Abstract
Background
Iatrogenic perforations related to endoscopic retrograde cholangiopancreatography (ERCP) are rare events, carrying with it a mortality of up to 8%. Given the rarity of this adverse event, there remains limited data and continued uncertainties when choosing therapeutic strategies. Our aims were to evaluate the management of ERCP-related perforations and compare outcomes based on timing of recognition.
Methods
The endoscopic databases of two tertiary care centers were interrogated to identify consecutive adult patients who sustained ERCP-related perforation over a 10-year period from 2006 to 2016. Electronic medical records were reviewed to extract demographic data, perforation type, management strategies, clinical data, and patient outcomes.
Results
14,045 ERCP’s were performed during our 10-year study period. Sixty-three patients (average age 62.3 ± 2.38 years, 76% female) with ERCP-related perforations were included. Stapfer I perforations were found in 14 (22.2%) patients, Stapfer II in 24 (38.1%), and Stapfer III and IV perforations were identified in 16 (25.4%) and 9 (14.28%), respectively. Forty-seven (74.6%) perforations were recognized immediately during the ERCP, whereas 16 (25.4%) were recognized late. Endoscopic therapy was attempted in 35 patients in whom perforations were identified immediately, and was technically successful in 33 (94.3%). In all, 4 (1 immediate/ 3 delayed) patients required percutaneous drainage and 9 (5 immediate/ 4 delayed) surgery. Length of hospital stay, ICU admission were significantly shorter and incidence of SIRS was significantly lower when perforation was recognized immediately.
Conclusions
Immediate recognition of ERCP-related perforations leads to more favorable patient outcomes; with lower incidence of SIRS, less need for ICU level care, and shorter hospital stay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iatrogenic perforations related to endoscopic retrograde cholangiopancreatography (ERCP) are an uncommon adverse event, occurring in 0.14–1.3% of cases, and are associated with a mortality of up to 8% [1,2,3,4,5,6,7,8,9,10]. Given the rarity of this adverse event, there remains limited high-quality data and continued uncertainties with regard to the optimal therapeutic strategy when a perforation is encountered. Thus, management tends to depend on institutional preference and endoscopist experience rather than an evidence-based approach.
Currently, some experts recommend basing the decision to pursue surgical repair on a patient’s dynamic markers including radiographic findings or vital signs [11,12,13], whereas others have proposed an algorithmic approach to management based on perforation mechanism and timing of recognition [14, 15]. The most widely utilized classification system was developed by Stapfer et al. and characterizes perforations based on mechanism and location [16]. Type I perforations are due to lateral duodenal wall perforation secondary to endoscope trauma, type II perforations are most common and related to endoscopic sphincterotmy, type III are due to perforation of the bile duct with endoscopic tools (wires, stents, baskets), and Type IV are usually miniscule and only identified by free air on fluoroscopic imaging. Prior studies utilizing this classification system demonstrate that patients with type III and IV perforation do well with conservative management alone, whereas patients with Type I and II perforations frequently require surgical management and are more likely to experience poor outcomes [14, 16,17,18]. However, there remain many unanswered questions pertaining to the exact role of surgical, endoscopic, and medical therapy; especially in patients with type I, II, and III perforations.
Predicting outcomes of duodenal perforations related to ERCP has also proven difficult [19]. Prior retrospective studies have related poor clinical outcomes to delay in diagnosis, presence of peritoneal signs, and the need for surgical management [12, 13]. However, “early” and “late” diagnoses have not been clearly defined; with “early” diagnosis being defined as anywhere between zero and 28 h after ERCP [13, 14]. To our knowledge, there is no study that has specifically compared perforations recognized immediately during ERCP versus those recognized post-procedurally.
Therefore, our aims were to evaluate the management of ERCP-related perforations and compare outcomes based on timing of recognition and therapeutic approach.
Materials and methods
The endoscopic databases of two tertiary care centers (Barnes Jewish Hospital and Barnes Jewish West County Hospital) were interrogated to identify consecutive adult patients who sustained ERCP-related perforation of the gastrointestinal tract over a 10-year period from 2006 to 2016. Patients were identified through the institutional endoscopy database (Provation MD, Minneapolis, MN) and review of our endoscopic nursing database obtained by direct patient contact (of all post-ERCP patients) within 24- to 72-h post-procedure. This study protocol was approved by the Human Research Protection Office (Institutional Review Board) at Washington University School of Medicine in Saint Louis. Electronic medical records (EMR) were reviewed by study members not involved with direct patient care (J.B., J.E.) to extract demographic data, perforation type, management strategies, clinical data, and patient outcomes. Those patients with ERCP-related esophageal and gastric perforations were excluded from our analysis.
Classification of perforations
ERCP-related perforations were classified based on the system proposed by Stapfer et al. [16] (Fig. 1). Type I perforations are lateral or medial wall perforations typically as a result of endoscope trauma, Type II (perivaterian) perforations are peri-ampullary and related to endoscopic sphincterotomy. Type III perforations represent ductal injuries due to passage of instrumentations such as guidewires, baskets, or stents. Type IV perforations are generally miniscule and are defined as the presence of retroperitoneal air alone and are thought to be due to over insufflation.
Definitions with endoscopic and fluoroscopic examples of ERCP-related perforations: A Type I—defect seen in lateral/medial duodenal wall secondary to endoscope trauma. B Type II—sphincterotomy related perforation (arrow) with the presence of pancreatic duct stent. C Type III—ductal or duodenal perforations secondary to instruments (i.e., guidewires, baskets, stents). Pancreatic duct leak seen on pancreatogram (arrow). D Type IV—presence of retroperitoneal air seen on fluoroscopy (arrows)
Management of perforations was at the discretion of the GI attending physician in collaboration with the consulting hepatobiliary surgeon and interventional radiologist. If recognized immediately, it is our standard of care for all patients with type I and II perforations to attempt endoscopic therapy with clip closure and/or biliary diversion with plastic or fully covered self-expanding metal stent (FCSEMS) placement (type II perforation only), bowel rest, intravenous antibiotics, and a nasogastric tube for luminal decompression. Patients failing endoscopic or medical management were escalated to surgery or percutaneous drainage; based on consensus of the multidisciplinary team.
Definitions
Immediately recognized perforation
This was defined by direct endoscopic visualization of a luminal defect, finding of extravasation of contrast, extraluminal passage of guidewire, or presence of retroperitoneal gas on fluoroscopic imaging during index ERCP.
Delayed recognized perforation
This was defined as perforation diagnosed based on imaging findings of pneumoperitoneum or presence of retroperitoneal gas with associated symptoms (abdominal pain requiring hospitalization) in the post-endoscopy recovery suite or were discharged to home and re-admitted with symptoms and imaging findings as above.
Patient characteristics
Comorbidities were assessed using the age-adjusted Charlson Comorbidity index (CCI) [20]. Systemic inflammatory response syndrome (SIRS) was defined as the presence of 2 or more of the following: body temperature < 36 °C or > 38 °C, tachycardia > 90 beats per minute, white blood cell count of < 4 × 10/L or > 12 × 10 9 / L, and tachypnea > 20 breaths per minute [21]. Post-ERCP pancreatitis (PEP) was defined according to consensus criteria [22, 23].
Statistical analysis
Demographic and clinical characteristics were reported using descriptive statistics: mean ± standard error of the mean and percentage for continuous variables. Univariate analysis was performed using the chi-square test or Fischer’s exact test where appropriate. Grouped data were compared using the Student t test. In all cases, p < 0.05 was required for statistical significance.
Results
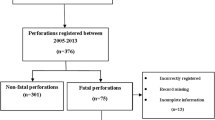
In total, 14,045 ERCP’s were performed during our 10-year study period from 2006 to 2016. Sixty five patients were identified with an ERCP-related iatrogenic perforation of the gastrointestinal tract; overall incidence of 0.46%. One patient was found to have an esophageal perforation and one patient had a gastric perforation, both were excluded from our current analysis given our focus on local–regional perforations. Therefore, 63 patients (average age 62.3 ± 2.38 years, 76% female) were included (Fig. 2). Forty-seven of the 63 (74.6%) perforations were recognized immediately during the ERCP, whereas 16 (25.4%) were classified as delayed. The baseline demographic, clinical, and procedural characteristics are listed in Table 1. Mean age-adjusted CCI was 3.4; similar in both groups (3.6 vs. 3.18, p = 0.32). Biliary obstruction was the most common indication for ERCP in 69.8% of patients, followed by Sphincter of Oddi dysfunction in 27%, ampullectomy and hemostasis in 2.1%, respectively. Three patients (4.7%) had undergone a Billroth II gastrectomy and 2 (3.2%) patients had large peri-ampullary diverticulum. Post-ERCP pancreatitis (PEP) developed in 9 patients (14.3%), 5 (10.6%) immediate vs 4 (25%) delayed, p = 0.16.
Classification and management of ERCP-related perforations
Stapfer I perforations were found in a total of 14 patients; 8 (61.5%) were recognized immediately, whereas 6 (42.8%) were recognized late (Table 2). Notably, 4 (30.7%) patients with type I perforations had documented altered anatomy (3 patient with Billroth II and one with peri-ampullary diverticulum). Nine (69.2%) patients in this group ultimately required surgery. Three (23.1%) patients had attempts at endoscopic repair with one patient treated with through the scope clips (TTS) and two with over-the-scope-clips (OTSC; Ovesco Endoscopy AG). One patient treated with OTSC required definitive surgical repair due to technical difficulties upon clip deployment. In the delayed group, two patients were determined to be poor surgical candidates due to advanced underlying malignancy. Of these, one patient was medically managed and one underwent percutaneous drainage of an intra-abdominal abscess, both died within 30 days of ERCP.
Stapfer II perforations were found in 24 (38.1%) patients of which 18 (72%) were recognized immediately and 6 (24%) recognized late. No patients required surgical management. Endoscopic therapy was attempted in 17 patients in the immediate recognition group (9 plastic stents, 8 FCSEMS and 2 TTS clips), and was successful in 16; none of these patients required surgery and one patient required percutaneous drainage. One patient did not receive diversion therapy due to the inability to obtain biliary access. In the delayed group, all patients were treated conservatively with bowel rest and antibiotics; no patients require percutaneous drainage or surgery.
Stapfer III and IV perforations were identified in 16 (25.4%) and 9 (14.3%), respectively. When recognized early, Stapfer III perforations were managed endoscopically in 9 patients (61.5%) (8 plastic stents, 1 FCSEMS) with none requiring percutaneous drainage or surgery. In the delayed recognition group, one patient required surgical management after an endoscopic ultrasound (EUS)-guided rendezvous resulted in bile duct perforation, 1 patient underwent bile duct stent placement, and one required percutaneous drainage. None of the patients with Stapfer IV perforations required percutaneous drainage or surgery, 4 patients (44.4%) had placement of biliary stents upon recognition of retroperitoneal air.
Patient-related outcomes of all ERCP-related perforations
When recognition of ERCP-related perforation was delayed, the presence of SIRS was significantly more common (4.3% vs. 31.2%, p = 0.003); Table 3. Overall mean length of stay in our cohort was 7.1 ± 1.25 days with a significant increase in length of stay when perforation was recognized late (5.15 ± 0.64 vs. 12.69 ± 4.34, p = 0.007). Number of patient days spent in intensive care was also significantly longer in those with delayed recognition (0.5 ± 0.21 vs. 3.6 ± 2.38, p = 0.04). Overall, non-operative management was successful in 52/63 cases, 82.5%. Post-ERCP pancreatitis was present in 9 (14.3%) patients; 5 (10.6%) immediate, 4 (24%) delayed (p = 0.16). Deaths were seen in 3 patients (4.8%); 1 in the early versus 2 patients in the late recognition group which was not statistically significant. Notably, all three patients who died within 30 days of index procedure had underlying metastatic malignancy and were deemed not to be surgical candidates.
Patient-related outcomes of Type I and II perforations (Table 4)
In a subgroup analysis of only type I and type II perforations (those effecting GI tract lumen), SIRS was significantly more common when the diagnosis of perforation was delayed (33.3% vs. 7.7%, p = 0.04). While length of stay (6.73 ± 0.98 vs. 13.0 ± 5.80, p value 0.69) and number of ICU days (0.73 ± 0.33 vs. 4.25 ± 3.14, p = 0.056) did not reach statistical significance. Mortality was more common in the delayed group (0% vs 16.7%, p = 0.015).
Patient-related outcomes of operative repair
Ten patients in our cohort required definitive surgical repair. Nine patients had type I perforations. Eight underwent primary repair by over sewing of the small intestinal defect and one patient underwent a choledochojejunostomy with gastroenterostomy in the setting of known pancreatic malignancy after being deemed not appropriate for resection. One patient with a type III perforation underwent repair of a distal common bile duct injury with primary closure and use of omental flap. The mean length of stay in the operative group was 17.5 ± 6.7 days, mean number of ICU days of 6.9 ± 3.5 days. There were no deaths within 30 days.
Discussion
In this study, we evaluated 63 consecutive patients with ERCP-related perforations over a 10-year study period. Our results indicate that immediate recognition of ERCP-related perforations leads to more favorable patient outcomes; with a lower incidence of SIRS, less need for ICU level care, and shorter hospital stay. Additionally, immediate recognition of Stapfer type I and II perforations is associated with decreased perforation-associated mortality. Furthermore, our data support endoscopic stent placement as a safe and effective treatment for Stapfer II, III, and IV perforations.
ERCP-related perforations remain a rare but serious adverse event [1, 3, 5,6,7,8]. Given the rarity and general limited high-quality data, management is generally guided by the endoscopist and institutional experience. Prior studies have demonstrated that earlier recognition may lead to improved outcomes [6, 24, 25]. It is hypothesized that early recognition and therapy limits the leakage of luminal contents and therefore results in less inflammatory reaction and potentially fewer complications. This hypothesis is supported by the literature pertaining to luminal perforations throughout the gastrointestinal tract [26]. In a recent study, examining patients with cervical esophageal and hypopharyngeal perforations, Zenga et al. found that patients who had per oral intake between the time of perforation and diagnosis and those that demonstrated signs of systemic toxicity (SIRS) were at higher risk of failing conservative management. Other studies, pertaining to colonic endoscopic mucosal resection, demonstrate that even with deep mural injuries and perforations, closure with endoscopic measures has a high rate of success in the setting of immediate identification and proactive management [27,28,29]. While thes data cannot be directly extrapolated to ERCP-related perforations, results would support this hypothesis, as those in whom perforation was not recognized immediately had on average a greater length of stay and higher likelihood of SIRS which may portend a poor prognosis and need for surgery.
Our study was the first to define delayed recognition as any patient where perforation was not recognized during endoscopy. This definition appears to have the most clinical relevance as the endoscopist, when recognized immediately, may be able to provide endoscopic therapy with stent placement, use of through-the-scope endoclips (TTS), or over-the-scope clips [30,31,32]. There is increasing literature, demonstrating efficacy of TTS and OTSC for repair of luminal perforation citing success rates of up to 90% [33, 34]. In our study, TTS and OTSC’s were utilized in 3 patients with type I perforations with a success rate of 66% (Fig. 3). In one patient, there was a technical failure upon deployment of the OTSC, and the patient ultimately required surgery; emphasizing the need for adequate training and surgical backup when endoscopic therapy is attempted. Furthermore, deployment of the OTSC in the duodenum can be extremely challenging due to the size of the outer diameter of the clip and sharp angulations in the duodenal sweep. Careful selection of patients is important and depends on various factors including underlying comorbidities and the size of luminal defect [34]. Despite attempts at endoscopic therapy in three cases, 7 out of 11 patients with lateral or medial duodenal perforations required definitive surgical repair. Therefore, unless the perforation is recognized immediately and endoscopic repair is thought to be successful, definitive surgical management should be urgently undertaken, to avoid further peritoneal contamination and resulting systemic toxicity.
It is our practice that all patients with type I and II perforations receive antibiotic therapy, bowel rest, and NG tube placement. The decision to pursue endoscopic management vs surgical management is based on the timing and location of perforation and also the patient’s clinical status. Recently, Khumbari et al. evaluated an algorithmic approach to manage ERCP-related perforations based on Stapfer classification [14]. They were able to retrospectively validate the approach demonstrating that type I perforations were better managed surgically and type II perforations managed medically unless they were deemed to clinically worsen. While our management strategy was similar, the timing of recognition differed greatly as 74.6% of perforations in our study were recognized immediately compared to less than 10% in the prior study. This primarily impacts the rate at which endoscopists are able to attempt endoscopic repair methods. Despite this, the majority of our patients with Type I perforations still underwent definitive surgical repair. Thus, a multidisciplinary approach in conjunction with hepatobiliary surgery remains the recommended treatment for lateral or medial duodenal wall perforations.
Type II perforations are most commonly encountered in clinical practice occurring when the sphincterotomy extends beyond the intramural portion of the bile duct or pancreatic duct. Similar to Type I perforations, the management approach should be individualized based on size of perforation and patient’s condition upon recognition. In the recently proposed algorithm, a surgical approach is only recommended if non-operative management fails [14]. In our study, no patient with a type II perforation in either group required definitive surgical repair. Our results further differ from a recent meta-analysis where patients with initial non-operative management of type II perforations demonstrated a failure rate near 30% [3]. Reasons for this difference are unclear but may be attributable to our high rate of biliary diversion therapy with the placement of either plastic biliary stents or FCSEMS. Recent data from Tringali et al. reported 100% success rate in preventing mortality and surgery with placement of FCSEMS in type II and III perforations [35]. In our study, 17 of 18 patients with type II perforations received endoscopic therapy with either FCSEMS or plastic stents (Fig. 4). Zero patients who received FCSEMS required a repeat intervention and one patient who received a plastic stent required percutaneous drainage. Our data suggest that in these patients, diversion therapy with stenting is beneficial, however, the superiority of FCSEMS versus plastic stents remains to be determined.
Overall, our data enhance the current treatment algorithms by emphasizing several important points. First, we have highlighted the importance of immediate recognition. Therefore, the endoscopist must have a high index of suspicion to recognize the defect and further needs expertise when attempting novel endoscopic repair methods. Although the majority of patients (69%) in this study with Stapfer I perforations required definitive repair, we anticipate that a greater number of lateral or medial duodenal perforations could be managed endoscopically, with high success rates, as experience with these techniques improves [33, 34]. Second, our data suggest that diversion therapy with stenting is beneficial in type II and III perforations. In type II perforations, no patient in our study required operative management which differs greatly from the 30% failure rate cited in prior publications [3]. Therefore, based on our results, we would propose attempts at endoscopic repair in all patients if the perforation is recognized immediately and would recommend that diversion therapy with plastic stents or FCSEMS be performed in patients with type II, III, and IV perforations.
There are limitations to our study. First, our study is retrospective in nature and limitations inherent to this design. Second, it is difficult for us to draw conclusions on the efficacy of endoscopic repair techniques given the relatively small number of patients, particularly with type I perforations. Finally, this study describes the practice pattern of a single group from a high volume tertiary care center and our practice may differ from other institutions making generalizability of our data unclear.
In conclusion, we have highlighted the importance of immediate recognition of ERCP-related perforations by showing a significant decrease in number of ICU days, LOS, and those presenting with signs of systemic toxicity when recognized during the endoscopic procedure. Furthermore, our data further support the role of attempting endoscopic therapy in immediately recognized ERCP-related perforations, especially in Types II and III.
References
Aliperti G (1996) Complications related to diagnostic and therapeutic endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am 6(2):379–407
Baillie J (1994) Complications of endoscopy. Endoscopy 26(1):185–203
Cirocchi R, Kelly MD, Griffiths EA, Tabola R, Sartelli M, Carlini L, Ghersi S, Di Saverio S (2017) A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon 15(6):379–387
Cotton PB, Garrow DA, Gallagher J, Romagnuolo J (2009) Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc 70(1):80–88
Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ et al (1996) Complications of endoscopic biliary sphincterotomy. N Eng J Med 335(13):909–918
Kodali S, Monkemuller K, Kim H, Ramesh J, Trevino J, Varadarajulu S, Wilcox CM (2015) ERCP-related perforations in the new millennium: a large tertiary referral center 10-year experience. United Eur Gastroenterol J 3(1):25–30
Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A (1998) Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc 48(1):1–10
Machado NO (2012) Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP 13(1):18–25
Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A et al (2001) Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol 96(2):417–423
Silviera ML, Seamon MJ, Porshinsky B, Prosciak MP, Doraiswamy VA, Wang CF, Lorenzo M, Truitt M, Biboa J, Jarvis AM et al (2009) Complications related to endoscopic retrograde cholangiopancreatography: a comprehensive clinical review. J Gastrointestin Liver Dis 18(1):73–82
Assalia A, Suissa A, Ilivitzki A, Mahajna A, Yassin K, Hashmonai M, Krausz MM (2007) Validity of clinical criteria in the management of endoscopic retrograde cholangiopancreatography related duodenal perforations. Arch Surg 142(11):1059–1064
Dubecz A, Ottmann J, Schweigert M, Stadlhuber RJ, Feith M, Wiessner V, Muschweck H, Stein HJ (2012) Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg 55(2):99–104
Jin YJ, Jeong S, Kim JH, Hwang JC, Yoo BM, Moon JH, Park SH, Kim HG, Lee DK, Jeon YS et al (2013) Clinical course and proposed treatment strategy for ERCP-related duodenal perforation: a multicenter analysis. Endoscopy 45(10):806–812
Kumbhari V, Sinha A, Reddy A, Afghani E, Cotsalas D, Patel YA, Storm AC, Khashab MA, Kalloo AN, Singh VK (2016) Algorithm for the management of ERCP-related perforations. Gastrointest Endosc 83(5):934–943
Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, Swack ML, Kopecky KK (1999) Classification and management of perforations complicating endoscopic sphincterotomy. Surgery 126(4):658–663 discussion 664–655.
Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, Garry D (2000) Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg 232(2):191–198
Miller R, Zbar A, Klein Y, Buyeviz V, Melzer E, Mosenkis BN, Mavor E (2013) Perforations following endoscopic retrograde cholangiopancreatography: a single institution experience and surgical recommendations. Am J Surg 206(2):180–186
Polydorou A, Vezakis A, Fragulidis G, Katsarelias D, Vagianos C, Polymeneas G (2011) A tailored approach to the management of perforations following endoscopic retrograde cholangiopancreatography and sphincterotomy. J Gastrointest Surg 15(12):2211–2217
Morgan KA, Fontenot BB, Ruddy JM, Mickey S, Adams DB (2009) Endoscopic retrograde cholangiopancreatography gut perforations: when to wait! When to operate!. Am Surg 75(6):477–483; discussion 483 – 474
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101(6):1644–1655
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS (2013) Acute pancreatitis classification working G: classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 62(1):102–111
Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N (1991) Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Eendosc 37(3):383–393
Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, Baillie J (2002) ERCP-related perforations: risk factors and management. Endoscopy 34(4):293–298
La Torre M, Velluti F, Giuliani G, Di Giulio E, Ziparo V, La Torre F (2012) Promptness of diagnosis is the main prognostic factor after colonoscopic perforation. Colorectal Dis 14(1):e23–e26
Zenga J, Kreisel D, Kushnir VM, Rich JT (2015) Management of cervical esophageal and hypopharyngeal perforations. Am J Otolaryngol 36(5):678–685
Burgess NG, Bassan MS, McLeod D, Williams SJ, Byth K, Bourke MJ (2016) Deep mural injury and perforation after colonic endoscopic mucosal resection: a new classification and analysis of risk factors. Gut. https://doi.org/10.1136/gutjnl-2015-309848
Ma MX, Bourke MJ (2016) Complications of endoscopic polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection in the colon. Best Pract Res Clin Gastroenterol 30(5):749–767
Swan MP, Bourke MJ, Moss A, Williams SJ, Hopper A, Metz A (2011) The target sign: an endoscopic marker for the resection of the muscularis propria and potential perforation during colonic endoscopic mucosal resection. Gastrointest Endosc 73(1):79–85
Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA (2012) Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc 76(1):202–208
Mangiavillano B, Viaggi P, Masci E (2010) Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis 11(1):12–18
Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P et al (2012) Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol 10(6):603–608
Honegger C, Valli PV, Wiegand N, Bauerfeind P, Gubler C (2017) Establishment of over-the-scope-clips (OTSC(R)) in daily endoscopic routine. United Eur Gastroenterol J 5(2):247–254
Verlaan T, Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P (2015) Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc 82(4):618–628
Tringali A, Cintolo M, Hassan C, Adler DG, Mutignani M (2017) Type II-III ERCP-related perforations treated with temporary Fully covered self-expandable stents. Dig Liver Dis 49:1169–1170
Author information
Authors and Affiliations
Contributions
JGB concept, design, data analysis, drafting the article; ZS manuscript revision; JB data collection and analysis; JE data analysis and collection; PH data collection, manuscript revision; GDL manuscript revision; DSE manuscript revision; KD manuscript revision; TH study design and data collection; MD manuscript revision; RCF manuscript revision; WGH manuscript revision; SMS manuscript revision; CH manuscript revision; WCC manuscript revision; SE manuscript revision; DKM concept, design, manuscript revision; VMK concept, design, data analysis, and critical revision of the article.
Corresponding author
Ethics declarations
Disclosures
Dr. Maria B. Majella Doyle receives speaking fees from Novartis; Dr William G Hawkins reports other from Accuronix; Dr. Chet Hammil reports personal fees from Medtronics; Dr. William C. Chapman receives personal fees from Novartis, XOR Labs Toronto and Pathfinder; Dr. Steven Edmundowicz receives personal fees from Olympus and Elsevier publications. Also receives personal fees from Check-cap, Motus, Freehold Surgical, Elira, Paion, Orchestra Medical, Medtronic and Spironetics, Dr. Daniel K Mullady receives personal fees from Boston Scientific; Jason G Bill, Zachary Smith, Joseph Brancheck, Jeffrey Elsner, Paul Hobbs, Gabriel D Lang, Dayna S Early, Koushik Das, Thomas Hollander, Ryan C. Fields, Steven M. Strasberg, and Vladimir Kushnir have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Bill, J.G., Smith, Z., Brancheck, J. et al. The importance of early recognition in management of ERCP-related perforations. Surg Endosc 32, 4841–4849 (2018). https://doi.org/10.1007/s00464-018-6235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6235-8