Abstract
Background
Complete macroscopic cytoreduction in patients with peritoneal carcinomatosis (PC) is the basic requirement for long-term survival. Diagnostic laparoscopy (DL) can be difficult and of limited clinical value secondary to postoperative or tumor-induced adhesions. The aim of this study was to evaluate the role of DL in patients with prior surgery and PC.
Methods
The database of the surgical department of the University Medical Center of Regensburg was reviewed (9/2010–10/2014) selecting for DL in patients with PC. The operative report had a standardized format allowing for the determination of the extent of the intra-abdominal visible area and the extent of tumor on the surface of the small intestine. For the classification we used our own developed score.
Results
DL was performed in 102 patients. The complete abdominal cavity was evaluable in 48%. At least two quadrants and the largest part of the small intestine could be assessed in 70%. 37% of the patients had massive tumor manifestation on the small intestine or its mesentery. PCI (Peritoneal Cancer Index) could not be calculated in 71% of the patients due to incomplete visualization of the abdominal cavity and/or multiple tumor manifestations on the small intestine. 54% of patients were classified as non-resectable and 85% who seemed suitable for cytoreductive surgery underwent a CCR-0 resection and HIPEC.
Conclusions
In spite of prior surgery and PC, DL is frequently possible and a useful tool to define the extent of tumor spread. Lots of patients can be prevented from needless open laparotomy. The extent of tumor involvement of the small intestine seems to be more relevant than calculation of the PCI to determine the potential for complete resection. Therefore, in the presence of adhesions, inspection of the complete abdominal cavity does not offer added clinical benefit and further adhesiolysis can be avoided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Untreated peritoneal carcinomatosis (PC) entails an ominous prognosis of just a few months, and survival rates after systemic chemotherapy alone are not satisfying. A multimodal therapeutic treatment concept including cytoreductive surgery (CRS) and hyperthermic perioperative chemotherapy (HIPEC) has been established over the last decades and should be now standard of care for peritoneal metastases from various tumor entities [1,2,3,4,5,6,7]. The most important prognostic factor in these patients is the completeness of macroscopic cytoreduction (CCR-0) [8]. Five-year survival rates after CRS and HIPEC of about 50% are reported in patients with colorectal primary [9]. The likelihood of achieving a complete cytoreduction depends on the extent and distribution of PC in the abdomen [10, 11]. Therefore, a correct preoperative assessment of the extent of PC has to be carried out, preventing an unnecessary laparotomy.
Imaging procedures like CT and/or PET-scans or magnetic resonance imaging (MRI) exclude approximately 20% of patients with PC from surgery due to extra-abdominal tumor burden. But these imaging modalities still fail to accurately assess the intra-abdominal extent and potential resectability of PC [12]. Especially imaging in patients who had previous abdominal surgery is challenging due do postoperative tissue alterations [13].
CT and MRI-scans has been shown to be a poor predictor of intraoperative PCI, in particular for tumor lesions smaller than 5 mm of size [14,15,16,17]. The removal of such small tumor nodules is a crucial factor for CCR-0 resection and a diffuse tumor affection on the surface of the small intestine and its mesentery is a contraindication for CRS and HIPEC [18]. If tumor affection of the small intestine and its mesentery remains uncertain after preoperative non-invasive diagnostics, diagnostic laparoscopy (DL) or laparotomy with direct tumor visualization is the only method available to reliably assess the extent of PC [19, 20].
Since PC often occurs secondarily, most of the patients have already undergone surgery of the primary tumor. The feasibility and value of DL is controversially discussed citing postoperative and tumorous adhesions. The working hypothesis of our study was that diagnostic laparoscopy does have an added value in the evaluation of peritoneal carcinomatosis prior to exploratory laparotomy. Thus, the aim of this retrospective study was (i) to evaluate the role of DL in patients with prior surgery and PC, (ii) to clarify whether the whole abdominal cavity and the complete small intestine must be inspected during DL to determine patient eligibility for CRS and HIPEC, and (iii) also, the role of the tumor infiltration of the small intestine seen during the DL was considered in view of the patients’ resectability.
Patients and methods
Patients
Hundred and eight diagnostic laparoscopies were performed in hundred and two patients for the assessment of peritoneal carcinomatosis between September 2010 and December 2014. Indications for DL were (i) confirmation of clinically and/or radiologically suspected PC and (ii) evaluation of the extent of PC as well as its potential resectability. Patients, after prior laparotomy or laparoscopy at another institution were re-evaluated laparoscopically, if the extent of the disease was unclear or poorly documented.
Written informed consent was obtained from all patients prior to all diagnostic and surgical procedures.
Surgical technique
At our center, two surgeons are routinely performing the diagnostic laparoscopy in patients with PC. The same surgeons are also performing the CRS and HIPEC. In our opinion, this approach is very important, because these surgeons have got the surgical experience to decide which peritoneal metastases can be completely removed, which may be borderline resectable or even irresectable.
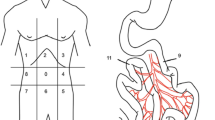
In patients without prior surgery, a midline mini-laparotomy about 2 cm distal from the umbilicus was performed. A 10 mm trocar was inserted through the incision and a pneumoperitoneum was established. The 30° laparoscope was introduced. For the examination of the abdominal cavity, a 5 mm trocar was positioned on the right or left side of the optical trocar. An additional 5 mm trocar was used, if necessary. The ‘Reverse Trendelenburg’ and ‘Trendelenburg’ positions were used to examine abdomen and pelvis. Adhesiolysis was performed as necessary, and biopsies were taken (Fig. 1).
Trocar positions in patients without any previous operations. Shown are the different trocar positions in patients without any previous operations. In the midline, the camera trocar and a 5 mm trocar were placed. Sometimes a second 5 mm trocar was needed for better exploration. This trocar was placed either in the medioclavicular line on the left or right side or also in the midline
In patients with prior surgery, the mini-laparotomy was done on the contralateral side of the primary operation (i.e., after a right hemicolectomy the mini-laparotomy was performed in the left upper abdominal quadrant or vice versa) to avoid complications due to adhesions along the scar. In these patients, lysis of adhesions at this time was limited to avoid tissue trauma prior to a potential CRS and HIPEC (Fig. 2).
Adhesions
The abdominal cavity was divided into four quadrants (right upper, right lower, left upper, and left lower quadrant). The amount of visible quadrants was classified as follows:
(a) adhesions stage I (more than two quadrants of the abdominal cavity and the main part of the small intestine was visible).
(b) adhesions stage II (less than two quadrants of the abdominal cavity and a minor part of the small bowel and its mesentery could be inspected).
Intraoperative classification of tumor manifestation on the small intestine (TMSI)
The tumor manifestation of the small intestine (TMSI) was assessed and classified as follows: TMSI grade 0 (no tumor manifestation), TMSI grade 1 (sporadic tumor spots), and TMSI grade 2 (multiple tumor spots on the small bowel).
Statistical analysis
Data analysis was performed using the SPSS statistical program (Statistical Package for Social Science, version 22.0). Proportions of variables were compared using the unpaired t test and the chi-squared-test, unless expected cell counts were below five in which case Fisher´s exact test was used.
The study was approved by the institutional review board of Regensburg University Medical Center.
Results
The study cohort included 48 men and 54 women. The median age was 54 years (range 28–77 years).
Hundred and eight DL were performed on Hundred and two patients. Six patients, four with a PC of a gastric cancer and two with a PC of a colon cancer, underwent DL twice, before and after 6 cycles of chemotherapy, because they were initially considered non-resectable. After the second laparoscopy, only one of these patients with a gastric cancer showed clear tumor regression and therefore a CCR-0 resection and HIPEC could be performed at that time.
As shown in our work-flow diagram (Fig. 3) we were able to exclude 46 patients (45%) from CRS and HIPEC by DL due to local tumor stage with advanced PC. 42 of these patients had massive tumor infiltration of the small intestine or its mesentery (TMSI grade 2). Two patients with gastric cancer had a PCI > 19 and two more patients were excluded due to chronic peritonitis of probable paraneoplastic origin.
In 9 patients, the suspicion of a PC could not be confirmed by histological examination and in one patient the histological result showed benign disease (Erdheim–Chester disease) (Table 1). A total of 56 patients (55%) could be preserved from a further surgical procedure. After DL was performed, 46 patients were scheduled for CRS and HIPEC at our institution. Of these, 39 patients (85%) received a complete macroscopic tumor resection (CCR0) and HIPEC. The remaining 7 patients showed irresectable stage of disease during explorative laparotomy (Fig. 3).
Prior operations and operation time of the DL
Eighty-three patients had undergone at least one prior laparotomy or laparoscopy before our DL. Fourteen patients had a history of smaller (e.g., hernia repair, appendectomy), 44 patients of moderate (e.g., hemicolectomy or rectum resection), and 25 patients of extended multivisceral resections (at least 3 abdominal organs).
The mean operating time for DL was 37 min (range 11–78 min). There was no significant difference in operating time between patients with prior surgery and those without (37 vs. 38 min; p = 0.817). This can be explained by the fact, that extensive adhesiolysis was avoided in all patients.
Morbidity and mortality
Complications occurred after two DL (1.9%). One patient with PC secondary to gastric cancer developed severe pain of the left abdominal wall. A subcutaneous emphysema was diagnosed which resolved spontaneously. The patient was discharged without any pain after four days. The other patient underwent a biopsy in proximity to the transverse colon. He developed septic symptoms after three days and was re-explored. Perforation of the transverse colon was found which was surgically closed, and a temporary diversion ileostomy was performed. The patient did well thereafter and was ultimately discharged home. No postoperative mortality was observed after DL.
Intraoperative classification of adhesions
More than two abdominal quadrants and the largest part of the small intestine (Grade I adhesions) were evaluable in 88% of the patients without prior surgery and in 70% of the patients with previous surgery, which was not significantly different (p = 0.07) (Table 2).
In 4 patients, the access to the abdominal cavity was impossible because of cancerous and/or postoperative adhesions. Therefore, the operation was converted to midline laparotomy. After conversion to open surgery, none of these patients showed resectable disease because of massive involvement of the small intestine. Two of these patients had PC of an appendiceal cancer and had previously undergone extended multivisceral resections; one patient underwent a moderate resection due to a primary colon cancer; and one patient with gastric cancer had no prior abdominal surgery (Table 3).
36 patients with grade I adhesions were considered as suitable for CRS and HIPEC after DL. In 31 patients (86%), a complete macroscopic tumor resection could be achieved. Patients with grade II adhesions showed a similar completion rate (80%; p = 0.634).
Intraoperative classification of tumor manifestation of the small intestine (TMSI)
46 patients with proven TMSI were considered eligible for CRS and HIPEC and a complete macroscopic cytoreduction could be performed in 39 of the cases (80%).
In four patients with TMSI Grade 0, the planned CCR0 and HIPEC was not feasible.
In one patient, as already mentioned above, the operation had to be stopped due to massive collateral circulation caused by liver cirrhosis. Three patients underwent incomplete tumor resection (CCR2). Two of them had prior operations, one with a recurrence of a PMP who had a history of previous CRS and HIPEC and grade 2 adhesions at the time of DL. The other patient had a moderate operation before and grade 1 adhesions at DL. In both patients, the intra-abdominal tumor spread was underestimated during diagnostic laparoscopy. The third patient with PMP was scheduled for palliative tumor reduction due to his poor general condition (Table 4).
Using DL we underestimated the intra-abdominal tumor spread in three patients with gastric cancer and TMSI grade 1. Two of them had minor prior operations and grade 2 adhesions. The third patient had no prior operation but grade 2 adhesions at DL. All three patients underwent palliative surgery.
Peritoneal cancer index
Due to multiple tumor spots on the small intestine (TMSI grade 2), which were considered as a contraindication for CRS and HIPEC, and/or an incomplete visualization of the abdominal cavity the PCI was not calculated in 77 DL (71%). In the remaining 31 DL the PCI ranged between 5 and 17 in 10 patients with gastric cancer, between 5 and 18 in 14 patients with colorectal cancer, between 12 and 15 in 4 patients with ovarian cancer, and at 16 in 2 patients with PMP. In one patient with a PCI of 10 and an inconclusive intraoperative pathology, benign Erdheim–Chester disease was established as the final diagnosis. Comparing the PCI of DL with open exploration, we underestimated the PCI in 3 of these 31 patients (10%).
Discussion
The likelihood of achieving a complete cytoreduction depends on the extent of the PC as well as on the skills of the surgeon. Our study demonstrates that diagnostic laparoscopy is a useful tool in selecting patients suitable for CRS and HIPEC, even if patients had previous abdominal surgery and/or present PC. 46 patients had shown non-resectable disease in the DL. In additional 10 patients, a PC could be ruled out by negative biopsies taken during laparoscopy. In total, a futile laparotomy could be avoided in 55% of the patients. Out of the patients who seemed to be suitable for CRS and HIPEC, 85% could be successfully treated with CCR0 and HIPEC. Interestingly, the grade of adhesions or prior abdominal surgery had no significant influence on the accurate prediction if a patient was suitable for CRS and HIPEC or not. In 86% of grade I and 80% of grade II adhesions, respectively, a correct prediction could be made. This supports our assumption that for evaluation of CRS and HIPEC, the distribution pattern of PC, in particular the small bowel involvement, seems to be more essential than the amount of visible abdominal cavity.
As indicated by Pomel et al. three major criteria affect the likelihood of complete resection. The extent of involvement of the small intestine, the depth of infiltration of the diaphragm, and the extent of infiltration of the PC between the celiac axis and the hepatic pedicle [19]. In our experience, we agree with other groups that the extent of the infiltration of PC between the celiac axis and the hepatic pedicle is difficult to assess during DL [19, 21].
The depth of tumor infiltration of the diaphragm cannot be evaluated by DL but the diaphragm can usually be resected without any complications as we did successfully in some patients during CRS. In our experience, the amount of tumor along the small intestine and its mesentery represents the decisive area determining resectability. If the length of tumor manifestation on the small intestine is too pronounced, a CCR0 situation will not be achieved because a short bowel syndrome should be generally avoided.
Some authors recommend a lysis of adhesions during the staging laparoscopy [20]. According to our data, for patient selection, it is not crucial to explore the whole abdominal cavity or the complete small intestine. We consider it adequate to explore enough loops (3–4 loops) of the small intestine to determine the distribution pattern of the tumor. In our study, we had to exclude 42 patients from CRS/HIPEC as a consequence of TMSI. This approach of limiting the lysis of adhesions might explain why we did not detect any difference in operation time between the two subgroups.
Moreover complications of the DL are very rare but can be potentially dangerous as the case of colon perforation in our series showed. Nevertheless, previous surgery and PC should not be contraindications for a DL.
In our study, DL underestimated the intraperitoneal tumor load in 5 of 46 patients and therefore missed the contraindication for the laparotomy. Regarding our data, the residual risk remains low, but has to be discussed with the patient before the open exploration for the scheduled CRS and HIPEC is done.
In order to prevent the risk of trocar metastasis, some groups recommend placing the trocars along the midline, so that these trocar sites can be resected at the time of laparotomy [19]. According to our experience this procedure is often not feasible, especially in patients with previous abdominal surgery via midline incision, because of major intra-abdominal adhesions along the incision line. Moreover, neoplastic seeding at trocar sites appears uncommon and is easily treatable. An Italian group observed no neoplastic seeding after 97 diagnostic laparoscopies in patients with PC [20].
In our opinion, the incidence of trocar site metastasis depends on the time elapsing between laparoscopy and definitive exploratory laparotomy. While they can be prevented by surgical resection of the access canals at the time of exploration, prognosis is not negatively affected in patients developing manifestations of their tumor at the trocar site and undergoing secondary resection of these metastasis.
The PCI is frequently used in the intraoperative evaluation of PC to estimate the feasibility of CRS and HIPEC. After complete laparoscopic lysis of adhesions, a correlation between open surgery PCI and laparoscopic PCI has been shown [20]. In our patient cohort, we limited the treatment of adhesions to avoid tissue trauma in preparation for CRS and HIPEC. However, we also revealed a good correlation between the PCI calculated during DL and at open exploration. But it has to be considered that we did not calculate the PCI in patients with adhesions stage II because of limited view of the abdominal cavity. Nevertheless, we were able to treat 8 of 10 patients with adhesion stage II successfully with CCR0 and HIPEC. None of the excluded patients was excluded due to adhesions but because of tumor involvement of the small intestine. Therefore, we conclude that the tumor manifestation on the small intestine and its mesentery is the key point for making the decision if a patient is more suitable for CRS/HIPEC than the PCI. Therefore, an adhesiolysis must not be enforced to calculate a PCI during the DL.
The PCI is a numeric value and a tool for quantifying the extent of PC. This is reflected by the correlation between PCI and patient outcome but nevertheless one will not refuse a CRS and HIPEC in a patient if the disease appears resectable.
In conclusion, the working hypothesis that DL has a clinical value in the assessment of PC in combination of previous surgery, prior to planned exploratory laparotomy, and complete surgical resection followed by HIPEC was confirmed based on the results of this study. We could clarify that it is not necessary to explore the whole abdominal cavity and the complete small intestine to determine if a patient is eligible for CRS and HIPEC.
References
Bakrin N, Bereder JM, Decullier E et al (2013) Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 39(12):1435–1443
Esquivel J, Piso P, Verwaal V et al (2014) American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol 110(7):777–778
Glehen O, Gilly FN, Boutitie F et al (2010) Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1290 patients. Cancer 116(24):5608–5618
Glehen O, Mithieux F, Osinsky D et al (2003) Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol 21(5):799–806
Spiliotis J, Halkia E, Lianos E et al (2015) Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Annal Surg Oncol 22(5):1570–1575
Sugarbaker PH (2006) New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 7(1):69–76
Verwaal VJ, van Ruth S, de Bree E et al (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21(20):3737–3743
Glehen O, Gilly FN (2003) Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am 12(3):649–671
Elias D, Lefevre JH, Chevalier J et al (2009) Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27(5):681–685
Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–374
Portilla AG, Shigeki K, Dario B, Marcello D (2008) The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol 98(4):228–231
Mohkam K, Passot G, Cotte E et al (2016) Resectability of peritoneal carcinomatosis: learnings from a prospective cohort of 533 consecutive patients selected for cytoreductive surgery. Ann Surg Oncol 23(4):1261–1270
Klumpp B, Schwenzer NF, Gatidis S et al (2014) Assessment of relapse in patients with peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy using F-18-FDG-PET/CT. RoFo 186(4):359–366
Esquivel J, Chua TC, Stojadinovic A et al (2010) Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 102(6):565–570
Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH (1993) Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 72(5):1631–1636
Koh JL, Yan TD, Glenn D, Morris DL (2009) Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 16(2):327–333
Rivard JD, Temple WJ, McConnell YJ, Sultan H, Mack LA (2014) Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis. Am J Surg 207(5):760–764. (Discussion 764–765).
Cotte E, Passot G, Gilly FN, Glehen O (2010) Selection of patients and staging of peritoneal surface malignancies. World J Gastrointest Oncol 2(1):31–35
Pomel C, Appleyard TL, Gouy S, Rouzier R, Elias D (2005) The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 31(5):540–543
Valle M, Garofalo A (2006) Laparoscopic staging of peritoneal surface malignancies. Eur J Surg Oncol 32(6):625–627
Garofalo A, Valle M (2003) [Staging videolaparoscopy of peritoneal carcinomatosis]. Tumori 89(4 Suppl):70–77
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Philipp von Breitenbuch, Thomas Boerner, Tonia Jeiter, Pompiliu Piso, and Hans J. Schlitt have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
von Breitenbuch, P., Boerner, T., Jeiter, T. et al. Laparoscopy as a useful selection tool for patients with prior surgery and peritoneal metastases suitable for multimodality treatment strategies. Surg Endosc 32, 2288–2294 (2018). https://doi.org/10.1007/s00464-017-5923-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5923-0