Abstract
Background
Clinical and experimental data indicate that neonates are sensitive to the CO2 pneumoperitoneum. An impaired splanchnic perfusion during laparoscopy in adults has been reported. We recently confirmed that intravenous colloids improve macrocirculatory function in neonates. We aimed to determine the impact of CO2 pneumoperitoneum on the perfusion of splanchnic organs in the young including effects of colloid application.
Methods
Male piglets (n = 25) were divided into four groups: (1) neonatal controls, (2) neonates with crystalloid restitution, (3) neonates with colloidal restitution, and (4) adolescents with crystalloid restitution. Animals were ventilated and subjected to a 3-h, 10 mmHg CO2 pneumoperitoneum followed by 2 h resuscitation. Hepatic, splanchnic, and arteriovenous shunt perfusion was assessed via central and portal venous catheters. Capillary organ flow was detected by fluorescent microspheres. The rate of bile flow was measured.
Results
The neonatal crystalloid group showed a significant decrease in the intestinal capillary perfusion at the end of the recovery period. This was not detectable in the adolescent and colloid group. There was a significant increase in microcirculatory arterioportal shunt flow during the CO2 pneumoperitoneum in both neonatal groups but not in the sham and adolescent groups (p < 0.05). Hepatic arterial perfusion increased after insufflation in all groups and dropped during capnoperitoneum to levels of about 70% baseline. There was no significant impairment of splanchnic perfusion or bile flow as a result of the pneumoperitoneum in all groups.
Conclusions
Capillary perfusion of the abdominal organs was stable during capnoperitoneum and recovery in adolescents and neonates with colloid restitution, but not with crystalloid restitution. Significant arterioportal shunting during capnoperitoneum could affect hepatic microcirculation in neonates. Our data confirm that moderate pressure capnoperitoneum has no major effect on the perfusion of abdominal organs in neonates with adequate substitution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic surgery has been established as a technical option in the neonatal and young patient. However, there are little data concerning the effects of pneumoperitoneum in this age group. The available data indicate that there is an increased sensitivity of the neonatal patient.
In a study conducted in our department, Gomez-Dammeier observed that the majority of the infants that underwent prolonged laparoscopic procedures developed anuria [1]. In a single center and a multicenter study, Kalfa and colleagues reported an increased rate of insufflation-related complications and incidences in neonates compared to older children [2]. The experiments of our group also indicated that the cardiovascular system of neonates is more sensitive to CO2 pneumoperitoneum than that of older children. In young rabbits, pneumoperitoneum led to a significant increase in superior and inferior vena cava pressure and cardiac output compared to baseline values [3]. In neonatal pigs, pneumoperitoneum induced a significantly more pronounced decrease in cardiac index and mean arterial pressure in comparison to adolescent pigs [4].
The effects of CO2 pneumoperitoneum in neonates in terms of local microcirculation remain unknown. To investigate the extent to which the intestinal and splanchnic microcirculation of the small infant is sensitive to pneumoperitoneum, we compared the splanchnic organ blood flow between neonatal and adolescent pigs during and following a prolonged CO2 pneumoperitoneum. As previous studies have shown that the application of plasma expander in addition to the intraoperative fluid substitution during surgery can prevent some of the unwanted side effects of pneumoperitoneum, we included one neonatal group that underwent this regimen.
Materials and methods
Animals
Methods and studies were approved by the Regional Animal Care and Use Committee of the Government of Lower Saxony (Protocol No. 33-42-502-06/1147). We used male German Landrace pigs in two different age cohorts: Adolescent piglets (age 123–141 days; mean body weight of 52.4 kg), and neonatal piglets (age 17–27 days; mean body weight of 6.3 kg). The neonates were randomly distributed across three further groups.
-
1.
Neonatal control (n = 5) Animals underwent a sham pneumoperitoneum followed by resuscitation. Fluid substitution was 10 ml/kg BW/h using a buffered electrolyte solution (Ringer’s acetate; Braun, Melsungen, Germany).
-
2.
Neonatal crystalloid (n = 5) Animals received full CO2-pneumoperitoneum and resuscitation. Fluid substitution was performed with 10 ml/kg BW/h using a buffered electrolyte solution (Ringer’s acetate; Braun, Melsungen, Germany).
-
3.
Neonatal colloid (n = 5) Animals received full pneumoperitoneum and resuscitation. In addition to the fluid substitution with 10 ml/kg BW/h with a buffered electrolyte solution (Ringer’s acetate; Braun, Melsungen, Germany), animals received 5 ml/kg BW/h hydroxyethyl starch 130/0.42/6:2 (Venofundin® 6%; B. Braun, Melsungen, Germany).
-
4.
Adolescent crystalloid (n = 5) Animals received full pneumoperitoneum and resuscitation. Fluid substitution was performed with 10 ml/kg BW/h using a buffered electrolyte solution (Ringer’s acetate; Braun, Melsungen, Germany).
Procedures
Animals were orotracheally intubated and mechanically ventilated with 2% isoflurane in oxygen/air, FiO2 0.5 to maintain an end-tidal carbon dioxide tension of 35–40 mmHg. Anesthesia was maintained by intravenous infusion of fentanyl and rocuronium. Body temperature was continuously measured and maintained using an infrared lamp (LP1, Lister, Germany) and a circulating water mattress (HICO Aquatherm 650; Hirtz, Cologne, Germany).
The carotid artery was cannulated, and a 4-French (4F) oximetry thermal dye dilution probe (Pulsiocath, 4F PV 2024L; Pulsion, Munich, Germany) was introduced and progressed via the distal ascending aortic artery to the left ventricle. A 22G single lumen central venous catheter (CVC) (Arrow) was introduced into the superior vena cava via the jugular vein. The left brachial artery was dissected and cannulated with a 4F catheter that was introduced towards the descending aortic arch. Likewise, the femoral artery was dissected, and a 4F thermodilution catheter (Pulsiocath, PV2014L16; Pulsion) was introduced to the distal aortic artery up to the left ventricle.
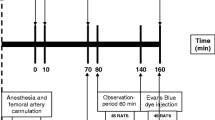
The abdomen was opened by a median laparotomy. A venous arcade of the small intestine was isolated, and a Silastic catheter (0.6 mm external diameter) was inserted and advanced into the portal vein. The hepatic bile duct was isolated, and a 14-French (14F) catheter was inserted and tunneled through the abdominal wall. Under fluoroscopic and manual control, another 22G catheter was inserted into the right jugular vein and progressed through the right atrium into the lower vena cava before being positioned in one of the hepatic veins. The positions of the catheters were confirmed fluoroscopically. A 4 mm trocar (30160H1; Karl Storz, Tuttlingen, Germany) was inserted in the lateral position and the abdominal wound was closed. The piglets were allowed to recover for 60 min before insufflation of CO2 (ENDOFLATOR 26430530; Karl Storz, Tuttlingen, Germany) with an insufflation pressure of 10 mmHg was initiated. The pneumoperitoneum was maintained for 3 h. Sham animals underwent the same procedures without the application of a pneumoperitoneum.
After decompression, monitoring continued for a further 120-min resuscitation period. After the final measurement, the piglets were euthanized by intravenous injection of pentobarbital (Fig. 1).
Endpoints
Microcirculatory parameter
Capillary blood flow was determined using 15 μm colored microspheres of different colors (orange, red, black, and pink, Dye-Trak Microspheres; Triton Technology, San Diego, CA, USA), according to the instructions of the manufacturer. Shortly thereafter, 2 ml of a rigorously mixed solution containing microspheres of one color per timepoint was injected into the left ventricle. The lines were flushed with 10 ml balanced electrolyte solution. Starting 10 s before the injection and continuing for 110 s, femoral and brachial arterial, as well as portal, blood were drawn through a heparinized syringe at a fixed rate of 2 ml/min by an automated system (Harvard Apparatus Infusion Pump #22; South Natick, MA, USA). The volume taken was replaced by a balanced electrolyte solution. At the end of the experiment, the wet and dry weight of each organ was measured. Defined portions of each organ were stored for 5–14 days at room temperature in a fume hood for autolysis. After digestion with 1M KOH and 10% Triton X-100, microspheres were recovered by specific washing and centrifugation steps. Dye was recovered from the microspheres by extraction with DMF (dimethylformamide) or CELLOSOLVE acetate. To quantify the microspheres, the dye content was then determined with a spectrometer at wavelengths of 673, 591, 546, 446, and 366 nm.
The mean fluorescent activity of both arterial samples served as the standard for the determination of the organ perfusion. The fluorescence of the organ samples was divided by the sample weight, then by the fluorescence of the standard, and then by 2 to calculate the capillary tissue perfusion of the samples. The organ perfusion of one animal was based on the median value of the multiple samples taken from one organ. To approximate total organ perfusion, this value was multiplied by the organ weight. The total flow of the small and large intestine, spleen, and pancreas were added together to approximate the portal flow. The portal shunt flow was calculated by dividing the fluorescence of the portal sample by the standard. The hepatic arterial perfusion was based on the total fluorescent activity, thereby including microspheres that bypassed the splanchnic capillaries and reached the liver via the portal vein.
Bile flow
The bile was collected extracorporeally, and the volume of bile produced was measured hourly.
Statistical analysis
We compared the response to pneumoperitoneum between neonates with and without colloidal restitution and adolescents. To prevent any general differences in the parameters between neonatal and adolescents from impairing the statistical analysis, the relative change of the parameters from the baseline value was used for the intergroup comparison. An intragroup comparison was calculated using analysis of variance ANOVA for repeated measures and a post hoc Holm–Sidak comparison. Intergroup comparison was calculated using analysis of variance ANOVA and Student–Newman–Keuls test for post hoc comparison.
Results
Intestinal perfusion
The baseline intestinal capillary perfusion was similar in all neonatal groups, and was about twice as high as that of adolescent pigs (data not shown). At the end of the pneumoperitoneum, the intestinal perfusion was not significantly altered compared to the baseline values in all groups (Fig. 2). At the end of the recovery period the intestinal perfusion decreased significantly in neonates with crystalloid fluid replacement. Neonates treated with plasma expanders, neonatal sham controls, and adolescents did not show a significant change in intestinal perfusion during the experiment.
Intestinal capillary perfusion. Changes of perfusion in the intestinal capillaries at different timepoints given as percent to baseline. In the neo crystalloid group, perfusion at the end of the recovery period was significantly decreased versus baseline (p > 0.05). Neo sham control group, neonatal pigs undergoing sham pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo crystalloid neonatal pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo colloid neonatal pigs undergoing full pneumoperitoneum and receiving hydroxyethyl starch in addition to crystalloid fluid substitution (n = 5), Adolescent adolescent pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5)
Hepatic arterial perfusion
The hepatic arterial perfusion was slightly higher in adolescents than in neonatal pigs at baseline levels. After the 3-h CO2 pneumoperitoneum and the 2-h recovery period, the hepatic arterial perfusion did not significantly change in all animals that had been subjected to CO2 insufflation (Fig. 3).
Hepatic arterial perfusion. Changes of perfusion in the hepatic artery at different timepoints given as percent to baseline. No significant changes of perfusion versus baseline in any group. Neo sham control group, neonatal pigs undergoing sham pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo crystalloid neonatal pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo colloid neonatal pigs undergoing full pneumoperitoneum and receiving hydroxyethyl starch in addition to crystalloid fluid substitution (n = 5), Adolescent adolescent pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5)
Splanchnic capillary perfusion and splanchnic arteriovenous shunt flow
There was no significant change in the splanchnic capillary blood flow in all groups throughout the experiment (Fig. 4).
Splanchnic capillary perfusion. Changes of perfusion in the splanchnic capillaries at different timepoints given as percent to baseline. No significant changes of perfusion versus baseline in any group. Neo sham control group, neonatal pigs undergoing sham pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo crystalloid neonatal pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo colloid neonatal pigs undergoing full pneumoperitoneum and receiving hydroxyethyl starch in addition to crystalloid fluid substitution (n = 5), Adolescent adolescent pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5)
The arteriovenous shunt perfusion in the splanchnic vessels was low at baseline. All animals subjected to CO2 insufflation exhibited an increase in the shunt perfusion during pneumoperitoneum, which reached statistical significance in both neonatal groups. Following deflation, the shunt perfusion was no longer elevated (Fig. 5).
Splanchnic arteriovenous shunt perfusion. Changes of perfusion in the splanchnic arteriovenous shunt vessels at different timepoints given as percent to baseline. In the neo crystalloid and in the neo colloid group, perfusion at the start and at the end of pneumoperitoneum was significantly increased versus baseline, respectively (p < 0.05). Neo sham control group, neonatal pigs undergoing sham pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo crystalloid neonatal pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5), Neo colloid neonatal pigs undergoing full pneumoperitoneum and receiving hydroxyethyl starch in addition to crystalloid fluid substitution (n = 5), Adolescent adolescent pigs undergoing full pneumoperitoneum and receiving crystalloid fluid substitution (n = 5)
Bile flow
There was no significant change in the bile flow in all groups throughout the observation period. Interestingly, the absolute rate of the bile flow, as given in ml/h kgBW, was higher in the neonatal group than it was in the adolescent group; however, there was no significant change in each group throughout the experiment.
Discussion
Since laparoscopic surgery was first introduced, many scientists have expressed concerns that the application of a pneumoperitoneum might impair blood flow to the abdominal organs. Numerous experimental and clinical studies have been conducted to investigate changes in splanchnic perfusion during laparoscopy (summarized in Table 1), and several authors have concluded that pneumoperitoneum has a significant effect on organ perfusion. However, the study designs, models, technical aspects, and results are heterogeneous. The majority of existing studies have observed a decrease in perfusion of about 30–40%, especially during high-pressure (> 12 mmHg) or prolonged (> 120 min) pneumoperitoneum.
Various techniques have been deployed to assess blood flow and microcirculation. Many previous studies, that have described distinct changes in perfusion used transonic or laser Doppler flow probes. We attempted to use Doppler flow probes positioned at the portal vein to measure portal blood flow and on the liver surface to measure superficial and deep liver flow in preliminary experiments to establish the best technique. However, we were not able to achieve the stability needed for accurate measurements during the insufflation period and to obtain valid data in neonates. Therefore, we decided to use colored microspheres to measure tissue perfusion. In contrast to probes, this method is better suited for the detection of microcirculatory disturbances around major vessels and is less affected by the movements that result from insufflation and deflation.
In our study, there was no change in intestinal capillary perfusion after initiation of the pneumoperitoneum. At the end of the long-lasting 10 mmHg pneumoperitoneum, we detected a small, but not significant, reduction in intestinal capillary perfusion in adolescent pigs. This was in line with the findings of other groups that have used microspheres and detected impaired organ perfusion only in high-pressure (15, 20 or 30 mmHg) pneumoperitoneum in different models [16, 19, 26].
The decrease was slightly more pronounced in infant piglets, however, due to the distinct variation of the values, did not reach significance during the period of the pneumoperitoneum. After deflation, intestinal capillary perfusion tended to decrease further, and we observed a significant drop in intestinal capillary perfusion (vs. baseline) in neonatal piglets supplemented with crystalloid infusion at the end of the 2-h recovery period. Nonetheless, the perfusion was about 70% that of the baseline values. We concluded that the pneumoperitoneum had no major effect on intestinal capillary perfusion.
In alternative models, our group has previously observed that some of the detrimental effects of pneumoperitoneum on cardiovascular functions only manifest after the release of the CO2 [3, 4]. This appears to be related to the compensatory effects of the increased abdominal pressure on intravascular pressure. Evaluating the total perfusion of the organs in the splanchnic vessel area, we observed a similar effect, with the pneumoperitoneum having only a minor, not significant, detrimental effect on organ perfusion.
An interesting observation that did not directly affect organ perfusion was an increase in microcirculatory shunt perfusion in the splanchnic vessel bed during the CO2 pneumoperitoneum in all groups. This increase only reached significance in neonates (p < 0.05). The phenomenon of microcirculatory shunting has been described for various organs with other conditions, such as sepsis [27] and ischemia [28], and is considered to represent a marker and cause of impaired tissue perfusion. In shunting, vessels connecting the afferent arterioles and efferent venules open, so that the blood flow is partially bypassing the capillary network. Several factors have been described to induce not only functional shunting, such as increased pressure and shear stress, but also metabolic stimuli [29]. It has also been shown that low oxygen levels evoke vasodilatation and thus increase of blood flow [30]. It is unclear whether the increased intra-abdominal pressure during capnoperitoneum or the metabolic effects leads to the functional shunting. Based on the vasodilatory effects of CO2 in the mesenteric vessel bed [31], it could be speculated that the insufflation of CO2 locally affects the vasculature. Due to a CO2-triggered vasodilatation and via vasodilator signaling, the perfusion of shunting vessels could be promoted, bypassing the capillary bed. The relevance of this arterioportal shunt perfusion during capnoperitoneum is not yet clear; however, it does not seem to be directly related to the changes in capillary perfusion.
To determine the effects of the capnoperitoneum on liver perfusion and function, we measured hepatic arterial perfusion and bile production. There was no significant change in bile production throughout the experiment indicating that the pneumoperitoneum does not have a significant effect on liver function. Hepatic arterial perfusion was calculated based on the microspheres trapped in the hepatic tissue and, thus, reflects both the arterial perfusion via the hepatic artery and microspheres transported to the liver via splanchnic shunting vessels. We observed an initial increase in the hepatic arterial perfusion of about 30% after CO2 insufflation in all groups. However, this dropped during the course of the pneumoperitoneum to levels of about 70% that of the baseline (n.s. vs. baseline). This initially puzzling observation could point toward a special susceptibility of hepatic perfusion to capnoperitoneum. It is reasonable to speculate that the increased splanchnic shunt perfusion during the capnoperitoneum initially increased the amount of arterial blood reaching the liver, as it adds up to the perfusion of the hepatic artery. Thereafter, and during the capnoperitoneum, the increased portal blood flow might reduce the perfusion of the hepatic artery via the hepatic arterial buffer response [32]. This theory appears worrisome because the reduction of hepatic global arterial perfusion displayed in Fig. 3 would be more severe during late pneumoperitoneum if shunt perfusion was subtracted, i.e., the display of the perfusion of the hepatic artery showed a very strong reduction at the end of the pneumoperitoneum. However, with the limited number of animals included in this study, we did not detect these values. The reported effects of capnoperitoneum on hepatic arterial and portal perfusion in the literature appear to be controversial. Some authors describe a reduced blood flow in the hepatic artery and portal vein during long-lasting, high-pressure pneumoperitoneum [12, 33], while others report an increase [8].
Looking at the data in detail, it becomes apparent that the effect of capnoperitoneum on hepatic blood flow seems not only to depend on the degree of intra-abdominal pressure, but also on other variables.
Junghans described an initial decrease in portal blood flow after the induction of capnoperitoneum, but found that, after increasing intravascular volume by infusion of a hydroxyethyl starch solution, the portal blood flow was increased beyond the baseline values [7]. We also observed this beneficial effect of plasma expanders, as infusion prevented a significant decrease in the intestinal capillary perfusion observed in neonates with a restrictive crystalloid fluid restitution.
Klopfenstein found that the hepatic arterial and portal vein blood flow were both decreased when the patient’s head was positioned in an upward tilt in the absence of pneumoperitoneum but not in the presence of abdominal CO2. No change in both hepatic blood flows was observed when the head was positioned in a downward tilt in the presence of abdominal CO2 [8]. This could be due to compression of the splanchnic capacity vessel beds or due to the vasodilatory effects of the CO2. In line with the second mode of action, Ali found that the inclusion of ethyl nitrite in the insufflation admixture during capnoperitoneum attenuated both the acute and prolonged liver blood flow decreased in comparison to the ethyl nitrite-free control group [18].
Other studies have found that the degree of impairment in global hepatic perfusion seems to be only present or more pronounced during pneumoperitoneum with particular high intra-abdominal pressures [22].
Nonetheless, even if total hepatic blood flow might be impaired only by lengthily, high-pressure pneumoperitoneum, the pneumoperitoneum seems to have an effect on the hepatic microcirculation prior to changes in global perfusion.
Sanchez-Etayo found that the total liver blood flow remained mostly unaltered at low intra-abdominal pressures and only decreased at an intra-abdominal pressure of > 20 mmHg [24]. However, the hepatic microvascular flow decreased at moderate intra-abdominal pressures. Hoekstra observed a disturbed microvascular perfusion during capnoperitoneum. After 6 h of capnoperitoneum, ICG (indocyanine green) clearance increased significantly, indicating a compensatory improvement of overall liver blood flow resulting in concomitantly improved global perfusion [25].
Taken together, these studies support our theory that an increase in splanchnic arterioportal shunt perfusion in combination with the increased abdominal pressure could lead to a marked reduction in flow in the hepatic artery and potential disturbance of hepatic microcirculation, despite only moderate changes in global hepatic perfusion. This is supported by clinical studies that have found that there is a significant increase in serologic markers of liver damage/dysfunction in patients who undergo laparoscopic cholecystectomy, despite a lack of clinical symptoms [34].
However, the special susceptibility of the hepatic microcirculation and its potential affection via the hepatic arterial buffer response require a special attention on the hepatic function during long-lasting laparoscopic procedures in neonates.
There are some limitations to our study. The power of our analysis is reduced by the limited number of animals per group, in combination with a comparison of multiple groups. Moreover, the standard deviation that is inherent in the technically demanding method made it impossible to detect the more minor effects the pneumoperitoneum had on the organ perfusion. Furthermore, the degree to which the preterm neonates with significantly lower arterial pressure than the animals used in our experiments could be affected by a pneumoperitoneum remains unknown.
In conclusion, our data suggest that there is no major impairment in splanchnic perfusion in adolescent and neonatal pigs that receive adequate fluid substitution during and following a prolonged CO2 pneumoperitoneum with moderate insufflation pressure. The observed increase in arterioportal shunt flow raises the possibility alterations in hepatic circulation and merits further investigation.
References
Gómez Dammeier BH, Karanik E, Glüer S, Jesch NK, Kübler J, Latta K, Sümpelmann R, Ure BM (2005) Anuria during pneumoperitoneum in infants and children: a prospective study. J Pediatr Surg 9:1454–1458
Kalfa N, Allal H, Raux O, Lopez M, Forgues D, Guibal MP, Picaud JC, Galifer RB (2005) Tolerance of laparoscopy and thoracoscopy in neonates. Pediatrics 6:785–791
Sümpelmann R, Schuerholz T, Marx G, Jesch NK, Osthaus WA, Ure BM (2006) Hemodynamic changes during acute elevation of intra-abdominal pressure in rabbits. Paediatr Anaesth 12:1262–1267
Metzelder ML, Kuebler JF, Huber D, Vieten G, Suempelmann R, Ure BM, Osthaus WA (2010) Cardiovascular responses to prolonged carbon dioxide pneumoperitoneum in neonatal versus adolescent pigs. Surg Endosc 3:670–674
Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglou E, Farmakis H, Dadoukis J (1996) Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc 3:324–326
Schilling MK, Redaelli C, Krähenbühl L, Signer C, Büchler MW (1997) Splanchnic microcirculatory changes during CO2 laparoscopy. J Am Coll Surg 4:378–382
Junghans T, Böhm B, Gründel K, Schwenk W, Müller JM (1997) Does pneumoperitoneum with different gases, body positions, and intraperitoneal pressures influence renal and hepatic blood flow? Surgery 121:206–211
Klopfenstein CE, Morel DR, Clergue F, Pastor CM (1998) Effects of abdominal CO2insufflation and changes of position on hepatic blood flow in anesthetized pigs. Am J Physiol 275:900–905
Takagi S (1998) Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc 12:427–431
Sala-Blanch X, Fontanals J, Martinez-Palli G (1998) Effects of carbon dioxide vs helium pneumoperitoneum on hepatic blood flow. Surg Endosc 12:1121–1125
Windberger UB, Auer R, Keplinger F, Längle F, Heinze G, Schindl M, Losert UM (1999) The role of intra-abdominal pressure on splanchnic and pulmonary hemodynamic and metabolic changes during carbon dioxide pneumoperitoneum. Gastrointest Endosc 49:84–91
Kotzampassi K, Paramythiotis D, Eleftheriadis E (2000) Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today 30:987–992
Pastor CM, Morel DR, Clergue F, Mentha G, Morel P (2001) Effects of abdominal CO2insufflation on renal and hepatic blood flows during acute hemorrhage in anesthetized pigs. Crit Care Med 29:1017–1022
Schmandra TC, Kim ZG, Gutt CN (2001) Effect of insufflation gas and intraabdominal pressure on portal venous flow during pneumoperitoneum in the rat. Surg Endosc 15:405–408
Yokoyama Y, Alterman DM, Sarmadi AH, Baveja R, Zhang JX, Huynh T, Clemens MG (2002) Hepatic vascular response to elevated intraperitoneal pressure in the rat. J Surg Res 105:86–94
Leister I, Schüler P, Vollmar B, Füzesi L, Kahler E, Becker H, Markus PM (2004) Microcirculation and excretory function of the liver under conditions of carbon dioxide pneumoperitoneum. Surg Endosc 18:1358–1363
Meierhenrich R, Gauss A, Vandenesch P, Georgieff M, Poch B, Schütz W (2005) The effects of intraabdominally insufflated carbon dioxide on hepatic blood flow during laparoscopic surgery assessed by transesophageal echocardiography. Anesth Analg 100:340–347
Ali NA, Eubanks WS, Stamler JS, Gow AJ, Lagoo-Deenadayalan SA, Villegas L, El-Moalem HE, Reynolds JD (2005) A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann Surg 241:256–261
Goitein D, Papasavas P, Yeaney W, Gagne D, Hayetian F, Caushaj P, Keenan R, Landreneau R (2005) Microsphere intestinal blood flow analysis during pneumoperitoneum using carbon dioxide and helium. Surg Endosc 19:541–545
Junghans T, Neudecker J, Dörner F, Raue W, Haase O, Schwenk W (2005) Effect of increasing cardiac preload, sympathetic antagonism, or vasodilation on visceral blood flow during pneumoperitoneum. Langenb Arch Surg 390:538–543
Szold A, Weinbroum AA (2008) Carbon dioxide pneumoperitoneum-related liver injury is pressure dependent: a study in an isolated-perfused organ model. Surg Endosc 22:365–371
Berger M, Goedeke J, Hubertus J, Muensterer O, Ring-Mrozik E, von Schweinitz D, Lacher M (2012) Physiological impact of pneumoperitoneum on gastric mucosal CO2pressure during laparoscopic versus open appendectomy in children. J Laparoendosc Adv Surg Tech A 22:107–112
Sánchez-Etayo G, Borrat X, Escobar B, Hessheimer A, Rodriguez-Laiz G, Taura P (2012) Effect of intra-abdominal pressure on hepatic microcirculation: implications of the endothelin-1 receptor. J Dig Dis 13:478–485
Hoekstra LT, Ruys AT, Milstein DM, van Samkar G, van Berge Henegouwen MI, Heger M, Verheij J, van Gulik TM (2013) Effects of prolonged pneumoperitoneum on hepatic perfusion during laparoscopy. Ann Surg 257:302–307
Chadi SA, Abdo H, Bihari A, Parry N, Lawendy AR (2015) Hepatic microvascular changes in rat abdominal compartment syndrome. J Surg Res 197:398–404
Adelsdorfer C, Taura P, Ibarzabal A, Vendrell M, Delitala A, Deulofeu R, Adelsdorfer W, Delgado S, Lacy AM (2016) Effect of transgastric natural orifice transluminal endoscopic surgery peritoneoscopy on abdominal organ microcirculation: an experimental controlled study. Gastrointest Endosc 83:427–433
Ince C, Sinaasappel M (1999) Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 27:1369–1377
Lauterbach M, Horstick G, Plum N, Weilemann LS, Münzel T, Kempski O, Pries AR (2006) Shunting of the microcirculation after mesenteric ischemia and reperfusion is a function of ischemia time and increases mortality. Microcirculation 13:411–422
Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW (2010) The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10:587–593
Reglin B, Pries AR (2014) Metabolic control of microvascular networks: oxygen sensing and beyond. J Vasc Res 51:376–392
Thomson IA, Fitch W, Hughes RL, Campbell D (1983) Effect of increased concentrations of carbon dioxide during halothane anaesthesia on liver blood flow and hepatic oxygen consumption. Br J Anaesth 55:1231–1237
Lautt WW (2007) Regulatory processes interacting to maintain hepatic blood flow constancy: vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration,escape from vasoconstriction. Hepatol Res 37:891–903
Richter S, Olinger A, Hildebrandt U, Menger MD, Vollmar B (2001) Loss of physiologic hepatic blood flow control (“hepatic arterial buffer response”)during CO2-pneumoperitoneum in the rat. Anesth Analg 93:872–877
Atila K, Terzi C, Ozkardesler S, Unek T, Guler S, Ergor G, Bora S, Gulay H (2009) What is the role of the abdominal perfusion pressure for subclinical hepatic dysfunction in laparoscopic cholecystectomy? J Laparoendosc Adv Surg Tech A 19:39–44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. JF Kuebler, Dr. N. Schukfeh, Dr. G. Vieten, Prof. AW Osthaus, Dr. D. Huber, Dr. N. Dennhard, Prof. R. Suempelmann, Prof. BM Ure, and Prof. ML Metzelder have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Kuebler, J.F., Schukfeh, N., Vieten, G. et al. Arterioportal shunting, splanchnic capillary perfusion, and the effects of colloids during capnoperitoneum in neonatal and adolescent pigs. Surg Endosc 32, 2923–2931 (2018). https://doi.org/10.1007/s00464-017-6005-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-6005-z