Abstract
Background
Although endoscopic resection (ER) may be sufficient treatment for early-stage esophageal cancer, additional treatment is recommended when there is a high risk of cancer recurrence. It is unclear whether delaying esophagectomy by performing and assessing the success of ER affects outcomes as compared with immediate esophagectomy without ER. Additionally, long-term survival after sequential ER and esophagectomy required further investigation.
Methods
Between 2011 and 2015, 48 patients with stage T1 esophageal cancer underwent esophagectomy after ER with curative intent at our institution. Two-to-one propensity score methods were used to identify 96 matched-control patients who were treated with esophagectomy only using baseline patient, tumor characteristics and surgical approach. Time from initial evaluation to esophagectomy, relapse-free survival, overall survival, and postoperative complications were compared between the propensity-matched groups.
Results
In the ER + esophagectomy group, the time from initial evaluation to esophagectomy was significantly longer than in the esophagectomy only group (114 vs. 8 days, p < 0.001). The incidence of dense adhesion (p = 0.347), operative time (p = 0.867), postoperative surgical complications (p = 0.966), and postoperative length of hospital stay (p = 0.125) were not significantly different between the groups. Moreover, recurrence-free survival and overall survival were also similar between the two groups (p = 0.411 and p = 0.817, respectively).
Conclusions
Treatment of stage T1 esophageal cancer with ER prior to esophagectomy did not increase the difficulty of performing esophagectomy or the incidence of postoperative complications and did not affect survival after esophagectomy. These results suggest that ER can be recommended for patients with stage T1 cancer even if esophagectomy is warranted eventually.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
When esophageal cancer is detected at an early stage, the survival rate can be as high as 85%; a stark contrast to a 5-year survival rate of <10% when diagnosed at an advanced stage [1]. Due to the importance of early detection, an increasing number of esophageal cancers are being diagnosed at an early and curable stage, particularly in patients who undergo surveillance endoscopy [2, 3].
Esophagectomy has traditionally been the standard treatment for all esophageal cancers, even for localized lesions. Esophagectomy is a radical surgery associated with substantial procedure-related mortality and long-term morbidity [4]. Even when performed at an experienced center by expert surgeons, esophagectomy for early-stage cancer is associated with a significant mortality (at least 2%) and postoperative morbidity in at least 40% of patients [5, 6]. In recent years, endoscopic resection (ER) has emerged as a potential alternative to surgery for early-stage, superficial, esophageal carcinoma [7]. ER completely removes the diseased mucosa by dissection through the middle or deep part of the submucosa. Because ER is minimally invasive and can be effective without compromising organ function, it is becoming the dominant treatment strategy for early-stage esophageal cancer and precancerous lesions [8].
Despite its promise, ER has intrinsic limitations. Important concerns include missed synchronous malignancy, misclassification of tumor depth of invasion, and an inability to obtain pathologic lymph node staging information. A particularly relevant disadvantage of ER is the risk of recurrent neoplasia, which in initial reports of ER was reported to be as high as 30% [2]. Early-stage esophageal cancer may be accompanied by lymph node metastasis [9], and metastases have been found in up to 4% of cancers limited to the epithelium and lamina propria, 0–22% of cancers with invasion of the muscularis mucosa, and 26–54% of cancers that invade the submucosa [3, 10, 11]. Some patients undergo ER treatment initially, but then undergo radical esophageal resection due to a high risk for cancer recurrence or lymph node metastasis. To date, it has been unclear whether initial treatment with ER significantly impacts the incidence of postoperative complications or long-term survival after esophagectomy. Therefore, we examined operative and postoperative outcomes in patients who underwent ER before an eventual esophagectomy to better understand the consequences of ER in this patient population.
Methods
Patients
This research was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center, Shanghai, China. All patients were treated by the Department of Thoracic Surgery, Fudan University Shanghai Cancer Center from March 2011 to March 2015. Data were collected prospectively into the patients’ medical and operative records and into a departmental database used to monitor outcomes in patients undergoing ER.
During the study period, 140 patients with T1 esophageal cancer underwent endoscopic resection with curative intent. To acquire tumor clinical stage before surgery, patients typically underwent preoperative examinations that included chest computed tomography (CT), abdominal ultrasound, electronic gastroscopy with narrow band imaging, endoscopic ultrasonography (EUS), and contrast esophagography. Treatment with ER was chosen by the patients after consultation with their surgeon regarding the pros and cons of ER, radiotherapy, and esophagectomy. Three patients with T1 esophageal tumors who underwent ER with curative intent were excluded from further analysis due to insufficient data on the characteristics we were examining, and five patients were screened with other malignant disease in previous 5 years. After ER, 65 patients chose follow-up with surveillance only and had not undergone esophagectomy as of their last telephone interview; 16 patients chose consolidation radiation directly after ER. The remaining 48 consecutive patients underwent esophagectomy after ER and were included into this study (Fig. 1; Table 1). Patients were excluded from the analysis using the following criteria: (1) tumor stage > T1 as determined in the ER specimen; (2) radiotherapy or chemotherapy before ER or during the interval between ER and esophagectomy; and (3) any other malignant disease in previous 5 years.
Esophagectomy after ER was recommended in these 48 patients by a multidisciplinary team based on the likelihood of occult residual tumor, as determined by positive resection margins in the ER specimen, and the risk of lymph node metastasis, as indicated by the presence of multifocal lesions, subclassification of superficial esophageal carcinoma ≥ m3, circular rather than longitudinal tumor spread, poor differentiation in the final pathologic analysis; stage T1b tumor, or the presence of lymphovascular invasion (LVI) [12,13,14].
Propensity-score matching
Propensity-score methods were used to identify 96 patients who were treated with esophagectomy only for comparison (Table 2). Propensity-score matching was performed with two-to-one nearest-neighbor matching without replacement to identify matched cohorts for the two treatment modalities (ER + esophagectomy and esophagectomy only). This method was adopted to balance the observed covariates between two groups. Age, sex, pathological tumor type, tumor location, tumor length, thickening of the esophageal wall, classification of superficial lesions, surgical procedure for esophagectomy, and whether or not the esophagectomy included video-assisted thoracoscopic surgery were selected as the observed covariates (Table 2). Based on this set of covariates, the propensity score was estimated using logistic regression. The statistical analyses were conducted using R version 3.3.1 software (R, CA, USA).
ER techniques
ER included endoscopic multi-band mucosectomy (EMBM) and endoscopic submucosal dissection (ESD). The Duette Multiband Mucosectomy Kit (DT-6, Cook Medical, Bloomington, IN) was used for EMBM. Overlapping resections were required to ensure complete resection. ESD was performed using a T-knife or VS knife (Erbe Elektromedizin, GmbH, Tübingen, Germany), under the ENDO CUT IQ model (Erbe platform system).
Surgical treatment
Esophagectomy was performed by the same surgical team that performed the ER, and the surgical procedure used (Table 2) was determined based on the features of lesion resected and the experience of the primary surgeon. Surgical patients had follow-up visits in the clinic 4 weeks and 6 months after esophagectomy, and annually thereafter.
Pathological diagnosis and staging
Surgical specimens from ER and esophagectomy were fixed in 5% formaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined by light microscopy. The margins of specimen were examined carefully to determine if an R0 resection was accomplished. The depth of invasion was examined by at least two independent pathologists.
Statistical analysis
Pearson’s Chi-square test was used to compare frequencies for categorical variables. Paired independent sample t tests or one-way analysis of variance (ANOVA) was used to compare continuous variables between the groups. The Kaplan–Meier method was used to analyze relapse-free survival (RFS) and overall survival (OS). The log-rank test was used to compare differences in RFS and OS. Follow-up of all patients was extended to May 2017. Statistical significance was set as p = 0.05. The statistical analyses were conducted using SPSS version 18.0 software (SPSS, Chicago, IL).
Results
Interval between initial evaluation and esophagectomy
The median interval from initial evaluation to esophagectomy was 114 days (range 4–863 days) in the patients who underwent ER followed by esophagectomy and was significantly longer than the interval between initial evaluation and esophagectomy in patients who underwent esophagectomy only (median 8 days, range 3–34 days) (Table 3). In more than half of the patients who underwent ER (31 patients, 64.6%), esophagectomy took place more than 30 days after ER. Additionally, none of the patients underwent emergency esophagectomy during ER due to complications.
Perioperative parameters
Operative parameters and early postoperative outcomes did not differ between patients who underwent ER prior to esophagectomy and those who did not (Table 3). Notably, a significant difference in the occurrence of dense adhesions (pleura adhesions noted in the surgical record) was not observed between the two groups (14.6% in ER + esophagectomy group vs. 9.4% in esophagectomy group, p = 0.347) operative time and postoperative hospital stay duration were also similar between the groups (Table 3). None of the patients who underwent esophagectomy died during their hospital stay. Major postoperative complication occurred in 16.7% of the patients who underwent esophagectomy after ER: pneumonia occurred in two patients (4.2%), anastomotic leak occurred in five patients (10.4%) but healed with conservative therapy, and pyloric obstruction occurred in one patient (2.1%) (Table 3) this was not significantly different from the occurrence of major postoperative complications in 12.5% of the patients who underwent esophagectomy only (p = 0.996).
Tumor pathology
Performing ER prior to esophagectomy did not significantly change the tumor profile as assessed by pathology after the esophagectomy. Lymph node metastases were detected in two patients who had undergone ER and three patients who had not (p = 0.748); LVI was detected in only one patient (Table 3). There were no significant differences observed in tumor differentiation or the final T stage diagnosed in the esophagectomy specimen between the groups (Table 3). The ER specimen was insufficient to diagnosis T and N stage in four patients who underwent ER (8%). After examination of the esophagectomy specimen, T status was upstaged in two patients and both T and N status were upstaged in the other two patients.
Analysis of survival and recurrence
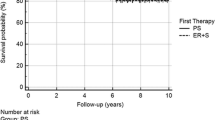
Performing ER before esophagectomy did not change either recurrence-free survival (Fig. 2A) or overall survival (Fig. 2B). Estimated survival did not differ between the patients who underwent ER before esophagectomy and those who did not (1-year survival, 97.9 vs. 98.9%; 2-year survival, 97.9 vs. 97.9%; 3-year survival, 91.6 vs. 90.9%).
Survival after esophagectomy. A Cumulative recurrence-free survival with abstracted number at risk displayed. B Cumulative overall survival with abstracted number at risk displayed. Solid line endoscopic resection plus esophagectomy group. Dotted line esophagectomy only group. ER endoscopic resection, HR hazard ratio
Two patients who underwent esophagectomy after ER had N1 tumors and were treated with adjuvant chemotherapy. At a mean follow-up of 43.4 ± 13.6 months (median 46 months, range 30–66 months), 41 patients were alive with no evidence of cancer recurrence (91.7%) as of last follow-up; one patient was alive with bone metastasis; one patient was alive with local recurrence and liver metastasis, and two patients were alive with lymph node metastases to the recurrent laryngeal nerve lymph nodes.
Among the propensity-matched patients with T1 tumors who underwent esophagectomy only, three patients with stage N1 tumors were treated with adjuvant chemotherapy. At a mean follow-up of 49.2 ± 12.6 months (median 47 months, range 26–75 months), 86 patients were alive with no evidence of cancer recurrence (89.5%) as of last follow-up; one patient was alive with right recurrent nerve lymph node metastasis; one patient was alive with right supraclavicular lymph node metastasis, and one patient was alive with lung metastasis.
Discussion
In this study, we investigated whether undergoing ER prior to esophagectomy affected procedure-related complications and long-term survival as compared with proceeding directly to esophagectomy in patients with early-stage esophageal cancer. We used propensity-score analysis to generate precisely matched patient cohorts with respect to epidemiologic and tumor parameters. The time to perform esophagectomy, postesophagectomy complications and hospitalization, RFS, and OS were comparable between the two groups. The delay between ER and esophagectomy was insignificant for prognosis. To the best of our knowledge, this is the first study with a propensity-matched control group documenting the impact of initial treatment with ER on the performance and therapeutic efficacy of a subsequent radical esophagectomy.
Previous studies have demonstrated that preoperative evaluation with endoscopic biopsy, endoscopic ultrasound, CT, and positron emission tomography are sometimes inadequate for accurate clinical staging. [10, 16,17,18,19]. ER is recognized as a relatively precise method for initial staging of esophageal cancer. ER specimens can be used to accurately determine the histopathological diagnosis and depth of tumor invasion in superficial lesions and yield clinically meaningful pathological staging in patients with early-stage esophageal cancer [10]. The current study provides reassurance that ER improves diagnosis and risk stratification without influencing operative parameters, postoperative complications, tumor recurrence, or survival if esophagectomy is later warranted.
If a T1 esophageal lesion is confined to the lamina propria without evidence of lymphovascular invasion, ER may be an appropriate curative therapy. According to guidelines put forth by the National Comprehensive Cancer Network (NCCN), ER and esophagectomy are equally recommended for the treatment of lesions limited to the mucosa [American Joint Committee on Cancer (AJCC) stage T1aN0M0] [20]. In contrast, when the lesion has invaded the submucosa or when LVI has occurred, the risk of lymph node metastasis is relatively high [18, 21, 22]. According to the NCCN guidelines only surgical resection (and not ER) is recommended for submucosal lesions (AJCC stage T1bN0M0) [20]. In our research, the pathological diagnosis of T stage determined in the esophagectomy specimen resulted in upstaging of the T status in 8% of the patients previously staged by ER, and 4.2% of patients had lymph node involvement that was detected pathologically after esophagectomy, even though they were diagnosed clinically as negative for metastasis. ER should not be considered as a valid curative alternative to esophagectomy in these cases [23, 24].
Although ER is associated with a relatively low risk of morbidity and mortality as compared with esophagectomy, ER can still result in severe complications such as perforation, bleeding, and postoperative stricture [25]. There are also procedure-related risks for insufficient, piecemeal resection [26]. This is worrisome because local recurrence can arise from small remnants of cancer left at the margins of a resection [27]. Minimizing the chance of local recurrence is an absolute priority when curing the cancer is the primary therapeutic goal. However, for some patients, especially those of advanced age or with comorbidities, a major surgery may carry the risk of overtreatment and diminish postoperative quality of life [8, 28]. To make the best choice for patients, we need to balance the risks and benefits of each treatment.
This study has some limitations, which should be considered when interpreting the findings. Although the data were collected prospectively into an institutional database and the patients’ medical records, the study still has drawbacks inherent to its retrospective nature including the potential for selection bias. The use of a propensity-matched analysis strengthens the findings, however, by normalizing some variables between the treatment groups. Also, this study has a follow-up of less than 5 years. Finally, this study was from single center and contains a relatively small sample size. A well-designed, randomized, and multicenter prospective study on esophagectomy after ER is desirable. Nevertheless, the results of this series should provide a useful foundation for future studies.
In conclusion, in patients with stage T1 esophageal cancer who underwent radical resection, ER before esophagectomy did not increase the difficulty of the esophagectomy operation or the incidence of postoperative complications. ER prior to esophagectomy also had no impact on survival or cancer recurrence during follow-up. These results suggest that ER can be recommended for patients with stage T1 esophageal cancer and does not compromise outcomes, including survival, even if esophagectomy is warranted eventually.
References
Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, Lin DM, Xie YQ, Yang L (2004) Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg 77(5):1740–1744
Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Holscher AH (2011) Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg 254(1):67–72
Kim DU, Lee JH, Min BH, Shim SG, Chang DK, Kim YH, Rhee PL, Kim JJ, Rhee JC, Kim KM, Shim YM (2008) Risk factors of lymph node metastasis in T1 esophageal squamous cell carcinoma. J Gastroenterol Hepatol 23(4):619–625
Spechler SJ (2015) Dysplasia in Barrett’s esophagus: limitations of current management strategies. Am J Gastroenterol 100(4):927–935
Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C (2007) Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy 39(1):30–35
Das A, Singh V, Fleischer DE, Sharma VK (2008) A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 103(6):1340–1345
Nealis TB, Washington K, Keswani RN (2011) Endoscopic therapy of esophageal premalignancy and early malignancy. J Natl Compr Cancer Netw: JNCCN 9(8):890–899
Choi JY, Park YS, Jung HY, Ahn JY, Kim MY, Lee JH, Choi KS, Kim do H, Choi KD, Song HJ, Lee GH, Cho KJ, Kim JH (2011) Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc 73(5):881–889
Park JS, Youn YH, Park JJ, Kim JH, Park H (2016) Clinical outcomes of endoscopic submucosal dissection for superficial esophageal squamous neoplasms. Clin Endosc 49(2):168–175
Qin X, He S, Zhang Y, Xue L, Lu N, Wang G (2014) Diagnosis and staging of superficial esophageal precursor based on pre-endoscopic resection system comparable to endoscopic resection. BMC Cancer 14:774
Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ (2014) Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst 106(7):dju133
Davison JM, Landau MS, Luketich JD, McGrath KM, Foxwell TJ, Landsittel DP, Gibson MK, Nason KS et al (2016) A model based on pathologic features of superficial esophageal adenocarcinoma complements clinical node staging in determining risk of metastasis to lymph nodes. Clin Gastroenterol Hepatol 14(3):369–377
Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L, Rabenstein T, Vieth M, Stolte M, Ell C et al (2014) Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 146(3):652–660
Gertler R, Stein HJ, Schuster T, Rondak IC, Höfler H, Feith M et al (2014) Prevalence and topography of lymph node metastases in early esophageal and gastric cancer. Ann Surg 259(1):96–101
Participants in the Paris Workshop (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 58 (6 Suppl): S3–S43
Van Westreenen HL, Westerterp M, Bossuyt PM, Pruim J, Sloof GW, van Lanschot JJ, Groen H, Plukker JT (2004) Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22(18):3805–3812
Lightdale CJ, Kulkarni KG (2005) Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 23(20):4483–4489
Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y, Kato H, Yamaguchi H, Saito D, Umemura S (2006) Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Modern Pathol 19(3):475–480
Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoleon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T (2007) Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy 39(1):24–29
NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. http://www.nccn.org. Version 1.2017. Updated March 21, 2017. Accessed 1 June 2017
Tomita N, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y, Tsurumaru M (2008) Lymphatic invasion according to D2-40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int 58(5):282–287
Sgourakis G, Gockel I, Lang H (2013) Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol 19(9):1424–1437
Holscher AH (2007) Endoscopic mucosal resection for early squamous-cell cancer of the esophagus–a dangerous game or standard treatment? Endoscopy 39(1):77–79
Badreddine RJ, Prasad GA, Lewis JT, Lutzke LS, Borkenhagen LS, Dunagan KT, Wang KK (2010) Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 8(3):248–253
Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M (2009) Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 70(5):860–866
Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H, Shinomura Y, Fujita M (2010) Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 72(2):255–264
Okada K, Tsuchida T, Ishiyama A, Taniguchi T, Suzuki S, Horiuchi Y, Matsuo Y, Yoshizawa N, Suganuma T, Omae M, Kubota M, Hirasawa T, Yamamoto Y, Inamori M, Yamamoto N, Nakajima A (2012) Endoscopic mucosal resection and endoscopic submucosal dissection for en bloc resection of superficial pharyngeal carcinomas. Endoscopy 44(6):556–564
Tang B, Bai JY, Zhao XY, Fan CQ, Yang X, Deng L, Yang SM, Yu J (2015) Endoscopic submucosal dissection for superficial esophageal cancer with near-circumferential lesions: our experience with 40 patients. Surg Endosc 29(8):2141–2148
Acknowledgements
This work is supported, in part, by Shanghai Science and Technology Commission Foundation key project (14JC140140), Grant from Science and Technology Commission of Shanghai Municipality (No. 15411951602; No. 16401970704), Trans-Century Training Programme Foundation for the Talents by the State Education Commission (NCET-13-0148), Grant from Health and Family Planning Commission of Shanghai Municipality (No. XYQ2011025; No. 2013ZYJB0301). We thank Shannon Wyszomierski, Ph.D. for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Shengfei Wang, Yangle Huang, Juntao Xie, Lingdun Zhuge, Longlong Shao, Jiaqing Xiang, Yawei Zhang, Yihua Sun, Hong Hu, Sufeng Chen, Toni Lerut, James Luketich, Jie Zhang, and Haiquan Chen have no conflicts of interest or financial ties to disclose.
Additional information
Shengfei Wang, Yangle Huang, and Juntao Xie contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, S., Huang, Y., Xie, J. et al. Does delayed esophagectomy after endoscopic resection affect outcomes in patients with stage T1 esophageal cancer? A propensity score-based analysis. Surg Endosc 32, 1441–1448 (2018). https://doi.org/10.1007/s00464-017-5830-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5830-4