Abstract
Background
Gastroparesis is difficult to treat and many patients do not report relief of symptoms with medical therapy alone. Several operative approaches have been described. This study shows the results of our selective surgical approach for patients with gastroparesis.
Materials and methods
This is a retrospective study of prospective data from our electronic medical record and data symptom sheet. All patients had a pre-operative gastric emptying study showing gastroparesis, an esophagogastroduodenoscopy, and either a CT or an upper GI series with small bowel follow-through. All patients had pre- and post-operative symptom sheets where seven symptoms were scored for severity and frequency on a scale of 0–4. The scores were analyzed by a professional statistician using paired sample t test.
Results
58 patients met inclusion criteria. 33 had gastric stimulator (GES), 7 pyloroplasty (PP), 16 with both gastric stimulator and pyloroplasty (GSP), and 2 sleeve gastrectomy. For patients in the GSP group, the second procedure was performed if there was inadequate improvement with the first procedure. There was no mortality. The follow-up period was 6–316 weeks (mean 66.107, SD 69.42). GES significantly improved frequency and severity for all symptoms except frequency of bloating and postprandial fullness. PP significantly improved nausea and vomiting severity, frequency of nausea, and early satiety. Symptom improvement for GSP was measured from after the first to after the second procedure. GSP significantly improved all but vomiting severity and frequency of early satiety, postprandial fullness, and epigastric pain.
Conclusion
All procedures significantly improved symptoms, although numbers are small in the PP group. GES demonstrates more improvement than PP, and if PP or GES does not adequately improve symptoms GSP is appropriate. In our practice, gastrectomy was reserved as a last resort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastroparesis is a syndrome in which there is delayed gastric emptying in the absence of a mechanical obstruction. The symptoms include nausea, vomiting, bloating, early satiety, and upper abdominal pain [1]. The incidence of gastroparesis as seen in a large population-based study is 2.4 per 100,000 person-years for men and 9.8 per 100,000 person-years for women, and the prevalence as seen in the same study is 9.6 per 100,000 persons for men and 38 per 100,000 persons for women [2]. The most commonly cited causes for gastroparesis include idiopathic (36%), diabetic (29%), or post-surgical (13%). Rare causes can include medication induced, infiltrative processes such as scleroderma or amyloidosis, spinal cord injury, or central nervous system disorder [3].
Primary treatment for gastroparesis is dietary modification by limiting foods that are fatty, acidic, spicy, or non-digestible fiber, as well as optimization of glycemic control [4]. For failed conservative treatment, medical treatment consists mainly of antiemetics, gastric motility agents, and antacids [5]. Surgery is considered for patients failing medical treatment and includes gastric tubes for venting, jejunal tubes for feeding, pyloroplasty, gastric stimulator placement, and various types of gastrectomy [6].
Gastric electrical stimulation has shown significant success. In a study in which 19 gastric stimulation devices were placed laparoscopically, vomiting frequency decreased in 75% of patients with gastroparesis secondary to diabetes mellitus, and 100% of patients with idiopathic gastroparesis [7]. Laparoscopic pyloroplasty has also proven effective. A study of 26 patients undergoing Heineke-Mikulicz pyloroplasty resulted in prokinetic use decreasing from 89 to 14% of patients, as well as significant improvements in nausea, vomiting, bloating, and GERD symptoms, with 83% of patients indicating improvement at 1-month follow-up [8]. Subtotal or total gastrectomy is often reserved for the cases that are refractory to all other options. This appears to work best for patients who have post-surgical gastroparesis; however, completion gastrectomy demonstrated success in only 43% of patients [9]. The purpose of our study is to determine best surgical treatment given our experience.
Materials and methods
This is a retrospective review of data from our electronic medical record for patients with gastroparesis treated at Ochsner Clinic between 2010 and 2016. This study includes 58 patients (47 female, 11 male) with a mean age of 48 (range 20–73). Of the 58 patients included in the study, 29 had gastroparesis due to diabetes, 25 had idiopathic gastroparesis, and 5 had post-surgical gastroparesis. There was no significant difference between the four treatment groups for BMI or baseline symptom scores. (Table 1) Patients underwent pyloroplasty only, insertion of a gastric stimulator only, both pyloroplasty and gastric stimulator, or sleeve gastrectomy. No patients underwent both pyloroplasty and gastric stimulator placement at the same time. A second procedure was only performed if there was poor resolution of symptoms with the first procedure. The mean length of follow-up ranged from 6 weeks to 80 months with an average length of follow-up of 17 months. All patients underwent a pre-operative gastric emptying study showing gastroparesis, esophagogastroduodenoscopy (EGD), and either CT or upper GI with small bowel follow-through.
Every patient filled out pre-operative symptom sheets, and post-operative symptom sheets were given at every follow-up appointment. Patients were called and a verbal symptom sheet was performed to improve the length of follow-up. The symptom sheet assesses frequency and severity of vomiting, nausea, early satiety, bloating, postprandial fullness, epigastric pain, and epigastric burning. Each symptom was given a score of 0–4. For severity, the scores are 0—absent, 1—mild (not influencing normal activities), 2—moderate (diverting from, but not urging modification of, usual activities), 3—severe (influencing usual activities severely enough to urge modification), and 4—extremely severe (requiring bed rest). For frequency, the scores are 0—absent, 1—rare (1 time/week), 2—occasional (2–4 times/week), 3—frequent (5–7 times/week), and 4—extremely frequent (>7 times/week).
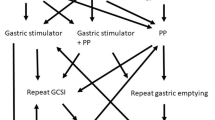
The main determinant for which operation was performed first was patient preference. Some patients were concerned about having a battery implanted with the gastric stimulator and elected to undergo pyloroplasty. Pyloroplasty was performed as a Heineke-Mikulicz pyloroplasty in which the pylorus was divided transversely using cautery and then sewn shut horizontally. Endoscopy was performed selectively following the pyloroplasty to assess for air leaks. If patients had post-operative symptoms of leak they underwent barium swallow or CT. This was performed either laparoscopically (n = 2), robotic-assisted laparoscopic (n = 15), or open (n = 3). For placement of the gastric stimulator, a distance of 10 cm was measured from the pylorus on the greater curvature of the stomach. Two neurogastric stimulator leads were placed 1 cm apart parallel to each other. Endoscopy was performed to make sure that the leads were not transmural; after this was verified the leads were sutured in place. The neurostimulator was placed in a subcutaneous pocket in the abdominal wall and sutured in place and turned on to basic levels. Gastric stimulator placement was performed either laparoscopically (n = 17) or open (n = 19). Sleeve gastrectomy was performed laparoscopically (1) or open (1).
We compared pre-operative scores for each symptom to the symptom score sheet from the longest length of follow-up for each patient. For those who were in the combination group, the comparison was made between the symptom sheet filled out pre-operatively for the second procedure and post-operatively to see any additional benefit separate from the first operation. The improvement in symptom score from baseline to follow-up was assessed using a paired sample, student’s t-test. Baseline symptom scores and BMI were analyzed using ANOVA or Kruskal–Wallis Test for data that was not normally distributed. A p value of <0.05 was considered statistically significant.
Results
There were 58 patients with completed pre-operative symptom sheets who could be included in the study. Of these, 33 had a gastric stimulator placed as their first or only procedure, 3 had a gastric stimulator placed as their second procedure, 7 had pyloroplasty as their first or only procedure, and 13 had pyloroplasty as their second procedure, and 2 underwent a sleeve gastrectomy. The combined group includes the 3 patients who had a gastric stimulator placed as their second procedure and the 13 patients who had a pyloroplasty as their second procedure.
The length of follow-up ranged from 6 weeks to 80 months with an average length of follow-up of 17 months. For the gastric stimulator only group (n = 33), there was statistically significant improvement in symptom severity and frequency for all symptoms (p < 0.05) except for frequency of bloating and postprandial fullness (p = 0.451 and p = 0.092, respectively). For the pyloroplasty only group (n = 7), there was statistically significant improvement in severity of vomiting (p = 0.007), severity and frequency of nausea (p = 0.038 and p = 0.015, respectively), and frequency of early satiety (p = 0.045); all other symptoms were not significantly improved. For the combined group (n = 16), symptom improvement was measured from after the first operation to post-operatively for the second operation to assess for additional improvement in symptoms. Of the 16 patients in this group, 13 had a pyloroplasty as their second operation and 3 had a gastric stimulator placed. There was statistically significant improvement in all symptoms except for severity of vomiting (p = 0.117), frequency of early satiety (p = 0.869), postprandial fullness (p = 0.758), and epigastric pain (p = 0.348) (Table 2). For the sleeve gastrectomy group, meaningful statistical analysis was unable to be performed due to the small sample size of two patients.
The average post-operative length of stay for the gastric stimulator only group was 2 days (±1.6); for the pyloroplasty only group the average length of stay was 2 days (±0.9), and for the combination group the average length of stay after the second operation was 5 days (±5).
There was no mortality during our follow-up. 30-day complications for the gastric stimulator only group were hematoma formation (1), pain at the stimulator site (2) with one patient having their stimulator removed a year after original placement, wound dehiscence (1), and post-operative DKA (1). Complications for patients who underwent gastric stimulator placement as their second operation were pain at the stimulator site (1) and superficial wound infection (1). There were no 30-day complications for the pyloroplasty group.
Discussion
The main surgical treatment options for gastroparesis include gastric stimulation, pyloroplasty, and subtotal gastrectomy. Gastrointestinal pacing through the use of intraluminal electrical stimulation has been performed since the early 1960s [10]. The device used today for treatment of gastroparesis, known as Enterra Therapy (Medtronic, Inc, Minneapolis, MN), was FDA approved in 2000 as a humanitarian device of exemption for gastroparesis refractory to medical management [11]. In 2003, the first double-blind cross-over trial was performed investigating the efficacy of gastric stimulation using Enterra Therapy. The results of this trial were promising, showing significant improvement in vomiting frequency and patient preference for the device being turned ‘on’ [12]. In this trial, weekly vomiting frequency decreased by ≥50% in 70–80% of patients [12].
Surgical pyloroplasty has been used for decades as a procedure to assist with gastric drainage in patients with obstruction, particularly from ulcer disease. More recently, it has been used for gastroparesis without stricture. This is based on the principle that increased pyloric tone slows gastric emptying and pyloric disruption improves forward flow. In a study of 28 patients, pyloroplasty has been shown to normalize GET in 71% with significant improvement in nausea, vomiting, bloating, and abdominal pain 3 months after pyloroplasty [8]. Additionally, in a recent large study of 177 patients, laparoscopic pyloroplasty alone was shown to improve gastric emptying time (GET) in 86% of patients [13].
Gastrectomy is traditionally reserved for patients with refractory post-surgical gastroparesis [14]. In a study of 62 patients with refractory post-surgical gastroparesis, subtotal or completion gastrectomy provided symptomatic improvement in 67% of patients [9]. Recently, there has been interest in sleeve gastrectomy for treatment of gastroparesis after increased gastric empting was observed in bariatric patients treated with sleeve gastrectomy [14]. To date, there have only been two small case series reporting sleeve gastrectomy results for gastroparesis. One series of four patients with diabetic gastroparesis reported resolution of nausea and vomiting for three of the patients [15]. The other study was a case series of nine patients who all reported subjective symptomatic improvement [16].
The mechanism of action through which the gastric stimulator improves symptoms is not well understood. It is unlikely that symptom improvement is through normalization of gastric emptying rate. In a study of 63 patients treated with gastric stimulation, only 22% of patients were shown to have a normal GET at 1 year. Overall, there was a 10% reduction in mean gastric retention at 2 h and a 7% reduction at 4 h. Similar symptom improvement was seen between those who had normalized GET and those who remained with delayed GET at 1 year [17]. Although there is no improvement in gastric slow wave or GET, stimulation with high frequency gastric stimulation has been shown to enhance slow wave amplitude and propagation velocity which improves nausea and vomiting [18]. Gastric stimulation has also been shown to increase the gastric maximal tolerable volume and symptom improvement may be due to modification of gastric sensation to distension [19].
While gastric stimulation alone does not normalize GET, addition of pyloroplasty has been shown to improve GET by 64% compared to the 7% improvement with gastric stimulator alone in a study of 49 patients with gastroparesis. There was also a significant improvement in symptoms, 71% improvement in total symptoms, with combined gastric stimulator and pyloroplasty without an increase in adverse events [20]. Historically pyloroplasty and gastric stimulator placement have not been performed at the same time over concerns for contamination of the stimulator leads at the time of surgery due to the entry into the stomach while performing the pyloroplasty. There have been reports of contaminated gastric stimulator leads being linked to delayed stimulator pocket infections [21]. However, a prospective study of 27 patients who underwent simultaneous gastric stimulator and pyloroplasty showed no increase in wound infection rate [22]. Although no increased infection rate was seen in this study, the sample size was small and there was no randomization or blinding.
Our study investigates the long-term efficacy of gastric stimulation, pyloroplasty, and combined gastric stimulation with pyloroplasty for the treatment of refractory gastroparesis. The gastric stimulator only group demonstrated the greatest symptomatic relief, with significant improvement in 12/14 symptoms. The only symptoms without significant improvement were frequency of bloating and frequency of postprandial fullness. In the 2003 double blind trial by Abell et al. assessing efficacy of gastric stimulation had similar findings. Abell et al. found significant improvement in severity of vomiting, nausea, early satiety, bloating, and epigastric pain at 6 or 12 months [12].
In our study, pyloroplasty alone demonstrated the least amount of improvement with symptom improvement in 4/12 categories. The four symptoms with significant improvement were severity of vomiting, severity and frequency of nausea, and frequency of early satiety. Other studies assessing the efficacy of pyloroplasty not only have found similar improvement in nausea and vomiting as seen in our study, but they have also found improvements in severity of bloating and abdominal pain that we did not [8, 13].
Combining both gastric stimulator and pyloroplasty demonstrated an additional symptomatic improvement in those patients who did not have adequate relief from the initial procedure. Our combined group demonstrated great symptomatic improvement with the majority of symptoms significantly improved (10/14). The four symptoms that were not significantly improved were severity of vomiting, frequency of early satiety, postprandial fullness, and epigastric pain. The significant improvement in symptom severity seen in our combined group is similar to the results of the recent prospective study of combined gastric stimulator and pyloroplasty operations by Davis et al. In this study, they showed significant improvement in severity of 6/7 symptoms: nausea, vomiting, early satiety, bloating, post-prandial fullness, and epigastric pain. In Davis et al., gastric stimulator and pyloroplasty were performed at the same time [22]. In the Davis et al. study, the entire population underwent simultaneous pyloroplasty and gastric simulator placement; therefore, they were not able to make any comparison to a control group. In our study, we were able to compare the combination group to each independent operation for symptom improvement. Additionally, since our operations were performed at separate times, we were able to assess for an additional improvement in symptoms with the addition of the second operation. This significant improvement we saw in 10/14 symptoms was not from baseline but is subsequent improvement from patient symptom scores after their first operation.
In our practice, gastrectomy is reserved as a last resort and only two patients underwent this procedure so we are unable to draw any conclusions regarding gastrectomy. The largest series assessing the efficacy of subtotal or completion gastrectomy was performed by Forstner-Barthell et al and includes 62 patients. They demonstrated significant reduction in nausea, vomiting, and postprandial pain [9].
Using the results of this project, we suggest gastric stimulator as initial treatment to patients unless there is a contraindication. Some patients have an aversion to the foreign body or difficulty with return for stimulator adjustments and in that case would perform pyloroplasty. Since some patients do not get significant improvement with initial treatment, we would go on to add pyloroplasty or stimulator but further work needs to be done on the best timing for this. We do not know how to assess when a patient should undergo the next procedure based on inadequate symptom improvement. In addition, further work needs to be done on simultaneous stimulator and pyloroplasty prior to suggesting such an approach.
One of the major limitations of this study includes the study design being retrospective chart review rather than a prospective randomized controlled trial. Additionally, the sample size for patients with a pyloroplasty alone was small with a sample size of seven. We are planning on continuing to collect patient data and hope to have a larger sample size for each group to analyze in the future.
Advances in the treatment of gastroparesis could benefit from a larger study of simultaneous pyloroplasty and gastric stimulator placement in those patients with refractory gastroparesis.
References
Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L (2013) Clinical guideline: management of gastroparesis. Am J Gastroenterol 108:18–37 (quiz 38)
Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ (2009) The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 136:1225–1233
Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW (1998) Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci 43:2398–2404
Wytiaz V, Homko C, Duffy F, Schey R, Parkman HP (2015) Foods provoking and alleviating symptoms in gastroparesis: patient experiences. Dig Dis Sci 60:1052–1058
Homko CJ, Duffy F, Friedenberg FK, Boden G, Parkman HP (2015) Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil 27:501–508
Borrazzo EC (2013) Surgical management of gastroparesis: gastrostomy/jejunostomy tubes, gastrectomy, pyloroplasty, gastric electrical stimulation. J Gastrointest Surg 17:1559–1561
McKenna D, Beverstein G, Reichelderfer M, Gaumnitz E, Gould J (2008) Gastric electrical stimulation is an effective and safe treatment for medically refractory gastroparesis. Surgery 144:566–572 (discussion 572–564)
Hibbard ML, Dunst CM, Swanstrom LL (2011) Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg 15:1513–1519
Forstner-Barthell AW, Murr MM, Nitecki S, Camilleri M, Prather CM, Kelly KA, Sarr MG (1999) Near-total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long-term results in 62 patients. J Gastrointest Surg 3:15–21 (discussion 21–13)
Bilgutay AM, Lillehei CW, Wingrove R, Griffen WO, Bonnabeau RC (1963) gastrointestinal pacing: a new concept in the treatment of ileus. Biomed Sci Instrum 1:377–383
Soffer EE (2012) Gastric electrical stimulation for gastroparesis. J Neurogastroenterol Motil 18:131–137
Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W (2003) Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 125:421–428
Shada AL, Dunst CM, Pescarus R, Speer EA, Cassera M, Reavis KM, Swanstrom LL (2016) Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc 30:1326–1332
Sarosiek I, Davis B, Eichler E, McCallum RW (2015) Surgical approaches to treatment of gastroparesis: gastric electrical stimulation, pyloroplasty, total gastrectomy and enteral feeding tubes. Gastroenterol Clin North Am 44:151–167
Bagloo M, Besseler M, Ude A (2010) Sleeve gastrectomy for the treatment of diabetic gastroparesis. In: Proceedings 12th world congress of endoscopic surgery april, pp 14–17
Meyer A, Pallati P, Shaligram A, Oleynikov D, Goede M (2012) Partial longitudinal gastrectomy: a novel curative approach for gastroparesis.In: Proceedings of the 2012 annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons: San Diego, pp 249
Lin Z, Hou Q, Sarosiek I, Forster J, McCallum RW (2008) Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil 20:464–470
Lin Z, Forster J, Sarosiek I, McCallum RW (2004) Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil 16:205–212
Gourcerol G, Ouelaa W, Huet E, Leroi AM, Ducrotte P (2013) Gastric electrical stimulation increases the discomfort threshold to gastric distension. Eur J Gastroenterol Hepatol 25:213–217
Sarosiek I, Forster J, Lin Z, Cherry S, Sarosiek J, McCallum R (2013) The addition of pyloroplasty as a new surgical approach to enhance effectiveness of gastric electrical stimulation therapy in patients with gastroparesis. Neurogastroenterol Motil 25:e134–e180
Gould JC, Dholakia C (2009) Robotic implantation of gastric electrical stimulation electrodes for gastroparesis. Surg Endosc 23:508–512
Davis BR, Sarosiek I, Bashashati M, Alvarado B, McCallum RW (2017) The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg 21:222–227
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Arthur, Richardson, and L Slattery have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Arthur, L.E., Slattery, L. & Richardson, W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc 32, 977–982 (2018). https://doi.org/10.1007/s00464-017-5775-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5775-7