Abstract
Background
Dedicated stents for treatment of cervical anastomotic leakage are currently unavailable. In this study, we aimed to assess the feasibility and efficacy of using custom-designed stents for treatment of cervical anastomotic leakage after esophagectomy.
Methods
The stents were designed according to the location and size of the leakage and the residual esophageal length as determined by esophagography in each case. It had a cup-shaped upper end and a globular lower end and a total height of 60–85 mm. The diameter of the upper cup-shaped part was 24–26 mm and the length 20–25 mm. The cup part and the stent main body were connected at a right angle. Data from cervical anastomotic leakage patients treated with these stents were retrospectively analyzed.
Results
Data from a total of 27 patients with cervical anastomotic leakage were retrospectively analyzed. The custom-designed esophageal covered stents were placed successfully at the first attempt in 24 cases (88.9%). The total operative time was 5–15 min. The stents were removed 7 days to 3 months after leakage healing. Follow-up showed no leakage recurrence; three patients had anastomosis scar strictures. Fifteen patients died (median survival 13.4 months) and nine survived.
Conclusion
Placement of the novel esophageal covered stent is a minimally invasive, efficacious treatment option for the patients with cervical anastomotic leakage after esophagectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgical resection of esophageal cancer tends to employ wide resection with cervical esophagogastric anastomosis, following which the incidence of postoperative leakage is much higher than with intrathoracic anastomosis [1, 2]. In patients with a small cervical anastomotic leakage with only mild exudate from the incision, the leakage will heal in a short period of time by conservative treatments such as incision debridement and dressing, anti-inflammatory therapy, and prohibition of oral food intake. However, in patients with a larger anastomotic leakage with greater amounts of surgical incision exudate, the leakage cannot be healed by conservative treatment alone, leading to decreased quality of life. Due to the presence of digestive enzymes in the saliva and esophageal secretions, it is difficult to heal a leakage by surgical debridement. Patients with cervical anastomotic leakage after esophagectomy combined with mediastinal abscess are at risk of life threatening descending mediastinitis, sepsis, and hemorrhage [3, 4].
There have been many reports on the use of esophageal covered stents in the treatment of esophageal leakage, esophageal rupture, and intrathoracic anastomotic leakage [5–7]; however, dedicated stents for treatment of cervical anastomotic leakage after esophagectomy are currently unavailable. The main reason is that the postoperative residual esophagus is too short for the placement of conventional esophageal covered stents, as they will tend to migrate, and therefore fail to effectively cover the leakage.
To overcome this problem and avoid some of the known adverse effects of the currently available esophageal covered stents, we designed a novel stent for cervical anastomotic leakage based on the individual postoperative cervical anatomy and lesion characteristics after esophagectomy. This study aimed to assess the efficacy and safety of this treatment in patients with cervical anastomotic leakage after esophagectomy.
Materials and methods
This study was approved by the Ethics Committee. Informed consent was obtained from the patients. Retrospective analysis was conducted on the clinical data of patients with cervical anastomotic leakage after esophagectomy treated with the novel esophageal covered stent in department of interventional radiology, including medical records, imaging data, operative records, and follow-up results. Cervical anastomotic leakage after esophagectomy was diagnosed and confirmed by esophagography with water-soluble contrast and chest computed tomography. We excluded patients with intrathoracic anastomotic leakage after esophagectomy and esophageal rupture, as well as patients with cervical anastomotic leakage after esophagectomy treated using other methods, i.e., reoperation, conservative treatment, or endoscopic intervention.
Stent design
The stents used in this study were modified versions of the conventional nitinol esophageal stent, and were custom designed according to the anatomic peculiarities and the lesion characteristics of individual patients. The stents comprised a single nickel–titanium shape-memory alloy wire (diameter 0.18 mm) with polyethylene film coated on the surface. The upper end was cup shaped and the lower end globular; the total height of the stent was 60–85 mm. The upper part of each stent was cup shaped with a diameter of 24–26 mm and length 20–25 mm and manufactured with a 2-mm polyethylene film soft side on the edge and a double-fold retrievable wire. The cup-shaped upper end and the stent main body were connected at a right angle. The stent body was tubular in shape with a diameter of 18–20 mm and length 10 mm. The stents were produced by Nanjing Micro-Tech Medical Company, Nanjing, China. After receipt of the specifications, it took 3–4 days for the company to manufacture an individual stent and supply it to our hospital. An example stent is shown in Fig. 1.
Stent placement procedure
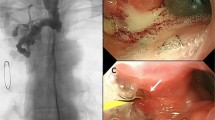
All interventional procedures were performed under conscious sedation, with oral lidocaine gel for local anesthesia. A positive oblique esophagogram obtained after oral administration of water-soluble iodinated contrast confirmed the location and size of leakage and the position of the inferior piriform recess. Under fluoroscopic guidance, a 5F catheter over a wire were inserted through the mouth into the esophagus and passed beyond the gastroesophageal anastomotic leakage into the proximal jejunum via the stomach and pylorus; the catheter was then withdrawn. The individually designed stent and its delivery system were placed over the stiff guidewire. The upper end of the stent was located in the upper esophagus (20 mm beneath the lower pole of the piriform recess) after slow release of the stent. After successful placement, the guidewire was kept in place while the delivery system was removed carefully (Fig. 2A–D).
Female, 64 years old, 7 days after esophageal cancer resection. A, B Positive oblique esophagography shows contrast agent overflow out of the neck through the gastroesophageal anastomosis (arrows show the leakage location). C, D Review esophagography shows that the leakage was completely blocked after stent placement
Anteroposterior and oblique esophagography were used to confirm esophageal leakage coverage and to check the stent position. The double-fold retrievable wire and proximal guidewire were brought into the nasal cavity from the mouth using an oral suction catheter if the stent position was appropriate. The double-fold retrievable wire was fixed at the side of the ear. A transnasal jejunal feeding tube was placed in the proximal jejunum over the guidewire.
In patients with cervical anastomotic leakage after esophagectomy combined with mediastinal abscess, a mediastinal abscess drainage catheter was placed through the esophageal leakage orifice via the nasal cavity before stent placement. A catheter over wire was inserted into the esophagus through the nose under fluoroscopic guidance, reaching the mediastinal abscess through the gastroesophageal anastomosis leakage orifice. The wire was withdrawn and 2 ml pus from the abscess was collected through the catheter for bacterial culture and sensitivity testing. The catheter tip was confirmed to be located at the distal end of the abscess cavity by contrast injection, and the catheter was then exchanged with a 5F straight catheter with multiple side holes. The abscess cavity was repeatedly and slowly rinsed with saline via the new catheter until the drainage fluid became clear. The catheter was then fixed externally (Fig. 3A–D).
Male, 59 years old, 3 days after esophageal cancer surgery. A, B Positive oblique esophagography shows contrast agent in the gastroesophageal anastomosis spilling out through the neck drainage tube. The contrast agent is overflowing into the right upper mediastinum (shown by the arrows). C, D Review positive oblique esophagography shows complete blockage of the leakage, with no contrast agent overflow
Postoperative management
Local dressing, anti-inflammatory drugs, and enteral nutrition were provided after the procedure. Patients were closely observed for pharyngeal foreign body sensation, pain, hoarseness, stuffiness, and bleeding.
Esophagograms were performed every 5–7 days. If cervical leakage showed sudden increase and imaging indicated contrast spillover from the neck or penetration into the mediastinal abscess cavity, indicating stent migration, the stent position was adjusted by slowly pulling the retrievable wire on the stent under fluoroscopic guidance. The stent was removed by interventional technique after the leakage had completely healed.
In patients with mediastinal abscess, irrigation with 5–20 ml saline (according to abscess size) via the drainage catheter was performed 1–2 times/day until the aspirate was clear. Subsequently, continuous negative pressure was maintained in the drainage catheter. The color of the drainage fluid, the degree of turbidity, and the drainage amount were recorded. Shrinkage of the abscess was monitored by contrast injection via the drainage catheter and radiography every 5–7 days, with additional CT performed if necessary. The mediastinal drainage catheter was removed after the abscess cavity had healed and then the stent was removed after 1 week.
Efficacy assessment and data analysis
Treatment was considered successful if the leakage healed and the patient resumed normal oral intake. Treatment was considered to have failed if the leakage did not heal, the stent could not be removed, or the leakage recurred after removal of the stent. Any need for further surgical treatment was also classified as failed treatment. GraphPad Prism 5.0 (San Diego, CA) was used for statistical analysis. All data were presented as the mean ± standard deviation.
Results
We retrospectively analyzed 27 cases of cervical anastomotic leakage in esophageal cancer patients with complete clinical and imaging data. The study sample comprised 19 males and eight females, aged 44–74 years (median 60.81 ± 7.01 years) (Table 1). In 22 patients, there were large amounts of saliva or purulent secretion spillover in the neck incision area, the fistula was difficult to heal, or surgical dressing, drainage, and other treatments would be ineffective according to the clinical assessment. Five patients had anastomotic leakage plus mediastinal abscess. All patients were referred to the department of interventional radiology (an independent treatment center in our hospital) from the department of thoracic surgery or from a local hospital. The available treatment options were explained to the patients by thoracic surgeons, and they were offered the option of treatment with the novel esophageal stent to cover the cervical anastomotic leakage. Clinical information for the 27 patients is shown in Table 1.
The individually designed esophageal covered stents were placed successfully at the first attempt in 24 cases (88.9%), with total operative time of 5–15 min. The stent was placed a little too high in one case (3.7%) but was removed and replaced successfully. The stent was replaced a little too far down in two cases (7.4%) but was adjusted to an appropriate location by pulling the double-fold retrievable wire. Thus, the total technical success rate was 100%. Jejunal feeding tubes were placed in all patients after successful stent implantation. In the five patients with cervical anastomosis leakage combined with mediastinal abscess, a transnasal mediastinal abscess drainage catheter was successfully placed, with daily aspiration of 20–60 ml of pus by continuous negative pressure suction (8–10 mm Hg).
The esophageal covered stents were kept in place for 7 days to 3 months (median 39.59 ± 23.14 days). All stents were removed via the interventional techniques after the leakage had healed, without obvious complications such as hemorrhage, esophagus rupture, or leakage recurrence.
Of the 27 patients, 24 were followed up for 6 months or more; three patients were lost to follow-up. The patients with follow-up showed no leakage recurrence. Anastomotic fibrotic stricture occurred in three patients, but food intake could be resumed after balloon dilatation in all three patients. Fifteen patients died with a median survival time of 13.4 months during the follow-up. However, these deaths were unrelated to the stent placement, eight patients died because of tumor recurrence, four deaths were caused by tumor progression-induced cachexia, two patients died of dyspnea, and there was one sudden death. Nine patients currently remain alive: two have mild gastroesophageal reflux, one suffers occasional choking with water but is eating normally, and six patients have returned to a normal life.
Stent placement complications and treatment
In this study, all patients complained of pharyngeal foreign body sensation; 10 (37%) patients experienced different levels of pain, but in all cases it gradually decreased or disappeared. One patient with severe pain was given intramuscular injection of tramadol. Gastroesophageal reflux occurred in four patients, in whom a nasogastric suction tube was placed in the gastric antrum. Twelve patients (44.4%) experienced stent dislocation. The stent migrated up to the pharynx in two cases (7.4%) because of intense vomiting, starting 1–3 days after stent placement. The stents were removed and replaced. In nine patients (33.3%) the stents were displaced downwards; in one patient (3.7%) the stent was displaced into the stomach. These cases were diagnosed at esophagography performed 3–7 days after stent placement; in all cases the location of stent was easily adjusted by pulling the double-fold retrievable wire under fluoroscopic guidance.
Discussion
For patients with large postoperative cervical anastomotic leakages and heavy local infections, conventional treatment alone cannot inhibit secretion of saliva. Corrosion from gastric juice and uncontrolled infection lead to failure of leakage healing in the long term. This not only reduces the patient’s quality of life but can also lead to anastomotic stricture resulting from granulation tissue overgrowth during the gradual leakage healing process [8]. Patients with cervical anastomotic leakages combined with mediastinal abscesses generally die from sepsis or hemorrhage as a consequence of inappropriate or delayed treatment [9].
Dedicated stents for cervical anastomotic leakage after esophagectomy are currently not commercially available. According to the literature, the failure rate is high with conventional esophageal covered stents [10]. The residual normal esophagus is short, with a length of 3–5 cm due to subtotal removal during the anastomosis procedure. After placement of conventional esophageal covered stents, normal neck movements (the mobility of neck is great), the complex cervical esophageal blood vessels and nerve distribution adjacent to the posterior wall of the trachea, and loose connective tissue in the surrounding area lead to severe foreign body sensation, and there are problems with fixation and stent shifting.
The novel stent used in this study was a fully coated, with a 2-mm polyethylene film soft edge above the stent that reduces irritation of the esophageal wall, relieves foreign body sensation, and prevents esophageal stricture at the upper edge of the stent. Each patient’s esophageal residual length and leakage location and size were measured by esophagography, and individualized stents were designed and produced. The long, cup-shaped upper part of these stents has greater apposition with the esophagus and reduces the risk of oral secretions passing into the leakage through the gap between the stent and esophagus. The tubular intermediate part of the stent acts to close the leakage. The lower part of the device was ball-shaped to avoid it cutting the gastric wall. The double-fold retrievable wire facilitates adjustment or removal of the stent.
Stent migration is a common problem with stents placed across esophagogastric anastomoses [11]. Bège et al. [12] reported migration of the stent in 59% of their patients, requiring replacement with either a longer stent or with two nested stents. The mean time until resolution of leakage was 86 days from the start of endoscopic management, and a mean of 4.4 endoscopies were performed per patient. In comparison with intrathoracic anastomosis, cervical esophagogastric anastomosis results in a shorter residual esophagus and, consequently, poorer stent stability and greater likelihood of stent migration. Adjuncts to stent placement, such as endoscopic clipping or suturing, have been advocated for prevention of stent migration [13]. Although the stent migration rate was high in the present study, the stent position was easily adjusted by the interventional technique.
The goal of stenting is closure of the anastomotic defect and maintenance of esophagogastric anastomotic integrity. The novel stent successfully covered the leakage and completely prevented saliva, food, gastric content, and bacteria from spilling out of the leakage orifice. Leakage resolution was accelerated when stent treatment was combined with complete leakage drainage, surgical dressing, and provision of sufficient enteral nutrition through a jejunal feeding tube. The possibility of stent migration can be reduced by (1) increasing the cup holder diameter and length according to patient tolerance; (2) prohibiting oral intake of water and providing enteral nutrition via a transnasal jejunal feeding tube; and (3) periodically reviewing the stent location by esophageal imaging.
There remain some unresolved issues with the technology presented in the current study. Issues such as how to reduce the stent migration rate and shorten the leakage healing time need further investigation due to the small number of patients.
In summary, the novel esophageal covered stent is a minimally invasive and efficacious option for the treatment of cervical anastomotic leakage after esophagectomy. Removing the stent or adjusting its position is easily done under fluoroscopic guidance.
References
Kassis ES, Kosinski AS, Ross P Jr, Koppes KE, Donahue JM, Daniel VC (2013) Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 96:1919–1926
Kayani B, Jarral OA, Athanasiou T, Zacharakis E (2012) Should oesophagectomy be performed with cervical or intrathoracic anastomosis? Interact Cardiovasc Thorac Surg 14:821–827
Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC (2004) Evolving options in the management of esophageal perforation. Ann Thorac Surg 77:1475–1483
Minnich DJ, Yu P, Bryant AS, Jarrar D, Cerfolio RJ (2011) Management of thoracic esophageal perforations. Eur J Cardiothorac Surg 40:931–937
Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje G, Hölscher AH, Metzger R (2009) Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 23:2258–2262
Han X, Zhao YS, Fang Y, Qi Y, Li X, Jiao D, Ren K, Wu G (2016) Placement of transnasal drainage catheter and covered esophageal stent for the treatment of perforated esophageal carcinoma with mediastinal abscess. J Surg Oncol 114:725–730
Gang W, Zhao YS, Fang Y, Qi Y, Li X, Jiao D, Ren K, Han X (2017) Treatment of spontaneous esophageal rupture with transnasal thoracic drainage and temporary esophageal stent and jejunal feeding tube placement. J Trauma Acute Care Surg 82(1):141–149
D’Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS (2011) Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg 142:39–46
Rajan PS, Bansal S, Balaji NS, Rajapandian S, Parthasarathi R, Senthilnathan P, Palanivelu C (2014) Role of endoscopic stents and selective minimal access drainage in oesophageal leaks: feasibility and outcome. Surg Endosc 28:2368–2373
Freeman RK, Ascioti AJ, Giannini T, Mahidhara RJ (2012) Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg 94:959–965
van Halsema EE, van Hooft JE (2015) Clinical outcomes of self-expandable stent placement for benign esophageal diseases: a pooled analysis of the literature. World J Gastrointest Endosc 7:135–153
Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Bradjanian S, Berdah SV, Brunet C, Grimaud JC, Barthet M (2011) An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 73:238–244
Winder JS, Pauli EM (2015) Comprehensive management of full-thickness luminal defects: the next frontier of gastrointestinal endoscopy. World J Gastrointest Endosc 7:758–768
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector. We thank the Nanjing Micro-Tech Medical Company for kindly providing the novel esophageal stents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Gang Wu, Meipan Yin, Yan Shi Zhao, Yi Fang, Yu Qi, Xiangnan Li, and Xinwei Han have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Wu, G., Yin, M., Zhao, Y.S. et al. Novel esophageal stent for treatment of cervical anastomotic leakage after esophagectomy. Surg Endosc 31, 5024–5031 (2017). https://doi.org/10.1007/s00464-017-5545-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5545-6