caused by the removal of hemostatic powder after tube positioning) and death. Afterward, autopsy showed that abdominal distension was due to perforation of distal esophagus (before or during Hemospray therapy), which brings to the recommendation of never using this device when a perforation is suspected or imminent.
Finally, it is opportune to comment on three specific situations. Four patients randomized for the Hemospray group presented with gastric tumor with oozing bleeding (one patient with a GIST in the gastric fundus and three patients with Adenocarcinoma). All of them were successfully treated and did not require any additional hemostatic procedure during the hospitalization period. Despite the short follow-up, we believe that this situation might become one of the main indications of hemostatic powder modality, as already published in previous reports [22, 25]. Another positive experience was the use of Hemospray in post-sphincterotomy bleeding. Two patients were successfully treated with hemostatic powder, and did not experience any obstruction of bile or pancreatic flow, and neither presented with rebleeding episodes during hospitalization. Lastly, it is notable that almost all the patients (except for one) who experienced rebleeding or needed another hemostatic procedure in the Hemospray group presented with an increase of the blood urea nitrogen at admission, which is lately assumed to be a significant predictor of poor outcome [26]. Perhaps this marker should indicate a mandatory and early second-look evaluation after the use of Hemospray so that definitive hemostatic therapy should be performed in better conditions if necessary, considering that hemostatic powder modality shall be used as a bridge treatment in those critical high-risk patients. According to our data, this last consideration should be emphasized in cases of peptic ulcer bleeding, which was the etiology responsible for more than a half of the cases of rebleeding in the Hemospray group.
Limitations
Despite the prospective and randomized character of this research, the study presents some limitations. First, the sample size was limited by the number of Hemospray devices available. This fact may have led to a type II error, slightly narrowing the generalizability of the results found. Another limitation may have been caused by the great and heterogeneous range of etiologies studied. In one hand, it enables a wide study on how this new hemostatic tool works in different settings. However, this fact limits a deeper analysis on specific situations, such as tumor or peptic ulcer bleeding or even in the post-papillectomy management, in which Hemospray may represent a great ally. Therefore, we believe that clinical trials exploring the hemostatic powder technique, assessing specific etiologies, are needed. Being the first randomized controlled trial using Hemospray on non-variceal bleeding, we opted for broad inclusion criteria, simulating the daily scenario of the endoscopist, which do not choose which bleeding lesion will be treated at a given moment. We did not perform cost analysis in our trial, since this was not the focus of this research. It is known that Hemospray presents higher costs compared to metallic clips; however, it is common to place more than one clip at the bleeding site when this technique is used, and this fact may balance costs between these modalities.
Conclusions
In conclusion, hemostatic powder presents similar hemostatic results when compared with conventional dual therapy (Hemoclip and epinephrine) in the treatment of patients with NVUGIB. Hemospray’s excellent primary hemostasis rate certifies it as a valuable tool in those arduous situations of severe bleeding or difficult location site. It also should be considered an optimum bridge hemostatic therapy, especially in high-risk patients, when a definitive endoscopic treatment may be performed in a more favorable condition.
References
Rockall TA, Logan RF, Devlin HB, Northfield TC (1995) Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 311:222–226
Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P (2010) International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 152:101–113
Baracat F, Moura E, Bernardo W, Pu LZ, Mendonça E, Moura D, Baracat R, Ide E (2016) Endoscopic hemostasis for peptic ulcer bleeding: systematic review and meta-analyses of randomized controlled trials. Surg Endosc 30(6):2155–2168
Ribeiro IB, Rezende DT, Madruga Neto AC, Ide E, Furuya CK, De Moura DTH, De Moura EGH (2018) Endoscopic dual therapy for giant peptic ulcer hemorrhage. Endoscopy. https://doi.org/10.1055/a-0665-4142
Barkun AN, Martel M, Toubouti Y, Rahme E, Bardou M (2009) Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc 69:786–799
Sreedharan A, Martin J, Leontiadis GI, Dorward S, Howden CW, Forman D, Moayyedi P (2010) Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev 7:CD005415
Laine L, Yang H, Chang SC, Datto C (2012) Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 107(8):1190–1195
Imperiale TF, Dominitz JA, Provenzale DT, Boes LP, Rose CM, Bowers JC, Musick BS, Azzouz F, Perkins SM (2007) Predicting poor outcome from acute upper gastrointestinal hemorrhage. Arch Intern Med 167(12):1291–1296
Al Dhahab H, McNabb-Baltar J, Al-Taweel T, Barkun A (2013) State-of-the-art management of acute bleeding peptic ulcer disease. Saudi J Gastroenterol 19(5):195–204
Smith LA, Stanley AJ, Bergman JJ, Kiesslich R, Hoffman A, Tjwa ET, Kuipers EJ, von Holstein CS, Oberg S, Brullet E, Schmidt PN, Iqbal T, Mangiavillano B, Masci E, Prat F, Morris AJ (2014) Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol 48(10):e89–e92
Holster IL, van Beusekom HM, Kuipers EJ, Leebeek FW, de Maat MP, Tjwa ET (2015) Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy 47(7):638–645
Sakai CM, Duarte RB, Baracat FI, Baracat R, Moura EGHM (2017) Endoscopic treatment of upper-GI ulcer bleeding with hemostatic powder spray. VideoGIE 2(1):12–13
Baracat FI, Tranquillini CV, Brunaldi VO, Baracat R, Moura EGHM (2017) Hemostatic powder: a new ally in the management of postsphincterotomy bleeding. VideoGIE 2(11):303–304
Pittayanon R, Rerknimitr R, Barkun A (2018) Prognostic factors affecting outcomes in patients with malignant GI bleeding treated with a novel endoscopically delivered hemostatic powder. Gastrointest Endosc 87(4):994–1002
Holster IL, Kuipers EJ, Tjwa ET (2013) Hemospray in the treatment of upper gastrointestinal haemorrhage in patients on antithrombotic therapy. Endoscopy 45:63–66
Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
SNOMED CT (2018) Systematised Nomenclature fo Medicine Clinical Terms. http://www.nlm.nih.gov/research/ulms/Snomed/snomed_main.html. Accessed 23 Sept 2018
Kwek BEA, Ang TL, Ong PLJ, Tan YLJ, Ang SWD, Law NM, Thurairajah PH, Fock KM (2017) TC-325 versus the conventional combined technique for endoscopic treatment of peptic ulcers with high-risk bleeding stigmata: a randomized pilot study. J Dig Dis 18(6):323–329
Kim YJ, Park JC, Kim EH, Shin SK, Lee SK, Lee YC (2018) Hemostatic powder application for control of acute upper gastrointestinal bleeding in patients with gastric malignancy. Endosc Int Open 6(6):E700–E705
Cahyadi O, Bauder M, Meier B, Caca K, Schmidt A (2017) Effectiveness of TC-325 (Hemospray) for treatment of diffuse or refractory upper gastrointestinal bleeding - a single center experience. Endosc Int Open 5(11):E1159–E1164
Haddara S, Jacques J, Lecleire S, Branche J, Leblanc S, Le Baleur Y, Privat J, Heyries L, Bichard P, Granval P, Chaput U, Koch S, Levy J, Godart B, Charachon A, Bourgaux JF, Metivier-Cesbron E, Chabrun E, Quentin V, Perrot B, Vanbiervliet G, Coron E (2016) A novel hemostatic powder for upper gastrointestinal bleeding: a multicenter study (the “GRAPHE” registry). Endoscopy 48(12):1084–1095
Yau AHL, Ou G, Galorport C, Amar J, Bressler B, Donnellan F, Ko HH, Lam E, Enns RA (2014) Safety and efficacy of Hemospray® in upper gastrointestinal bleeding. Can J Gastroenterol Amp Hepatol 28:72–76
Thomson M, Urs A, Narula P, Rao P, Belsha D (2018) The Use and Safety of a Novel Haemostatic Spray in the Endoscopic Management of Acute Nonvariceal Upper Gastrointestinal Bleeding in Children. J Pediatr Gastroenterol Nutr 67(3):e47–e50
Chen Y-I, Barkun A, Nolan S (2015) Hemostatic powder TC-325 in the man- agement of upper and lower gastrointestinal bleeding: a two-year ex- perience at a single institution. Endoscopy 47:167–171
Kumar NL, Claggett BL, Cohen AJ, Nayor J, Saltzman JR (2017) Association between an increase in blood urea nitrogen at 24 hours and worse outcomes in acute nonvariceal upper GI bleeding. Gastrointest Endosc 86(6):1022–1027
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Felipe Iankelevich Baracat, Diogo Turiani Hourneaux de Moura, Vítor Ottoboni Brunaldi, Caio Vinicius Tranquillini, Renato Baracat, Paulo Sakai, and Eduardo Guimarães Hourneaux de Moura declare that they have no conflict of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baracat, F.I., de Moura, D.T.H., Brunaldi, V.O. et al. Randomized controlled trial of hemostatic powder versus endoscopic clipping for non-variceal upper gastrointestinal bleeding. Surg Endosc 34, 317–324 (2020). https://doi.org/10.1007/s00464-019-06769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06769-z