Abstract
Background
The long-term outcomes of robotic-assisted laparoscopic lateral lymph node dissection (RALLD) have not been fully investigated. This study aimed to assess the oncological and long-term outcomes of RALLD for rectal cancer through comparison with those of open lateral lymph node dissection (OLLD) in a retrospective study.
Methods
Between September 2002 and October 2014, the medical data of 426 patients who underwent total mesorectal excision with lateral lymph node dissection for primary rectal cancer were collected. Of these, 115 patients were excluded after data collection (stage IV, n = 61; total pelvic exenteration, n = 31; multiple cancer, n = 20; conventional laparoscopic surgery, n = 3). Before matching, 311 patients with clinical stage II/III were analyzed. Using exact matching, patients were stratified into RALLD (n = 78) and OLLD (n = 78) groups. Pathological findings and long-term outcomes were compared between the groups.
Results
The pathological stage and number of harvested lymph nodes showed no significant differences between the groups. The rate of positive resection margin in the RALLD group tended to be lower compared with that of the OLLD group (p = 0.059). The median follow-up duration was 54.0 months in 156 patients. The 5-year overall survival rate was 95.4 and 87.8% in the RALLD and OLLD groups, respectively (p = 0.106). The 5-year relapse-free survival rate was 79.1 and 69.9% in the RALLD and OLLD groups, respectively (p = 0.157). The 5-year local relapse-free survival rate was 98.6 and 90.9% in the RALLD and OLLD groups, respectively (p = 0.029).
Conclusions
The short- and long-term outcomes indicated that RALLD may be a useful modality for locally advanced low rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In Japan, total mesorectal excision (TME) with lateral lymph node dissection (LLD) is indicated for patients with clinical T3–4 low rectal cancer, in accordance with the Japanese Society for Cancer of the Colon and Rectum guidelines for the treatment of colorectal cancer [1], and neoadjuvant chemoradiotherapy (CRT) is performed only for selected patients. A randomized controlled trial (RCT) comparing open TME with and without LLD for clinical stage II/III low rectal cancer showed that postoperative complications were comparable between the two groups, although LLD is associated with longer operative times and greater blood loss [2]. Recently, several retrospective studies have demonstrated the safety and feasibility of conventional laparoscopic LLD (CLLLD) [3,4,5,6,7,8]. Meanwhile, Liang [9] reported that the morbidity was not particularly low (21.7%) and the short-term recurrence rate was quite high (27.3%) and concluded that the technical feasibility of CLLLD was suitable only for a few selected patients. CLLLD has a technical problem with straight and inflexible instruments and it has inadequate visualization caused by its unstable camera and the assistant’s traction in the narrow and anatomically complex pelvic cavity. To standardize CLLLD, these technical problems of conventional laparoscopic surgery (CLS) should be improved. Robotic-assisted laparoscopic surgery (RALS) is a promising advanced technology that can overcome the inherent limitations of CLS. Compared with CLS, RALS has advantages such as free-moving multijoint forceps, a motion scaling function, high-quality three-dimensional imaging, stable camera work by an operator, greatly improved ergonomics, and short learning curve [10]. A few retrospective case series reported that robotic-assisted laparoscopic LLD (RALLD) was safe and feasible [11,12,13,14]. We reported the short-term outcomes of RALLD by comparing with those of open LLD (OLLD) and concluded that the short-term outcomes of RALLD may be superior to those of OLLD [15]. A couple of reports have focused on the long-term outcomes of RALLD, and they included only a few patients [12, 14]. Therefore, the long-term outcomes of RALLD were not fully investigated. Moreover, no report has compared the long-term outcomes between the RALLD and OLLD groups. The aim of the present study was to assess the oncological and long-term outcomes of RALLD through comparison with those of OLLD.
Materials and methods
Patients and study design
The data of consecutive primary rectal adenocarcinoma patients who underwent TME with LLD at Shizuoka Cancer Center Hospital between September 2002 and October 2014 were retrospectively reviewed. Before data collection, biopsies of the lateral lymph nodes were excluded. A prospective colorectal database, containing information regarding patient characteristics, preoperative assessment, operative characteristics, postoperative complications, pathological characteristics, and oncological outcomes, maintained at the hospital was used for the analysis. We already reported the short-term outcomes of RALLD retrospectively [13, 15], and these patients were included in this study. All study protocols were approved by our institutional review board (29-J7-29-1-3).
Treatment strategy for rectal cancer
TME with bilateral LLD is indicated for patients with clinical T3–4 low rectal cancer on preoperative images, in accordance with the Japanese guidelines [1]. Our indications for LLD were either low rectal cancer with clinical T3–4 or T1–2 rectal cancer with metastasis to lateral lymph nodes on preoperative images. Patients without lateral lymph node metastasis on preoperative images who were older than 75 years or with severe comorbid conditions did not undergo LLD. Unilateral (involved side) LLD was performed in patients diagnosed with lateral lymph node metastasis on preoperative imaging but who were older than 75 years or with severe comorbid conditions. Lateral lymph nodes with a short-axis diameter of ≥ 6 mm, irregular shapes, and heterogeneous internal intensity on preoperative MRI were regarded as clinically metastatic lateral lymph nodes. Stage III patients with pathological lymph node metastasis were recommended for adjuvant chemotherapy for 6 months after the operation. Neoadjuvant CRT (50.4 Gy in 25 fractions for 5 weeks with systemic capecitabine chemotherapy) was used for clinical T4 patients in whom obtaining a clear resection margin without CRT was difficult. Preoperative tumor staging was performed by colonoscopy, computed tomography, magnetic resonance imaging, and barium enema. Low rectal cancer was defined as the lower border of the tumor being located distal to the peritoneal reflection. Patients were staged using the tumor node metastasis classification [16]; however, lateral lymph nodes (internal iliac, obturator, and common iliac lymph nodes) were considered regional lymph nodes, as reported previously [17].
Indications for robotic-assisted laparoscopic surgery
Up until December 2011, when RALS was starting (da Vinci® surgical system; Intuitive Surgical, Sunnyvale, CA, USA), TME with LLD was performed using the open method. Since then, TME with LLD have also been performed using robotic-assisted methods. We performed RALS for all patients who opted for it, regardless of sex, body mass index, tumor location, clinical stage, or type of operation. RALS was performed by four surgeons (T.Y., Y.K., A.S., and H.K.) with extensive experience in open and conventional laparoscopic colorectal surgeries. OLLD was performed by 10 surgeons including the above four surgeons.
Operative technique
We have described in detail our technique of RALLD previously [13, 15]. LLD involves complete removal of the lateral pelvic lymph nodes in the fat tissues outside the pelvic plexus, around the common iliac artery, internal iliac artery, and obturator space, preserving all autonomic nerves. The procedure of OLLD was done in accordance with previously reported methods [2]. The removal area of OLLD was the same as that of RALLD.
Pathological findings and long-term outcomes
Pathological parameters that could influence the quality of rectal surgery and oncological outcomes, including the number of harvested lymph nodes and positive resection margin status, were evaluated. A positive resection margin included a positive surgical dissection plane and a positive proximal or distal margin of the resected specimen. Long-term oncological outcomes such as overall survival, relapse-free survival, and local relapse-free survival rates were analyzed for the patients who underwent RALLD or OLLD.
Statistical analysis
To reduce covariate imbalance between the groups, one-to-one exact matching of patients in the RALLD group with those in the OLLD group was performed, according to clinical T, clinical N, and neoadjuvant CRT. Comparative analyses of the patients in the RALLD and OLLD groups were performed on the 1:1 matched cohort. Mann–Whitney U tests were performed to compare the continuous variables between the two groups. The categorical variables were analyzed using Fisher’s exact tests or Chi-square tests. Overall survival, relapse-free survival, and local relapse-free survival rates were calculated using the Kaplan–Meier method and compared using the log-rank test. Data differences between groups were considered statistically significant at p < 0.05. All statistical analyses were conducted using the Statistical Package for the Social Sciences for Windows, version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
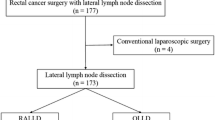
In total, 426 patients who underwent LLD were included in this study. Of these, 115 patients were excluded after data collection. Exclusion criteria included stage IV (n = 61), total pelvic exenteration (n = 31), multiple cancer such as synchronous or metachronous malignant lesions (within 5 years) other than carcinoma in situ (n = 20) and CLS (n = 3). Before matching, 311 patients with clinical stage II/III (83 patients who were treated with RALLD and 228 patients who were treated with OLLD) were analyzed. Using the exact matching method, patients matched according to clinical T, clinical N, and neoadjuvant CRT were stratified into RALLD (n = 78) and OLLD (n = 78) group (Fig. 1).
The baseline characteristics of the overall and matched cohorts of patients included in the RALLD and OLLD groups are summarized in Table 1. Before matching, clinical T and N tended to be different between the groups. After matching, clinical T, clinical N, and neoadjuvant CRT became well balanced. Moreover, other characteristics, such as age, sex, carcinoembryonic antigen, and tumor distance from the anal verge were not significantly different between the groups.
Surgical characteristics and pathological findings
The surgical characteristics and pathological findings are listed in Table 2. The type of procedure was not significantly different between the groups. The rate of poorly differentiated adenocarcinoma or mucinous carcinoma was significantly higher in the OLLD group compared with the RALLD group. In both groups, 36 (23.1%) patients had clinical lateral lymph node metastasis and 20 (12.8%) patients had pathological lateral lymph node metastasis. In patients with pathological T3/4, 28/120 (23.3%) were diagnosed with clinical lateral lymph node metastasis, and 8/36 (22.2%) were diagnosed with pathological T1/2. The results of the prediction of pathological lateral lymph node metastasis by MRI finding were as follows: sensitivity, 75.0% (15/20); specificity, 84.6% (115/136); positive predictive value, 41.7% (15/36); negative predictive value, 95.8% (115/120); accuracy, 83.3% (130/156). In the 12 neoadjuvant chemoradiotherapy patients, five had clinical lateral lymph node metastasis and four had pathological lateral lymph node metastasis. Pathological T, pathological N, pathological lateral lymph node metastasis, pathological stage, and the number of harvested lymph nodes showed no significant differences between the groups. Although the rate of positive resection margin tended to be lower in the RALLD group than in the OLLD group, it was not statistically significant. There were no p/yp stage IV patients in the two groups. In terms of postoperative chemotherapy, in the RALLD group, fluorouracil or capecitabine was administered in 18 patients, and fluorouracil or capecitabine combined with oxaliplatin was administered in 22 patients. Meanwhile, in the OLLD group, fluorouracil or capecitabine was administered in 30 patients, and fluorouracil or capecitabine combined with oxaliplatin was administered in 11 patients.
Long-term outcomes
The median follow-up duration was 54.0 (range 13.6–135.0) months (RALLD group, 41.6, range 13.6–63.3 months; OLLD group, 59.6, range 15.6–135.0 months) in 156 patients. The 5-year overall survival rate was 95.4 and 87.8% in the RALLD and OLLD groups, respectively (p = 0.106) (Fig. 2). The 5-year relapse-free survival rate was 79.1 and 69.9% in the RALLD and OLLD groups, respectively (p = 0.157) (Fig. 3). The 5-year local relapse-free survival rate was 98.6 and 90.9% in the RALLD and OLLD groups, respectively (p = 0.029) (Fig. 4). In terms of local recurrence, in the RALLD group, one patient had lateral lymph node recurrence. Meanwhile, in the OLLD group, three patients had lateral lymph node recurrence; three patients had central pelvic recurrence, and one patient had anastomotic recurrence. The patient with central pelvic recurrence in the OLLD group was the one with positive resection margin at initial operation. The three patients with lateral lymph node recurrence and one patient with central pelvic recurrence in the OLLD group were those with pathological lateral lymph node metastasis at initial operation.
Discussion
In this study, we compared the long-term outcomes of the RALLD group with those of the OLLD group for locally advanced low rectal cancer. To our knowledge, this is the first study to compare long-term outcomes between patients who underwent RALLD and OLLD. Since clinical T, clinical N, and neoadjuvant CRT have been considered to have impact on recurrence, one-to-one exact matching was performed to adjust for differences in these factors between the two groups. Recently, several studies of RALLD have been reported [11,12,13,14,15]. Park et al. [11] first reported the safety and feasibility of RALLD based on a series of 8 patients treated with RALLD. Bae et al. [12] also reported the safety and feasibility of RALLD based on a series of 11 patients treated with RALLD and 10 patients treated with CLLLD. They concluded that minimally invasive techniques for LLD in selected patients can be an acceptable alternative to OLLD. Moreover, we already reported the short-term outcomes of RALLD (n = 85) through comparison with those of OLLD (n = 88) [15]. Although operative time was significantly longer, blood loss was significantly lesser in the RALLD group than in the OLLD group. The rates of wound infection, small bowel obstruction, anastomotic leakage, and urinary retention were significantly lower in the RALLD group than in the OLLD group. We concluded that the short-term outcomes of RALLD may be superior to those of OLLD for locally advanced low rectal cancer. However, efficacy and long-term oncological outcomes have not been fully investigated.
The present study showed that the overall survival rate and the relapse-free survival rate of the RALLD group tended to be better compared with those of the OLLD group. The local relapse-free survival rate of the RALLD group was significantly better compared with that of the OLLD group. We considered the reasons for the lower local recurrence rate in the RALLD group despite the same rate of clinical T and neoadjuvant CRT between the groups. Our previous study showed extremely low blood loss in the RALLD group compared with that in the OLLD group (25 vs. 637 mL, p < 0.001) [15], thus providing a clear surgical field, leading to precise rectal mobilization and LLD. Consequently, less urinary dysfunction and less positive resection margin of the RALLD could be achieved. These reasons might explain the lower local recurrence rate in the RALLD group. These short- and long-term outcomes indicated that RALLD may be a useful modality for locally advanced low rectal cancer.
In Western countries, TME with CRT is considered the standard treatment for locally advanced low rectal cancer and LLD is hardly performed because TME with CRT reduced the local recurrence rate, compared with TME alone [18]. Moreover, lateral lymph node metastasis is generally considered a systemic metastatic disease. In Japan, TME with LLD is indicated for patients with clinical T3–4 low rectal cancer, in accordance with the Japanese guidelines [1] and neoadjuvant CRT is performed only for selected patients. Japanese surgeons consider LLD as the standard treatment for locally advanced low rectal cancer because lateral lymph node metastasis is considered a regional disease with an incidence of 14.6–20.1% in patients with locally advanced low rectal cancer [1, 17, 19]. In a retrospective multicenter study, Sugihara et al. [19] reported that the risk of local recurrence was reduced by 50.3% and the 5-year survival rate improved by 8.0% when LLD was performed in patients with pT3–4 low rectal cancer. An RCT comparing TME with and without LLD for clinical stage II/III low rectal cancer without lateral lymph node enlargements was performed in Japan [20], and it showed lateral lymph node metastasis in 7.4% of the patients in the TME with LLD group and the non-inferiority of TME alone to TME with LLD was not confirmed. Therefore, the efficacy of TME with LLD was supported in Japan. We did not perform neoadjuvant CRT in > 90% patients who underwent TME with LLD. Even for our strategy, the rate of local recurrence was 1.6 and 9.0% in the RALLD and OLLD groups, respectively.
Recently, a few oncological long-term outcomes of RALLD or CLLLD were reported. Shin et al. [14] reported that the 5-year cumulative local recurrence rate was 3.6% for patients who underwent LLD, paraaortic lymph node dissection, or multivisceral en bloc resection using RALS with a median follow-up of 30 months. They concluded that for selected patients, robotic-assisted extended rectal surgery had acceptable long-term oncological outcomes. In terms of CLLLD, Nagayoshi et al. [8] reported that the 3-year overall survival and the 3-year relapse-free survival rates did not differ significantly between CLLLD and OLLD.
The present study focused on TME with LLD. Several studies on the comparison of oncological outcomes of RALS versus open surgery (OS) or RALS versus CLS from Western surgeons who mainly performed TME with CRT were reported. Ghezzi et al. [21] compared the use of RALS with OS for rectal cancer and reported that the overall survival and disease-free survival rate were not significantly different between the two groups. However, the cumulative local recurrence rate was significantly lower in the RALS group than in the OS group (RALS: 3.4% vs. OS: 16.1%, p < 0.024). Comparing RALS with CLS for rectal cancer, several previous reports demonstrated comparable oncological outcomes [22,23,24,25,26]. Meanwhile, Kim et al. [27], reporting the results of a multivariate analysis, indicated that RALS was a significant and good prognostic factor for overall survival and cancer-specific survival and suggested that RALS has potential oncological benefits.
The present study includes several limitations. First, although we used one-on-one matching to reduce covariate imbalance between the two groups, the retrospective design of the study has inherent limitations. Moreover, the surgeons and postoperative chemotherapy regimens were different between the two groups because of the difference in historical background. Therefore, a prospective comparative study in terms of long-term oncological outcomes among RALLD, CLLLD, and OLLD is needed. Second, the “circumferential resection margin” is associated with local recurrence, which, however, cannot be evaluated with the Japanese method [28]. Instead, the microscopically positive resection margin status was evaluated by pathologists, and no positive resection margin was observed in the RALLD group. Third, the total cost of RALLD and OLLD was not analyzed. Since the high cost of RALS is a major problem [22], the short- and long-term outcomes including cost-effectiveness should be considered and analyzed.
The present study showed that the rate of positive resection margin tended to be lower and the overall survival and relapse-free survival rate tended to be better in the RALLD group than that in the OLLD group. Furthermore, the local relapse-free survival rate was significantly better in the RALLD group than in the OLLD group. These short- and long-term outcomes indicated that RALLD may be a useful modality for locally advanced low rectal cancer.
References
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34
Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, Moriya Y (2012) Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 13:616–621
Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M, Kuroyanagi H (2017) Feasibility of laparoscopic total mesorectal excision with extended lateral pelvic lymph node dissection for advanced lower rectal cancer after preoperative chemoradiotherapy. World J Surg 41:868–875
Park JS, Choi GS, Lim KH, Jang YS, Kim HJ, Park SY, Jun SH (2011) Laparoscopic extended lateral pelvic node dissection following total mesorectal excision for advanced rectal cancer: initial clinical experience. Surg Endosc 25:3322–3329
Furuhata T, Okita K, Nishidate T, Ito T, Yamaguchi H, Ueki T, Akizuki E, Meguro M, Ogawa T, Kukita K, Kimura Y, Mizuguchi T, Hirata K (2015) Clinical feasibility of laparoscopic lateral pelvic lymph node dissection following total mesorectal excision for advanced rectal cancer. Surg Today 45:310–314
Liu T, Zhang C, Yu P, Chen J, Zeng D, Gan L, Lv W, Liu L, Yan X (2011) Laparoscopic radical correction combined with extensive lymphadenectomy and pelvic autonomic nerve preservation for mid-to-low rectal cancer. Clin Colorectal Cancer 10:183–187
Matsumoto A, Arita K (2017) A technique of laparoscopic lateral pelvic lymph node dissection based on vesicohypogastric fascia and ureterohypogastric nerve fascia for advanced low rectal cancer. Surg Endosc 31:945–948
Nagayoshi K, Ueki T, Manabe T, Moriyama T, Yanai K, Oda Y, Tanaka M (2016) Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach. Surg Endosc 30:1938–1947
Liang JT (2011) Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol 18:153–159
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29:1679–1685
Park JA, Choi GS, Park JS, Park SY (2012) Initial clinical experience with robotic lateral pelvic lymph node dissection for advanced rectal cancer. J Korean Soc Coloproctol 28:265–270
Bae SU, Saklani AP, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2014) Robotic and laparoscopic pelvic lymph node dissection for rectal cancer: short-term outcomes of 21 consecutive series. Ann Surg Treat Res 86:76–82
Kagawa H, Kinugasa Y, Shiomi A, Yamaguchi T, Tsukamoto S, Tomioka H, Yamakawa Y, Sato S (2015) Robotic-assisted lateral lymph node dissection for lower rectal cancer: short-term outcomes in 50 consecutive patients. Surg Endosc 29:995–1000
Shin US, Nancy You Y, Nguyen AT, Bednarski BK, Messick C, Maru DM, Dean EM, Nguyen ST, Hu CY, Chang GJ (2016) Oncologic outcomes of extended robotic resection for rectal cancer. Ann Surg Oncol 23:2249–2257
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H (2016) Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc 30:721–728
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, Oxford
Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K (2012) Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 255:1129–1134
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T (2006) Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 49:1663–1672
Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y (2017) Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 266:201–207
Ghezzi TL, Luca F, Valvo M, Corleta OC, Zuccaro M, Cenciarelli S, Biffi R (2014) Robotic versus open total mesorectal excision for rectal cancer: comparative study of short and long-term outcomes. Eur J Surg Oncol 40:1072–1079
Park EJ, Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 261:129–137
Lim DR, Bae SU, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2016) Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg Endosc 31:1728–1737
Saklani AP, Lim DR, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2013) Robotic versus laparoscopic surgery for mid-low rectal cancer after neoadjuvant chemoradiation therapy: comparison of oncologic outcomes. Int J Colorectal Dis 28:1689–1698
Feroci F, Vannucchi A, Bianchi PP, Cantafio S, Garzi A, Formisano G, Scatizzi M (2016) Total mesorectal excision for mid and low rectal cancer: laparoscopic vs robotic surgery. World J Gastroenterol 22:3602–3610
Law WL, Foo DCC (2017) Comparison of short-term and oncologic outcomes of robotic and laparoscopic resection for mid- and distal rectal cancer. Surg Endosc 31:2798–2807
Kim J, Baek SJ, Kang DW, Roh YE, Lee JW, Kwak HD, Kwak JM, Kim SH (2017) Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum 60:266–273
Japanese Classification of Colorectal Carcinoma (2009) Japanese society for cancer of the colon and rectum, 2nd edn. Kanehara & Co, Tokyo
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Tomohiro Yamaguchi, Yusuke Kinugasa, Akio Shiomi, Hiroyasu Kagawa, Yushi Yamakawa, Akinobu Furutani, Shoichi Manabe, Yusuke Yamaoka, and Hitoshi Hino have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Kinugasa, Y., Shiomi, A. et al. Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surg Endosc 32, 4498–4505 (2018). https://doi.org/10.1007/s00464-018-6197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6197-x