Abstract
Background
Pancreaticoduodenectomy (PD) is a complex operation with high perioperative morbidity and mortality, even in the highest volume centers. Since the development of the robotic platform, the number of reports on robotic-assisted pancreatic surgery has been on the rise. This article reviews the current state of completely robotic PD.
Materials and Methods
A systematic literature search was performed including studies published between January 2000 and July 2016 reporting PDs in which all procedural steps (dissection, resection and reconstruction) were performed robotically.
Results
Thirteen studies met the inclusion criteria, including a total of 738 patients. Data regarding perioperative outcomes such as operative time, blood loss, mortality, morbidity, conversion and oncologic outcomes were analyzed. No major differences were observed in mortality, morbidity and oncologic parameters, between robotic and non-robotic approaches. However, operative time was longer in robotic PD, whereas the estimated blood loss was lower. The conversion rate to laparotomy was 6.5–7.8%.
Conclusions
Robotic PD is feasible and safe in high-volume institutions, where surgeons are experienced and medical staff are appropriately trained. Randomized controlled trials are required to further investigate outcomes of robotic PD. Additionally, cost analysis and data on long-term oncologic outcomes are needed to evaluate cost-effectiveness of the robotic approach in comparison with the open technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic cancer is widely recognized as one of the most aggressive solid tumors and one of the most lethal in Western society. Despite considerable advances in surgical and oncologic treatment over the last 50 years, the median survival is still only approximately 21–24 months and the 5-year survival for all patients is only 5% [1]. Furthermore, only a minority of patients presenting with pancreatic cancer are candidates for surgical therapy due to the presence of either distant metastasis or locally invasive disease.
Pancreaticoduodenectomy (PD) has been universally accepted as the only chance for cure for patients with cancerous tumors of the head of the pancreas, malignant periampullary tumors, distal cholangiocarcinoma, cancer of the first and second portions of the duodenum and malignant or premalignant cystic pancreatic neoplasms, such as intraductal papillary mucinous neoplasms (IPMNs) or neuroendocrine pancreatic tumors (PNETs), when indicated [2]. The PD procedure was first described by Allessandro Codivilla in 1898 [3] and later popularized by Allen O. Whipple in 1935 [4] and is considered one of the most complex operations of the alimentary track owing to the combined challenge of careful dissection in close proximity to critical vascular structures and the restoration of enteric continuity with three anastomoses (pancreaticojejunostomy, hepaticojejunostomy and gastrojejunostomy) [5, 6]. Not surprisingly, the surgery has a high perioperative morbidity of 30–40% and mortality rate of 1–6% even at the highest volume centers [7].
In an effort to reduce the historically high rate of perioperative morbidity, minimally invasive surgical (MIS) approaches were applied to the field of pancreatic surgery. Gagner and Pomp [8] described the first laparoscopic PD over 20 years ago; however, this technique has not gained widespread popularity [9], due to the retroperitoneal location of the pancreas, its close relationship with major vascular structures and the tedious nature of the dissection required to optimize oncologic margins in pancreatic cancer. Perhaps the most significant barrier to widespread adoption of laparoscopic PD is the challenge of reconstruction, as three separate anastomoses are required [10].
The development of the Da Vinci robotic platform (Intuitive Surgical®, CA, USA) has drastically altered the paradigm of minimal invasive pancreatic surgery. The Da Vinci® surgical system consists of a three- or four-armed robot operated by a surgeon who sits at a separate console. Robotic surgery overcomes many of the key shortcomings of traditional laparoscopy, which include monocular vision, limited degrees of freedom and the effects of pivot and fulcrum, which make suturing particularly difficult to master. In contrast, the robotic approach affords the surgeon a three-dimensional stereoscopic view of the operating field and restores hand–eye coordination [11]. The Endowrist® instrumentation replicates the movements of the human hand with seven degrees of freedom and eliminates hand tremor. The ease and precision of dissection and suturing represent a real advance over the traditional laparoscopic approach [12].
Since the development of the robotic platform, the challenge of minimally invasive pancreatic surgery has been taken up with renewed enthusiasm, with the result that the number of reports on robotic-assisted (RA) pancreatic surgery has been on the rise. The aim of the present review is to evaluate the current state of total robotic PD.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statements were followed to conduct this systematic analysis [13].
Literature search
A systematic literature search was performed in PubMed, Embase, Cochrane Central Register of Controlled Trials and BioMed Central for studies performed between January 1, 2003, and July 31, 2016. The following terms were used to perform the search: “robotic” OR “robotics” OR “da Vinci” OR “minimally invasive” AND “pancreaticoduodenectomy” OR “Whipple procedure” OR “pancreatic surgery.” All titles and abstracts were analyzed to select those concerning robotic PD. Subsequently, full text articles were independently screened by 2 authors for eligibility. When multiple articles were published by the same study group and no difference in the study period was described, only the most recent article was considered to avoid double counting.
Study selection
Inclusion criteria included studies written in English, with human subjects, reporting at least one of the outcomes of interest for PDs undertaken for various types of pancreatic pathology and in which all the surgical steps were performed robotically (dissection, resection and reconstruction). Comparative studies including randomized controlled trials (RCTs) and non-randomized controlled trials (non-RCTs) were considered. Comparative studies with open and/or laparoscopic comparator groups were included. Non-comparative studies, such as case reports and case series, irrespective of their size, bearing the outcomes of interest were also considered. Studies in which the outcomes of interest were neither reported nor directly or indirectly inferable were excluded.
Data extraction and outcomes of interest
One reviewer (M.K.) evaluated all retrieved studies to determine whether they met inclusion criteria, to assess study quality and extract data. The study team resolved all the disagreements through discussion to reach a consensus. All studies were reviewed for the following data:
-
1.
Author’s surname and year of publication, origin of study, study design, study period, type of robotic system.
-
2.
Patient characteristics: number of patients, age, BMI, sex, tumor size.
-
3.
Operative outcomes: technical details of robotic PD (pancreatic stump treatment, anastomosis techniques), operative time (defined as the time from skin incision to placement of the surgical dressing), estimated blood loss (EBL), length of hospital stay (LOS), conversion to laparotomy, transfusion, rate of reoperation.
-
4.
Complications: bleeding, presence and grade of pancreatic fistula, biliary leak, delayed gastric emptying, mortality.
-
5.
Oncologic outcomes: number of lymph nodes harvested, number of incomplete resections (R1).
-
6.
Cost.
Assessment of literature quality
The (modified) Newcastle–Ottawa Quality Assessment star scoring system [14] was used to evaluate the quality of all included studies. The scale is comprised of seven elements that assess patient population and selection, study comparability, follow-up and outcome of interest. In assessing comparability between groups, focus was on variables that might affect primary endpoints, such as patient age, pathologic tumor-node-metastasis stage, types of PD, resection margin, tumor size, histologic type and type of reconstruction. Studies were scored using an ordinary star scale so as to compare their quality, with higher scores representing higher quality. A maximum of one star was awarded to a study for each numbered item within the selection and outcome assessment. A maximum of two stars was awarded for the comparability of the two groups. The maximum total score was 9 stars, and the quality of each article was graded as level 1/low quality (0–5 stars) or level 2/high quality (6–9 stars).
Results
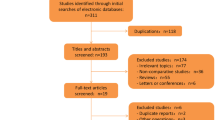
The PRISMA flow diagram for systematic review is presented in Fig. 1. The initial search yielded 56 potentially relevant articles. After the titles and abstracts were screened for relevance, 28 remaining articles were further assessed for eligibility. Thirteen studies were included in the systematic review. Their characteristics are listed in Table 1. The indication for exclusion and characteristics of excluded studies were also analyzed and are presented in Table 2.
Study quality
The quality of all 13 non-RCTs was level 2 (6–9 stars) on the modified Newcastle–Ottawa scale and good for the RCT according to the Jadad composite scale [15].
Characteristics of included studies
All thirteen of the included studies were non-RCTs and were published between January 1, 2000, and July 31, 2016. The systematic review included a total of 738 patients for whom total robotic PD was planned [16–28]. Overall, the procedure was successfully performed in 692 patients (93%). Five hundred and twenty-three patients were operated in the USA, 88 in China, 119 in Italy, 5 in Brazil and 3 in Japan. The majority of PDs were classic Whipple operations, and fewer were pylorus-preserving PD (PPPD). The management of the pancreatic stump was described in most cases: mainly end-to-side pancreaticojejunostomy [16–28], fewer pancreaticogastrostomy [16] and one fibrin glue occlusion of the main pancreatic duct [16].
Table 1 shows the results of the current review of totally robotic PD.
Intraoperative outcomes
Operative time (OT)
All studies reported the median OT. Except for Zhou et al. [18] and Boggi et al. [25], none of the studies specified whether the OT included the setup, draping and docking phases. The mean OT varied between 356 and 718 min, with a longer operative time reported early in the experience. Boone et al. [27] demonstrated that there was an important difference in mean OT between the first 80 robotic PD and the last 120 (581 min vs 417 min). In the comparative studies, the OT was significantly longer in robotic PD compared to open PD (OPD) [17, 18, 20, 22, 25].
Estimated blood loss (EBL)
EBL was available also in all 13 studies. Analysis of comparative studies found that the robotic approach significantly minimizes blood loss when compared with the open group [17, 18, 20].
Conversion rate
Regarding the feasibility of the robotic approach, the overall rate of conversion to laparotomy ranged from 0 to 18.3%. The most common reported causes were failure to progress, hemorrhage and unexpected vascular involvement [16, 21]. Boone et al. [27] showed that after 20 procedures the conversion rate dropped from 33 to 3.3%.
Table 3 summarizes intraoperative findings of the analyzed studies.
Overall postoperative complications
Morbidity
Overall morbidity rates reported in comparative studies ranged from 25 to 73%. The most compelling contrasts were reported in Zhou et al. [18] and Baker et al. [21] (25% robotic PD vs 75% open PD and 40% robotic PD vs 67% open PD). It is important to emphasize that in robotic PD, total morbidity is not represented because of the absence of data from large series.
Pancreatic fistula (POPF)
Based on the International Study Group for Pancreatic Fistula (ISGPF) [29], POPF is described as drain output of any measurable volume of fluid on or after postoperative day (POD) 3 with amylase content over 3 times the serum amylase activity. Except the comparative study of Lai et al. [17] (35% robotic PD vs 17% open PD) and Boggi et al. [25] (33% robotic PD vs 16% open PD), POPF rates were comparable between the minimally invasive and open group.
Delayed gastric emptying, postoperative hemorrhage and bile leak
Delayed gastric emptying is defined by the ISGPS [30] as need for maintenance of nasogastric tube for 3 days, the need to reinsert the nasogastric tube for persistent vomiting after POD 3, or inability to tolerate a solid diet by POD 7.
Postoperative hemorrhage was available in 6 [16–18, 20, 22, 25] studies and bile leak in 4 studies [16, 17, 20, 22, 24, 26]. The results showed that the postoperative hemorrhage and bile leak rates were comparable between groups but that the robotic PD group tended to fewer incidence of delayed gastric emptying [16, 17, 21, 22, 25].
Reoperation and mortality
Ten studies [16–18, 21, 24, 25] reported incidence of mortality ranging from 1 to 12.5% in robotic PD, which was comparable to the mortality rate in open PD. Most postoperative deaths reported were related to hemorrhagic complications of POPF or cardiac events. Eight studies [16–20, 22, 23] reported incidence of reoperation. Primary causes for reoperation were intra-abdominal hemorrhage and severe POPF (Grade C) [16]. Overall, no significant differences were found.
Length of stay (LOS)
Pooling data from 12 studies including 588 patients, length of stay analysis showed a difference favoring robotic PD. In Lai et al. [17] and Zhou et al. [18], the robotic group had a significantly shorter LOS in comparison with the open group (mean 13.7 vs 25.8 and 16.38 vs 24 days, respectively) (Table 4).
Operative oncologic outcomes
Most robotic PD was performed for malignant diseases. The most frequent malignancy was pancreatic adenocarcinoma, followed by ampullary adenocarcinoma and distal cholangiocarcinoma. Eight studies reported the number of harvested lymph nodes (Table 5). The number of lymph nodes harvested and ability to achieve an R0 resection are related to prognosis. The number of lymph nodes harvested was comparable between groups, but the minimally invasive group tended to have less positive margins. In Lai et al. [17] study, the R1 ratios were robotic PD 26% versus open PD 64%, whereas in the Boggi et al. [25] trial the R1 ratio was 12.5% in robotic PD and 45% in open PD.
Table 4 summarizes postoperative findings of the analyzed studies.
Discussion
Allen Oldfather Whipple is the uncontested father of North American pancreatic surgery. Although both Alessandro Codivilla in Italy and Walther Kausch in Germany had performed PD decades before [3], Whipple’s presentation at the American Surgical Association meeting in 1935 of 3 patients who underwent a 2-staged operations and his successful performance of a 1-stage PD 5 years later set the stage for further development of this operation in the USA and Canada [4]. The current version of the operation that bears his name is now performed throughout the world and, although still fraught with potentially serious complications, is a common operation in many major medical centers.
In an effort to reduce the historically high rate of postoperative morbidity, minimally invasive approaches to PD are being explored. To date, minimally invasive PD is thought to be a feasible operation in selected patients being treated at selected centers with improved outcomes compared with the open approach. The lack of randomized trials or high-quality, non-randomized prospective studies as well as data on long-term outcomes, cost-effectiveness and learning curve analysis do not allow for firm conclusions to be drawn, so minimally invasive PD cannot be considered superior or standard at this time [41, 42]. In 1994, Gagner and Pomp [8] described the first laparoscopic PD, but the level of evidence concerning the technique is still low as less than 300 cases performed were identified in the reviewed studies [43, 44]. The high level of complexity of the operation and the high level of skill required for intracorporeal anastomoses have led to a growing interest in RA surgery.
Robotic surgery assists the surgeon in overcoming many of the obstacles to widespread application of laparoscopic pancreatic surgery. The superior visualization, improved 3-dimensional imaging, enhanced dexterity, improved ergonomics and the restoration of hand–eye coordination help surgeons to complete complex procedures and reconstructions, with at least equivalent results to the open approach.
In the current literature, the definition of robotic PD has not been standardized, since in many studies the technique is defined as robotic, robotic-assisted, robotic-assisted laparoscopic and robotic hybrid. The current review aims to evaluate the current state of total robotic PD, which includes robotic dissection, resection and reconstruction.
Safety and feasibility of a new surgical approach are of paramount importance. The findings in this study indicate that robotic PD is a feasible procedure, with some high-volume centers reporting 6.5 and 7.8% conversion rates [24, 27]. The Pittsburg group reported a steep decline in conversion rate after 20 procedures were performed (35 vs 3.3%) [24].
Minimally invasive surgery has always been associated with longer operative times when compared to open techniques. The overall duration of robotic PD was significantly longer in all studies compared with open PD. Time for setup, draping and docking the robot has a significant impact on the overall OT, and whether the documented OT include these factors is not defined in most studies. Nonetheless, the lengthy operative times observed in robotic PD can be mentally and physically exhausting for the surgical team. Again, the Pittsburg team showed the importance of the learning curves impact on OT, reporting reduction in the mean OT from 581 min for cases 0–80 to 417 min for cases 81–200 [23, 24, 34, 36].
Operative blood loss was shown to be lower in robotic PD when compared to open PD [17, 18, 21], especially after the learning curve [22]. This may be attributed to the magnified view of small vessels that the robotic camera allows for, particularly during dissection of the plane between the uncinate process and the superior mesenteric vessels. This finding indicates that the robotic approach has advantages without compromising safety.
Analyzing morbidity after PD, there were no major differences [22, 27, 34] between the open and minimally invasive approaches. Theoretically, robotic procedure lead to faster recovery, reduced respiratory complications, reduced wound infections and shorter postoperative stay compared to open surgery [17, 27]. The postoperative morbidity rate ranged between 20 and 73% and suggests that robotic PD is as safe as open PD. Severe complications requiring reoperations ranged between 3 and 11%, in high-volume centers [23, 24, 34, 36]. Zureikat et al. [34] reported 4 reoperations after 132 robotic PD and Boggi et al. [24] reported 9 reoperations after 83 robotic PD mainly because of postoperative hemorrhage. The reoperation rate of 3–11% after robotic PD is higher than the 3% reoperation rate reported in high-volume centers after open PD [45].
Delayed gastric emptying was reported in 4 comparative studies indicating an important advantage favoring robotic PD compared to open PD. Regarding bile leak, Lai et al. [17] reported a difference between the approaches in 20 patients (robotic PD 15 vs 6% open PD), but Chen [22], Zureikat [34] and Guilianotti [16] (270 patients) did not find any major difference between the two approaches.
Pancreatic fistula (POPF) is the most common postoperative complication after PD and the inciting event for many downstream complications that result in longer length of stay, need for reinterventions, readmissions and deaths. Variations in the precise definition of POPF have historically led to widely different rates of reported leak rates, from as low as 2% to more than 35% [36]. In this study, the overall rate of POPF after robotic PD was 20–32%, comparing favorably to most open PD series that report fistulae in the post-ISGPF era [36, 37, 46]; most of them had low output and were conservatively managed (Grade A). Larger series of robotic PD [24] with documented risk factors for POPF (pancreatic texture, pancreatic duct size, ASA score, EBL, OT, tumor size, BMI) will allow us to determine whether the Braga and Callery scores [47, 48] for open PD apply to robotic PD.
The mortality rate was low in the robotic PD group (1.6%), similar to mortality rates of high-volume centers for open PD (1–4%) [16, 24, 25]. This might be explained by the fact that robotic PD is performed only in very high volume hospitals [49] and in highly selected patients.
As far as length of hospital stay is concerned, reported outcomes varied widely in patients undergoing robotic PD. One could expect that robotic surgeries would reduce hospital stay; however, this is not observed in the majority of the series. This might be explained by differences in national health systems between countries and differing hospital policies regarding discharge [16]. It is very likely that the overall LOS is similar between robotic PD and open PD in most centers, with a slight advantage in robotic PD [17, 18].
Oncologic outcome is the major concern regarding robotic PD among patients suffering from malignancies. R0 resections and lymph nodes retrieved are two indicators of the oncologic adequacy of robotic PD. Microscopic infiltration of the pancreatic stump (R1) was considerably lower (10%) for patient undergoing robotic PD, lower rate comparing large series of open PD [50]. Again, one possible explanation for this outcome could be the preoperative selection of patients at low-risk for positive margin status. The number of lymph nodes harvested varied, ranging from 10 to 35, with the highest numbers reported in studies with the largest number of robotic PD [16, 21, 23, 25, 27]. Considering these factors, plus the utility of MIS in decreasing the pro-inflammatory and immunologic response to surgical trauma [51, 52] and decreasing time to adjuvant therapy, robotic PD seems to be at least comparable and perhaps better than open PD for malignancies, but long-term outcomes are as yet unknown.
One important question surrounding the use of MIS is whether or not the benefits will offset the significantly increased operative costs. The robotic platform is expensive with an initial capital cost of 1–2.5 million dollars (USD); annual maintenance liabilities well over 100,000 dollars and many single-use instruments [53–55]. Four studies [21–23, 25] have attempted to address this question. Not surprisingly, all found operating room costs to be greater for robotic PD. However, when the total hospital costs were taken into account (including costs of hospital stay and readmission) the robotic approach tended to be less expensive than the open approach. Baker et al. [21] showed that there was no significant difference in overall cost ($176,931 robotic PD vs $182,552 open PD) in 71 PDs. In another study [54] including 76 patients, total robotic costs were $150,473, while cost of the open approach was $142,149. Chen et al. [22] reported overall cost results for 180 patients, which demonstrated that robotic PD was more expensive than open PD (robotic PD $19,755 vs open PD $12,110), but was associated with significantly lower postoperative costs ($8529 robotic PD vs open PD $10,559 OPD) although it should be noted that average length of hospital stay in China was approximately 3–4 weeks and the patients usually opted to discharge after full recovery. Boggi et al. [25] documented excess mean operative cost for robotic PD of 6.193 euros, whereas Cunningham et al. [23] concluded that a standard policy of omitting a postoperative ICU admission on postoperative day 0 after robotic PD can result in overall savings in total hospital costs. These data demonstrate that robotic related costs can be cushioned by the shorter stay and faster recovery of patients. What is more, as the number of robotic procedures increases, the costs of technology are likely to proportionally decrease.
Conclusions
In summary, it is rational to conclude that robotic PD is safe and feasible in a high-volume institution where surgeons are experienced and medical staff are appropriately trained. Randomized controlled trials are certainly the best way to investigate this important question further. Data on cost analysis and long-term oncologic outcomes are needed to evaluate the cost-effectiveness of the robotic approach in comparison with the open technique.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics, 2006. CA Cancer J Clin 56:106–130
Sarr MG, Murr M, Smyrk TC, Yeo CY, Fernandez del Castillo C, Hawes RH (2003) Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state of the art and unanswered questions. J Gastrointest Surg 7:417–428
Schnelldorfer T, Adams DB, Warshaw AL, Lillemoe KD, Sarr MG (2008) Forgotten pioneers of pancreatic surgery: beyond the favorite few. Ann Surg 247:191–202
Whipple AO, Parsons WB, Mullins CR (1935) Treatment of carcinoma of the ampulla of Vater. Ann Surg 102:763–779
Papalampros A, Niehaus K, Moris D, Fard-Aghaie M, Stavrou G, Margonis AG, Angelou A, Oldhafer K (2016) A safe and feasible “clock-face” duct-to-mucosa pancreaticojejunostomy with a very low incidence of anastomotic failure: a single center experience of 248 patients. J Visc Surg. doi:10.1016/j.jviscsurg.2016.05.004
Moris D, Papalampros A, Vailas M, Petrou A, Kontos M, Felekouras E (2016) The hepaticojejunostomy technique with intra-anastomotic stent in biliary diseases and its evolution throughout the years: a technical analysis. Gastroenterol Res Pract. doi:10.1155/2016/3692096
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single institution experience. J Gastrointest Surg 10:1199–1210
Gagner M, Pomp A (1994) Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 8:408–410
Mabrut JY, Fernandez-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, Fabre JM, Boulez J, Baulieux J, Peix JL, Gigot JF, Hepatobiliary and Pancreatic Section (HBPS) of the Royal Belgian Society of Surgery, Belgian Group for Endoscopic Surgery (BGES), Club Coelio (2005) Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery 137:597–605
Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S, Mosca F (2015) Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 29:9–23
Bodner J, Augustin F, Wykypiel H, Fish J, Muehlmann G, Wetscher G, Schmid T (2005) The da Vinci robotic system for general surgical applications: a critical interim appraisal. Swiss Med Wkly 135:674–678
Velasquez CA, Navkar NV, Alsaied A, Balakrishnan S, Abinahed J, Al-Ansari AA, Jong Yoon W (2016) Preliminary design of an actuated imaging probe for generation of additional visual cues in a robotic surgery. Surg Endosc 30:2641–2648
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med 6:e1000100
Wells GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Dept of Epidemiology and Community Medicine, University of Ottawa, Ottawa
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y (2001) Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord 12:232–236
Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, Caravaglios G, Coratti A (2010) Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 24:1646–1657
Lai EC, Yang GP, Tang CN (2012) Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy—a comparative study. Int J Surg 10:475–479
Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, Chen XF (2011) Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot 7:131–137
Horiguchi A, Uyama I, Ito M, Ishihara S, Asano Y, Yamamoto T, Ishida Y, Miyakawa S (2011) Robot-assisted laparoscopic pancreatic surgery. J Hepatobiliary Pancreat Sci 18:488–492
de Vasconcellos Macedo AL, Schraibman V, Okazaki S, Mauro FC, Epstein MG, Goldman SM, Lustosa SA, Matos D (2011) Treatment of intraductal papillary mucinous neoplasms, neuroendocrine and periampullary pancreatic tumors using robotic surgery: a safe and feasible technique. J Robot Surg 5:35–41
Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, Vrochides D, Martinie JB (2015) Robotic pancreaticoduodenectomy for pancreatic adenocarcinoma: role in 2014 and beyond. J Gastrointest Oncol 6:396–405
Chen S, Chen JZ, Zhan Q, Deng XX, Shen BY, Peng CH, Li HW (2015) Robotic-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 29:3698–3711
Cunningham KE, Zenati MS, Petrie JR, Steve JL, Hogg ME, Zeh HJ 3rd, Zureikat AH (2016) A policy of omitting an intensive care unit stay after robotic pancreaticoduodenectomy is safe and cost-effective. J Surg Res 204:8–14
Polanco PM, Zenati MS, Hogg ME, Shakir M, Boone BA, Bartlett DL, Zeh HJ, Zureikat AH (2016) An analysis of risk factors for pancreatic fistula after robotic pancreaticoduodenectomy: outcomes from a consecutive series of standardized pancreatic reconstructions. Surg Endosc 30:1523–1529
Boggi U, Napoli N, Costa F, Kauffmann EF, Menonna F, Iacopi S, Vistoli F, Amorese G (2016) Robotic-assisted pancreatic resections. World J Surg. doi:10.1007/s00268-016-3565-3
Rashid OM, Mullinax JE, Pimiento JM, Meredith KL, Malafa MP (2015) Robotic Whipple procedure for pancreatic cancer: the Moffitt cancer center pathway. Cancer Control 22:340–351
Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, Zeh HJ, Zureikat AH (2015) Assessment of quality outcome for robotic pancreaticoduodenectomy; Identification of the learning curve. JAMA Surg 150:416–422
MacKenzie S, Kosari K, Sielaff T, Johnson E (2011) The robotic Whipple: operative strategy and technical considerations. J Robot Surg 5:3–9
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Giulianotti P, Gorodner V, Kinzer K, Benedetti E, Oberholzer J (2012) Robot-assisted pancreatoduodenectomy with preservation of the vascular supply for autologous islet cell isolation and transplantation: a case report. J Med Case Rep 6:74
Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784
Chalikonda S, Aguilar-Saavedra JR, Walsh RM (2012) Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 26:2397–2402
Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ 3rd (2013) 250 robotic pancreatic resections: safety and feasibility. Ann Surg 258:554–559
Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC (2011) Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 35:2739–2746
Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ (2012) Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol 19:864–870
Zeh HJ, Bartlett DL, Moser AJ (2011) Robotic-assisted major pancreatic resection. Adv Surg 45:323–340
Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F (2013) Feasibility of robotic pancreatoduodenectomy. Br J Surg 100:917–925
Chan OC, Tang CN, Lai EC, Yang GP, Li MK (2011) Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci 18:471–480
Bao PQ, Mazirka PO, Watkins KT (2014) Retrospective comparison of robotic-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg 18:682–689
Wang M, Cai H, Meng L, Cai Y, Wang X, Li Y, Peng B (2016) Minimally invasive pancreaticoduodenectomy: a comprehensive review. Int J Surg 35:139–146
Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, Fisher S, Viñuela EF, Kingham TP, Fong Y, DeMatteo RP, D’Angelica MI, Jarnagin WR, Allen PJ (2014) Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg 218:129–139
Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, Berry DP (2009) Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 13:1129–1137
Addeo P, Guilianotti PC (2010) Update on laparoscopic pancreatectomy in 2010. Minerva Chir 65:655–666
Buchs NC, Volonte F, Pugin F, Bucher P, Jung M, Morel P (2010) Robotic pancreatic resection: how far can we go? Minerva Chir 66:603–614
Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP, Lillemoe KD, Warshaw AL (2012) Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 152:S56–S63
Denbo JW, Orr WS, Zarzaur BL, Behrman SW (2012) Toward defining grade C pancreatic fistula following pancreaticoduodenectomy: incidence, risk factors, management and outcome. HPB (Oxford) 14:589–593
Braga M, Capretti G, Pecorelli N et al (2011) A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 254(5):702–707 (discussion 707–708)
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr (2013) A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216(1):1–14
Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN (1999) Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 126:178–183
Yeo CJ, Cameron JL (1999) Improving results of pancreaticoduodenectomy for pancreatic cancer. World J Surg 23:907–912
Goldfarb M, Goldfarb M, Brower S, Schwaitzberg SD (2010) Minimally invasive surgery and cancer: controversies part 1. Surg Endosc 24:304–334
Moris D, Felekouras E, Chrousos GP (2016) No cytokine is an island: IL-6 alone is not sufficient to predict morbidity after a major abdominal surgery. Ann Surg. doi:10.1097/SLA.0000000000001977
Barbash GI, Glied SA (2010) New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 363:701–704
Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, Vrochides D, Martinie JB (2016) Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot 12:554–560
Author information
Authors and Affiliations
Contributions
Author contributions
MK, MV, AP contributed to study concept and design. MK, DM, DS helped with acquisition of data. MK, DM, AP, AM contributed to analysis and interpretation of data. DM, EF, AP helped in drafting of the manuscript. DM, AMitrousias, AMichalinos, EF contributed to critical revision of the manuscript for important intellectual content. EWB helped in editing and Linguistic control. MK, DM contributed to statistical analysis. MV helped with administrative, technical or material support. EWB, MCM helped with revision. AP contributed to supervision.
Corresponding author
Ethics declarations
Disclosures
Drs. Michail Kornaropoulos, Demetrios Moris, Eliza W. Beal, Marinos C. Makris, Apostolos Mitrousias, Athanasios Petrou, Evangelos Felekouras, Adamantios Michalinos, Michail Vailas, Dimitrios Schizas and Alexandros Papalampros have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Kornaropoulos, M., Moris, D., Beal, E.W. et al. Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surg Endosc 31, 4382–4392 (2017). https://doi.org/10.1007/s00464-017-5523-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5523-z