Abstract
Background
Primary cytoreduction is the mainstay of treatment for advanced ovarian cancer (AOC). We developed and prospectively evaluated an algorithm to investigate the possible role of laparoscopic primary cytoreduction (LPC) in carefully selected patients, with AOC.
Methods
From June 2007 to July 2015, all patients with stage III–IV ovarian cancer and clinical conditions allowing aggressive surgery were candidate to primary cytoreduction with the aim of achieving residual tumor (RT) = 0. The possibility of attempting laparoscopic cytoreduction was carefully evaluated using strict selection criteria. The other patients were approached by abdominal primary cytoreduction (APC). At the end of LPC, an ultra-low pubic mini-laparotomy was performed to extract surgical specimens and to accomplish a laparoscopic hand-assisted exploration of the abdominal organs, in order to confirm complete excision of the disease.
Results
Of the included 66 patients, 21 were considered eligible for LPC; the remaining 45 underwent APC. Optimal cytoreduction (i.e., RT = 0) was obtained in 95 and 88.4% in the LPC and APC groups, respectively. No intra-operative complication and 4 (19%) early post-operative complications were observed among patients who received LPC. Patients who underwent APC had 17.8 and 46.7% intra- and early post-operative complications, respectively. Median time to initiation of chemotherapy was 15 (range, 10–30) days in the LPC group and 28 (20–35) days in the APC group. After a median follow-up of 51 months, 2-year disease-free survival was 76.2% in the LPC group and 73.4% in the APC group.
Conclusions
After strict selection, a group of patients with AOC may undergo LPC with extremely high rates of optimal cytoreduction, satisfactory perioperative morbidity, a short interval to chemotherapy, and encouraging survival outcomes.
Clinical trial registration NCT02980185
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The US Cancer Statistics show that, in 2017, one woman will die of ovarian cancer for every 1.59 who will be diagnosed with this malignancy [1]. These figures are likely due both to the aggressive biological behavior of the disease and to the fact that the majority of patients are diagnosed at an advanced stage, i.e., when ovarian cancer has spread beyond the ovaries and the pelvis.
The standard approach for the initial treatment of patients with advanced ovarian cancer (AOC) consists of surgical removal of all the visible implants of the disease through a generous midline laparotomy, followed by combination platinum-based chemotherapy [2]. Through the years, solid evidence has accumulated regarding the crucial role of optimal cytoreduction as a major determinant of prognosis, and it is now widely accepted that no residual visible disease at the end of surgery represents one of the most important prognostic factors in AOC patients [3, 4].
While minimally invasive surgery has gained an important role in the comprehensive surgical staging of early-stage ovarian cancer, [5] the use of laparoscopy for advanced forms is usually limited to the pre-operative evaluation of resectability of the disease, in order to discriminate those patients in whom an extensive surgical effort may lead to optimal cytoreduction from those who may benefit more from neoadjuvant chemotherapy, followed by interval debulking and then completion of cytotoxic treatment [6]. In recent years, laparoscopy has been further applied to AOC as a successful tool for secondary cytoreduction in case of limited recurrent disease [7]. Although only retrospective series have been published on this type of approach, results appear promising and deserve further investigation.
In the last decade, improvements in operators’ skills, surgical technique, and minimally invasive instrumentation have allowed the accomplishment of highly complex procedures in gynecologic surgery such as laparoscopic pelvic exenteration [8, 9] and upper abdominal debulking [10, 11]. The same has happened also for non-gynecological subspecialties: nowadays, pancreaticoduodenectomy, [12] multiple bowel resections, [13] and partial hepatectomies [14] can be safely accomplished by laparoscopy, while only few years ago they appeared as something that could not even be imagined.
As a logical consequence, some authors have recently suggested a possible role of laparoscopic debulking surgery in secondary cytoreduction after neoadjuvant chemotherapy [15, 16]. However, primary laparoscopic cytoreduction for AOC is still regarded almost as a taboo and up to now it has been reported only in limited retrospective series with a low number of patients included and a short follow-up [17,18,19]. It is well known that open debulking is associated with an inevitably high rate of threatening intra- and post-operative complications and long-lasting hospital admissions. The expectable advantages of applying minimally invasive surgery to primary cytoreduction for AOC include better quality of life, earlier initiation of adjuvant therapy, and lower overall morbidity.
Many criticisms have been raised regarding the possibility of laparoscopic cytoreductive treatment of AOC. Among them, the two most relevant are (1) the technical difficulty in eradicating all the disease through a minimal-access approach; and (2) the impossibility to adequately explore and palpate the peritoneal surface and retroperitoneal structures by laparoscopy, in order to assess the real extent of the disease. Refinements in surgical technique may at least theoretically allow to overcome the first objection; for the second point, some series have suggested a hand-assisted technique, to improve radicality and to reduce the intra-operative bias due to the impossibility of direct manual palpation of the peritoneum/retroperitoneum [20, 21].
The purpose of the present study has been to prospectively analyze the safety, efficacy, and oncological outcomes of laparoscopic primary cytoreduction for AOC (with hand-assisted exploration of the abdomen at the end of the procedure), in strictly selected patients. We also provided data regarding the outcomes of women not eligible for laparoscopic surgery, who underwent traditional open surgery.
Materials and methods
All patients with primary AOC undergoing major gynecologic surgery by the first author (MC) at the Division of Gynecologic Oncology of the Sacred Heart Hospital, Negrar, Verona, Italy, were entered since 2007 into a prospective research-quality surgical database, regularly updated by trained residents under direct consultants’ supervision. The quality of the data entered was continuously monitored during the study period with annual audits. Demographics were obtained and entered pre-operatively, surgical outcomes were entered immediately post-operatively, and follow-up was collected after each office examination.
The protocol of the present study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, [22] and it was approved by the Ethical Committee of the Hospital of Negrar.
Pre-operatively, all patients were submitted to an abdomino-pelvic clinical examination and ultrasound and a total body PET-CT scan with FDG. Assessment of CA125 was performed in each patient, and clinic-anamnestic data were collected. Every case was discussed within the Gynecologic Oncological Team, a panel of experts with two Gynecologic Oncologic surgeons, the Medical Oncologist, the General and Nuclear Radiologist, the General Surgeon, the Urologist, and the Anesthesiologist.
All patients with presumed FIGO stage IIIA1–IV disease and no anesthesiological/clinical contraindications for aggressive surgery underwent diagnostic laparoscopy to evaluate the feasibility of complete cytoreductive surgery (whether open abdominal or laparoscopic). In all cases, surgery was initiated with a laparoscopic exploration of the abdomino-pelvic cavity, starting in a clock-wise direction from the upper abdomen and carefully exploring the diaphragmatic surfaces and the splanchnic viscera. Adhesiolysis was accomplished when indicated; section of the hepatic ligaments was performed to mobilize the liver and to obtain a better access to the right diaphragmatic surface; a 30-degree laparoscope was also used for better anatomical visualization. As mentioned, all the procedures were performed by a single surgeon (MC) with extensive experience in surgical anatomy, gynecologic surgery, minimally invasive techniques, gynecologic oncology, and open/laparoscopic cadaveric dissection. The first operator has accomplished more than 2000 major gynecologic operations, with more than 500 gynecologic oncology cases.
Selection of patients
The possibility of complete cytoreduction was assessed during diagnostic laparoscopy; massive infiltration of the mesenteric root or of the hepatic hilum was considered as absolute contraindications to primary cytoreductive effort. The Fagotti score was then used to further predict the likelihood of optimal debulking surgery [6]. In case of Fagotti score < 8, and if the patient was considered adequate for extensive surgery from the clinical and anesthesiological point of view, a primary debulking procedure was performed.
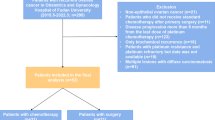
At this time, a specific evaluation to discriminate patients eligible for laparoscopic versus open abdominal approach was performed taking into account also pre-operative imaging and using the following strict criteria: patients did not undergo laparoscopic cytoreduction in the presence of “massive omental cake,” obliterated Morison’s pouch, the need of more than two liver resections, involvement of the retrohepatic diaphragm, multiple and/or bilateral diaphragmatic carcinomatosis, massive infiltration of more than three organs, bulky upper abdominal masses > 5 cm, pelvic bulky disease > 20 cm, the necessity of more than two bowel resections, > 2 bulky nodes, and obliterated spleno-colic ligament. A summary of our prospective treatment algorithm for advanced ovarian cancer and of the criteria used in this study to select patients for primary laparoscopic cytoreduction is provided in Fig. 1.
After this evaluation, patients were divided in two groups, according to the surgical approach used: laparoscopic primary cytoreduction (LPC) group and abdominal primary cytoreduction (APC) group.
Surgical technique
Total extrafascial hysterectomy was performed following the Clermont–Ferrand technique, and radical hysterectomy was performed following the Querleu–Morrow classification [23]. In cases of extensive pelvic infiltration of the peritoneum, a total Hudson–Delle Piane radical oophorectomy with or without en-bloc rectal resection was performed [24, 25]. When needed, segmental rectal resection was accomplished using the nerve-sparing technique previously described by our group [26]. Nodal dissection was performed only in case of bulky nodes or suspicious retroperitoneal disease at pre-operative imaging.
Every surgical procedure included a final laparoscopically assisted hand-palpation phase: a ultra-low, mini-laparotomic (< 4 cm) pubic incision was performed, to extract specimens and to allow the insertion of a surgeon’s hand for systematic evaluation of possible residual disease under laparoscopic guidance. The different diagnostic and operative phases are illustrated in Figs. 2, 3, and 4.
Laparoscopic assessment and management of advanced ovarian cancer, A laparoscopic view of pelvic carcinomatosis in FIGO stage IIIC AOC; B laparoscopic view of diaphragmatic carcinomatosis with “bulky” left diaphragmatic metastasis; C laparoscopic metastasectomy of right diaphragmatic nodule infiltrating the muscular layer; D laparoscopic view of pelvic carcinomatosis with “frozen pelvis” and bowel infiltration in FIGO stage IIIC AOC; E pubic transverse ultra-low mini-laparotomy in course of totally laparoscopic radical oophorectomy with en-bloc bowel resection for FIGO stage IIIC AOC; F image of the surgeon’s left arm inserted intra-abdominally by the mini-laparotomic access, during the laparoscopic-assisted hand-palpation phase of LPC
Different intra-operative phases of the laparoscopic cytoreductive surgery for advanced ovarian cancer: A laparoscopic vision of diaphragmatic palpation by the surgeon’s left hand inserted intra-abdominally through the mini-laparotomic access, during the laparoscopic-assisted hand-palpation phase of LPC; B completion of mesenteric node resection in course of the mini-laparotomic phase; C transection of the rectosigmoid during the mini-laparotomic phase; D and E view of the surgical specimen after totally laparoscopic en-bloc posterior exenteration with segmental rectosigmoid resection and pelvic peritonectomy in AOC with diffuse pelvic carcinomatosis; F laparoscopic view of the surgical field after totally laparoscopic posterior exenteration with end-to-end colo-rectal anastomosis
Optimal cytoreduction was defined as “no visible disease” (i.e., RT = 0) at the end of the procedure. Post-operative complications were classified as “early” if they occurred before 30 days and “late” if they occurred after 30 days from the operation.
Every patient underwent adjuvant chemotherapy; the time interval between primary cytoreduction and the initiation of the medical treatment was counted in days. As part of the protocol and in accordance with Chi et al. [27], all patients were submitted to a post-operative CT scan to confirm the completeness of cytoreduction before the initiation of chemotherapy. Women were included in the institutional follow-up program: follow-up evaluations were scheduled monthly for the first 3 months, then every 3 months for the first 2 years, and every 6 months thereafter. Markers tests were accomplished every 4 months post-operatively, a CT scan was scheduled 1 month after the end of chemotherapy and then yearly, and a PET-CT scan was performed in case recurrence was suspected. A platinum-based combination was chosen for adjuvant treatment. Bevacizumab was added to frontline or second-line therapy in 2013.
Statistical analysis
Continuous variables are reported as median (range). Categorical variables are reported as absolute numbers (percentage). Median time-to-relapse, progression-free survival (PFS), and overall survival (OS) were calculated. Kaplan–Meyer curves and log-rank test were calculated for PFS and OS data.
Statistical comparisons between groups (although biased by the intrinsic imbalance between groups) have been provided in Supplementary Tables. Normality testing (D’Agostino and Pearson test) was performed to determine whether continuous variables were sampled from a Gaussian distribution. Afterwards, comparisons between two group of continuous variables were accomplished using independent samples t test or Mann–Whitney U test, as appropriate. Categorical covariates were compared with Chi-square test. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows 16.0 package (SPSS, Inc., Copyright IBM Corporation 2010, Somers, NY) and GraphPad version 5.00 for Windows (GraphPad Software, San Diego, CA).
Results
From June 2007 to July 2015, a total of 147 patients with FIGO stage IIIA1-IV AOC were treated at our Institution. Of these patients, 13 (8.8%) were not eligible for primary cytoreduction, due to anesthesiological contraindications, 67 (45.6%) were excluded at primary laparoscopic evaluation, because they were considered unsuitable for primary debulking (according to the Fagotti score) and underwent neoadjuvant chemotherapy, and 1 (0.7%) refused enrolment. Of the residual 66 patients, 21 (31.8%) were eligible for LPC, whereas 45 (68.2%) patients underwent APC. Demographic and histological characteristics are shown in Table 1. Patients submitted to LPC had a lower BMI and a tendency towards a higher rate of ASA score 1, with respect to patients submitted to APC. Intra-operative procedures are listed in Table 2. No conversion from laparoscopy to open surgery was registered. Pelvic/para-aortic nodal debulking was accomplished in 19% of patients in the LPC group and in 48.9% of patients in the APC group. Optimal RT at the end of surgery was obtained in 95.3% of patients after LPC and in 88.4% after APC. Median estimated blood loss was 250 mL (range, 100–500) in the LPC group and 600 mL (200–4500) in the APC group. Intra-operative complications were observed in 8 (17.8%) patients in the APC group versus none in the LPC group.
Early post-operative complications (Table 3) were observed in 19% of cases in the LPC group and in 46.7% of patients in the APC group. One (2.2%) post-operative death was observed in the APC group (due to sepsis and cardiac failure), and 0 in the LPC group.
Median hospital stay was 15 days in the APC group and 9 days in the LPC group. Interval between cytoreduction and initiation of the first chemotherapy cycle was 15 (10–30) days in the LPC and 28 (20–35) days in the APC group.
Every patient (excluding the subject with early post-operative death in the APC group) was submitted to six cycles of post-operative adjuvant chemotherapy by the standard carbo-taxol regimen. All patients completed the six cycles of first-line chemotherapy in a period of maximum 6 months. Late complication rate was 15 and 15.6% for the LPC and APC groups, respectively. The rate of late post-operative reoperation was 10% after LPC and 8.8% after APC.
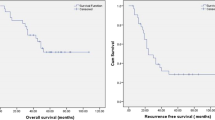
After a median follow-up period of 47.3 and 52.3 months in the LPC and APC groups, respectively, 33.3% of patients recurred after laparoscopic surgery versus 35.5% after open abdominal surgery. Two-year disease-free survival was 76.2% in the LPC group and 73.4% in the APC group. Details regarding the specific sites of recurrence are provided in Table 4. PFS and OS were comparable between groups (log-rank test 0.135 and 0.339, respectively, for OS and PFS), with 100% of overall survival in the LPC group. Median time to relapse was 17.4 months (range 9.3–30.6) after LPC, versus 18.3 months (range 6.6–28.9) after APC. Survival curves are shown in Figs. 5 and 6. A subanalysis of disease-free and overall survival in patients with optimally debulked disease (RT = 0) is provided in Supplementary Figs. 1 and 2. Six (28.6%) and 12 (26.7%) patients received Bevacizumab in LPC and APC groups.
Supplementary Tables 1–4 provide details of the statistical comparisons between LPC and APC groups.
Comment
The present article shows that, in strictly selected patients, laparoscopic primary debulking for AOC is feasible and allows very high rates of optimal cytoreduction to RT = 0 (95%) that well compare with those of open abdominal surgery in referral centers [28].
To date, only small, retrospective series with ill-defined inclusion criteria have been published regarding the use of minimally invasive surgery for the surgical comprehensive treatment of advanced ovarian cancer [17,18,19]. This paucity of data likely reflects on the one hand the high technical difficulty of performing advanced laparoscopic procedures that allow complete cytoreduction, and on the other hand the almost ubiquitous prejudice that minimally invasive surgery is not adequate for this type of disease and may be even detrimental for the prognosis of patients.
Regarding the first objection, it should be recognized that laparoscopic surgery has intrinsic limits due to the need of gaining enough space to mobilize the anatomical structures and to allow adequate movements of the instruments, which may be impaired by the positioning and the fulcrum-effect of the ports. However, the impressive diffusion of laparoscopic surgery has determined a dramatic and probably unexpected expansion of its indications, so that procedures once considered as impossible are nowadays regarded as routine everyday clinical practice.
Regarding the second criticism, many detractors of laparoscopy in the past have claimed that the use of CO2 may promote the seeding of tumoral cells to the peritoneum and the laparoscopic ports [29]. However, no evidence supports this statement; in fact, many centers now consider diagnostic laparoscopy as a crucial part of preventive evaluation of women, to identify AOC patients who may benefit from primary aggressive cytoreduction rather than neoadjuvant chemotherapy [6].
An actual limitation of laparoscopic cytoreduction is the impossibility to visualize certain abdominal regions (such as the epiploon retrocavity, the posterior aspect of the liver, etc.). To avoid the risk of missing malignant localizations, laparoscopic-assisted hand-palpation technique was used in the present study at the end of LPC. This approach allowed us also to be sure regarding the correct estimation of RT. A possible area concern is the difficulty in exploring the retroperitoneal structures (in particular the para-aortic lymph nodes) as well as all aspects of the bowel and its mesentery, even with the hand-assisted method. The risk of missing disease spread in these areas should translate into a higher site-specific recurrence rate. As reported in Table 4, the rate of nodal and bowel recurrences tended to be higher in the LPC group, compared to the APC group, although not reaching statistical significance (Supplementary Table 4). This particular issue deserves much attention and close monitoring in the next future. On the other hand, it must be remembered that the laparoscope magnifies small lesions, which may be missed at the time of laparotomic exploration.
The use of laparoscopy in AOC has possible extremely promising implications, including the dramatic decrease in the overall morbidity of surgical treatment (with reduction of pain, post-operative length of hospital stay, and complications), and an earlier initiation of adjuvant chemotherapy. Interestingly, in our series we observed a 13-day anticipation in starting adjuvant treatment among women treated with laparoscopic surgery. Moreover, we emphasize that only 19% of patients experienced a complication in the LPC group, thus corroborating the assumption that laparoscopic surgery may help in decreasing adverse events related to primary cytoreduction.
We remark that the present series is not a comparative study: patients in the two groups are not comparable in terms of baseline characteristics. In fact, patients in the LPC group had a lower BMI and tended to have lower ASA score. As a consequence, women who underwent laparoscopic surgery showed more favorable conditions, from a medical and surgical point of view. Even more importantly, the two study groups are not comparable also in terms of initial tumor load and localization, due to the presence of strict exclusion criteria for laparoscopic debulking. As a result, in the APC group women had larger tumor masses, and/or more upper abdomen/retroperitoneal localizations, and/or more likelihood of massive diaphragmatic/retrohepatic involvement. However, many reports have demonstrated that the major determinant for prognosis in AOC is residual tumor and not its initial diffusion;[30,31,32] in other words, it is how much tumor is “left behind” at the end of surgery rather than the initial spread of the disease to exert an impact on survival. Some authors correctly point out that less widespread tumors may have a more indolent biological behavior and may tend to recur more slowly. However, studies which stratifying on the intrinsic aggressiveness of the disease have shown that the radicality of surgery and RT remain the strongest predictors of recurrence [33]. The limited number of patients included prevents us from providing definitive conclusions, particularly in terms of survival. However, due to the high incidence of relapse in AOC, the median follow-up of this study (51 months) appears long enough to detect possible evident detrimental effects of laparoscopic surgery among patients in the LPC group. Nevertheless, only comparative and possibly randomized studies with well-balanced baseline characteristics will provide answers regarding the real impact of laparoscopic surgery in the field of AOC. Another possible limitation of the present study is that all women were operated by a single surgeon with extensive experience and high skills in oncologic surgery and minimally invasive techniques. The external validity of our findings may be scarce, and we believe that, for the moment, our results could be replicated only in dedicated settings with adequate background in complex laparoscopic procedures.
The present study represents a preliminary and pioneering work and, as such, it is logical to accept that it is exposed to several possible criticisms. However, in our opinion it may represent a milestone in the treatment of advanced ovarian cancer and possibly the origin of a deep rethinking in the approach to this disease, provided that our results will be replicated in larger independent series. Of course, this type of surgery requires particular and uncommon skills, and for the moment, generalizability of our results may be scarce. However, as it happened in the past for other types of operations, laparoscopic skills and refinements in technique can be taught and developed. Moreover, we may speculate that the advent of robotic surgery and the possible refinements in this new minimally invasive approach may have the potential to overcome the limitation of scarce reproducibility and applicability of laparoscopic surgery to AOC. We strongly believe that, if the main goal in AOC is to completely eradicate the tumor up to RT = 0, it does not matter if this is achieved by laparotomy or laparoscopy. Surgery for AOC is associated with the risk of severe, life-threatening complications. Minimally invasive techniques may be extremely important, to try to minimize morbidity at least in a subgroup of well-selected patients with a pattern of disease spread that can be approached by laparoscopy. Of course, our results do not represent a definitive endorsement of laparoscopic cytoreduction for AOC, but rather they strongly encourage further research, possibly randomized and on a larger sample size, to evaluate the real impact of minimally invasive surgery in this setting.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, ESMO Guidelines Working Group (2013) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 (Suppl 6):vi24–vi32. doi:10.1093/annonc/mdt333
Bristow RE, Montz FJ, Lagasse LD et al (1999) Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol 72:278
Peiretti M, Zanagnolo V, Aletti GD et al (2010) Role of maximal primary cytoreductive surgery in patients with advanced epithelial ovarian and tubal cancer: surgical and oncological outcomes. Single institution experience. Gynecol Oncol 119:259–264. doi:10.1016/j.ygyno.2010.07.032
Ghezzi F, Malzoni M, Vizza E (2012) Laparoscopic staging of early ovarian cancer: results of a multi-institutional cohort study. Ann Surg Oncol 19:1589–1594. doi:10.1245/s10434-011-2138-9
Fagotti A, Vizzielli G, De Iaco P et al (2013) A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol 209:462.e1–462.e11. doi:10.1016/j.ajog.2013.07.016
Gallotta V, Fagotti A, Fanfani F et al (2014) Laparoscopic surgical management of localized recurrent ovarian cancer: a single institution experience. Surg Endosc 28:1808–1815. doi:10.1007/s00464-013-3390-9
Martínez A, Filleron T, Vitse L et al (2011) Laparoscopic pelvic exenteration for gynaecological malignancy: is there any advantage? Gynecol Oncol 120:374–379. doi:10.1016/j.ygyno.2010.11.032
Puntambekar S, Rajamanickam S, Agarwal G, Joshi S, Rayate N, Deshmukh A (2011) Laparoscopic posterior exenteration in advanced gynecologic malignant disease. J Minim Invasive Gynecol 18:59–63. doi:10.1016/j.jmig.2010.09.003
Menderes G, Schwab C, Black J, Silasi DA (2016) Laparoscopic resection of the diaphragmatic tumor nodule for management of recurrent endometrial cancer. J Minim Invasive Gynecol 23:473–474. doi:10.1016/j.jmig.2016.01.006
Trinh H, Ott C, Fanning J (2004) Feasibility of laparoscopic debulking with electrosurgical loop excision procedure and argon beam coagulator at recurrence in patients with previous laparotomy debulking. Am J Obstet Gynecol 190:1394–1397
Stauffer JA, Coppola A, Villacreses D, Mody K, Johnson E, Li Z, Asbun HJ (2017) Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc 31:2233–2241. doi:10.1007/s00464-016-5222-1
Taggarshe D, Attuwaybi BO, Matier B, Visco JJ, Butler BN (2015) Hand-assisted laparoscopic (HAL) multiple segmental colorectal resections: are they feasible and safe? Int Surg 100:632–637. doi:10.9738/INTSURG-D-14-00208.1
Cherqui D (2016) Evolution of laparoscopic liver resection. Br J Surg. doi:10.1002/bjs.10252
Tozzi R, Gubbala K, Majd HS, Campanile RG (2016) Interval laparoscopic En-Bloc resection of the pelvis (L-EnBRP) in patients with stage IIIC-IV ovarian cancer: description of the technique and surgical outcomes. Gynecol Oncol 142:477–483. doi:10.1016/j.ygyno.2016.07.003
Corrado G, Mancini E, Cutillo G et al (2015) Laparoscopic debulking surgery in the management of advanced ovarian cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer 25:1253–1257. doi:10.1097/IGC.0000000000000491
Nezhat FR, Datta MS, Lal N (2008) Laparoscopic cytoreduction for primary advanced or recurrent ovarian, fallopian tube, and peritoneal malignancies. Gynecol Oncol 108:S60
Fanning J, Yacoub E, Hojat R (2011) Laparoscopic-assisted cytoreduction for primary advanced ovarian cancer: success, morbidity and survival. Gynecol Oncol 123:47–49. doi:10.1016/j.ygyno.2011.06.020
Magrina JF, Zanagnolo V, Noble BN, Kho RM, Magtibay P (2011) Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol 121:100–105
Krivak T, Elkas J, Rose G et al (2005) The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol 96:72–76
Varnoux C, Huchon C, Bats AS et al (2013) Diagnostic accuracy of hand-assisted laparoscopy in predicting resectability of peritoneal carcinomatosis from gynaecological malignancies. Eur J Surg Oncol 39:774–779
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative (2014) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12:1495–1499. doi:10.1016/j.ijsu.2014.07.013
Querleu D, Morrow CP (2008) Classification of radical hysterectomy. Lancet Oncol 9:297–303
Hudson CN, Chir MA (1968) A radical operation for fixed ovarian tumors. J Obstet Gynaecol Br Commonw 75:1155–1156
Benedetti Panici P, Maneschi F, Scambia G, Cutillo G, Mancuso S (1996) The pelvic retroperitoneal approach in the treatment of advanced ovarian carcinoma. Obstet Gynecol 87:532–538
Ceccaroni M, Clarizia R, Bruni F et al (2012) Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc 26:2029–2045
Chi DS, Ramirez PT, Teitcher JB, Mironov S, Sarasohn DM, Iyer RB, Eisenhauer EL et al (2007) Prospective study of the correlation between postoperative computed tomography scan and primary surgeon assessment in patients with advanced ovarian, tubal, and peritoneal carcinoma reported to have undergone primary surgical cytoreduction to residual disease 1 cm or less. J Clin Oncol 25:4946–4951
Aletti GD, Dowdy SC, Gostout BS et al (2009) Quality improvement in the surgical approach to advanced ovarian cancer: the Mayo clinic experience. J Am Coll Surg 208:614–620. doi:10.1016/j.jamcollsurg.2009.01.006
Canis M, Rabischong B, Botchorishvili R et al (2001) Risk of spread of ovarian cancer after laparoscopic surgery. Curr Opin Obstet Gynecol 13:9–14
Aletti GD, Dowdy SC, Gostout BS et al (2006) Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 107:77–85
Eisenkop SM, Spirtos NM, Lin WC (2006) “Optimal” cytoreduction for advanced epithelial ovarian cancer: a commentary. Gynecol Oncol 103:329–335
Sioulas VD, Schiavone MB, Kadouri D, Zivanovic O, Roche KL, O’Cearbhaill R et al (2017) Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol 145:15–20. doi:10.1016/j.ygyno.2017.02.023
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Marcello Ceccaroni, Giovanni Roviglione, Francesco Bruni, Roberto Clarizia, Giacomo Ruffo, Matteo Salgarello, Michele Peiretti, and Stefano Uccella have no conflict of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ceccaroni, M., Roviglione, G., Bruni, F. et al. Laparoscopy for primary cytoreduction with multivisceral resections in advanced ovarian cancer: prospective validation. “The times they are a-changin”?. Surg Endosc 32, 2026–2037 (2018). https://doi.org/10.1007/s00464-017-5899-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5899-9