Abstract
Introduction
Theoretically, reducing the number of ports required in minimally invasive surgery for gastric cancer would further minimize trauma associated therewith. Advances in single-site surgery have afforded surgeons the ability to perform reduced-port distal gastrectomy via a robotic approach using the Single-Site™ system, eliminating restrictions on the movement of surgical instruments.
Methods
This phase I/II study was designed as a single-arm prospective trial of reduced-port robotic distal gastrectomy (RRDG) by a single surgeon (NCT02347956). From January to October 2015, 40 individuals scheduled to undergo robotic surgery for early gastric cancer were asked to participate in the trial. Nineteen were enrolled and underwent RRDG. The primary endpoints were 30-day morbidity and mortality.
Results
No intraoperative event requiring conversion to laparoscopic or open surgery occurred, and no major complication was observed following RRDG (0.0% [80% CI (0.0–11.4%)]). Medians of operation time, blood loss, the number of retrieved lymph nodes, days until gas passing, and hospital stay were 190 min, 20 mL, 48, 3, and 5 days, respectively.
Conclusions
Deemed safe and feasible through the present trial, RRDG could be a valid alternative to conventional robot distal gastrectomy for managing early gastric cancer. Our reduced-port robotic surgery using the Single-Site system and a third robotic arm could potentially be applicable as a highly advanced, minimally invasive surgery for other solid organ diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrectomy with lymph node dissection offers the best chance of curing gastric cancer. With advances in instruments and technique, laparoscopic gastrectomy has emerged as a preferred treatment for early gastric cancer, showing favorable short-term surgical outcomes and comparable long-term oncologic outcomes to open surgery [1–3].
To minimize trauma associated with laparoscopic surgery, surgeons have developed procedures, wherein a majority of the instruments required for surgery are introduced via a single periumbilical port and the ports along the flank are eliminated, which are called reduced-port laparoscopic surgery [4–7]. Inevitably, however, crowding of multiple surgical instruments within the periumbilical port, restricting the surgeon’s ability to move the instruments, emerged as a major limitation of reduced-port surgery for gastric cancer, especially in performing lymph node dissection.
Exhibiting the potential to improve upon limitations to the movement of surgical instruments during reduced-port surgery, robotic surgical systems equipping the da Vinci® Single-Site™ instrumentation (Intuitive Surgical, Sunnyvale, CA, USA) offer surgeons a unique surgical environment in which a scope, two robotic arms, and an assistant instrument can be employed via a single port. Introducing the robotic system facilitates more intuitive control of the instruments within the operative environment by compensating for the crossing of the two robotic arms within the Single-Site port. Application of this system has proven successful in cholecystectomy, hysterectomy, and colectomy [8–11].
The aim of this prospective study was to assess the safety and feasibility of reduced-port robotic distal gastrectomy (RRDG) using the Single-Site system for early gastric cancer. To clarify the value of RRDG, we compared the surgical outcomes of the novel procedure with those for conventional robotic distal gastrectomy (CRDG).
Methods
Study design
This study was conducted as a prospective, single-arm, phase I/II clinical trial by a single surgeon, and was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (4-2014-0864). The study protocol was registered at clinicaltrial.gov (NCT02347956).
Patients
From January 2015 through October 2015, 40 patients with pathologically confirmed adenocarcinoma of the middle to distal stomach who were scheduled to receive distal robotic gastrectomy were asked to participate in the study. After receiving explanations on the clinical trial, patients who agreed to participate in the trial underwent RRDG; those who declined underwent CRDG. Inclusion criteria were tumors clinically confined to the mucosa or submucosa that did not fulfill criteria for endoscopic mucosal dissection. Exclusion criteria were patients with advanced cancer deeper than the submucosa or early lesions curable by endoscopic resection; patients who had received systemic chemotherapy, radiotherapy, or both; and patients who required D2 lymphadenectomy and other organ resection were excluded. Finally, 19 patients who agreed to participate in the trial underwent RRDG. 21 patients who declined RRDG underwent CRDG.
Clinical patient characteristics and outcomes included age, gender, American Society of Anesthesiologists score (ASA), location of tumor, reconstruction method, T and N classification, operation time, docking time, intraoperative bleeding, conversion to laparoscopy or open surgery, the number of retrieved lymph nodes, pathologic margin status, days until gas passing, hospital stay, visual analogue scale (VAS) score, white blood cell (WBC) count, C-reactive protein (CRP) level, morbidity, mortality, and re-admission to the hospital. Operation time was defined as skin-to-skin time. Patients were followed up every 3 months for 2 years and every 6 months thereafter for the duration of the scheduled follow-up period. We also compared the surgical outcomes of CRDG as an internal control to clarify the clinical impact of RRDG.
Surgical technique
All robotic distal gastrectomies were performed using the da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). RRDGs were performed using the Single-Site system and a third robotic arm. For comparison, schematic illustrations of reduced-port laparoscopic distal gastrectomy and RRDG using the Single-Site system are provided in Fig. 1A, B: Note the criss-crossing of the cannulae. As described above, equipping the da Vinci Single-Site system allows for intuitive control of ipsilateral instruments. The first and third robotic arms can be controlled with the surgeon’s left hand, with the right hand controlling the second robotic arm.
Schematic illustration. Illustrations of A reduced-port laparoscopic distal gastrectomy and B reduced-port robotic distal gastrectomy using the Single-Site™ system and a third robotic arm. Comparison of instrumentation for C conventional and D reduced-port robotic distal gastrectomy. *DOF degree of freedom
In CRDG, the first and second arms are utilized as the right and left hands of the surgeon, respectively (Fig. 1C). The third arm is used for traction of the stomach. In RRDG, the first robotic arm is used for traction, while the second and third robotic arms are used as the right and left hands of the surgeon, respectively (Fig. 1D).
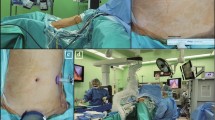
The reduced-port approach was begun by making a transumbilical incision of about 2.5 cm in length, through which a Single-Site™ port (428,065, Intuitive Surgical) was inserted. The Single-Site™ port comprises four lumens for instrumentation: one lumen for an 8.5-mm endoscope (8.5 mm Endoscope Cannula, 400,263, Intuitive Surgical), two for 5-mm curved cannulae (5 × 250 mm curved cannula, 428,071 and 428,072, Intuitive Surgical), and one for a 10-mm accessory port (10 mm Accessory Cannula, 428,076, Intuitive Surgical) for use by an assistant (Fig. 2A). Next, for equipping ultrasonic shears, an 8-mm straight cannula (8-mm instrument cannula, 420,002, Intuitive Surgical) was inserted along the right flank (Fig. 2B) in a port-in-port fashion via a 12-mm port (XCEL®, Ethicon Endo-surgery, Cincinnati, OH, USA). Finally, a 12-mm assistant port (XCEL®, Ethicon Endo-surgery) was placed along the left flank. In patients with a thin body habitus and when less complicated surgery was anticipated, the left assistant port was not inserted.
After port insertion, the robotic cart was placed over the patient along the midline. After docking the camera cannula to the robotic camera arm, two curved cannulae for robotic arms 1 and 2 were inserted through the Single-Site port and docked to robotic arms 1 and 2: the camera was used to visualize the tips of the inserted curved cannulae to ensure that they did not cause injury to intra-abdominal organs during their insertion. Once completed, Cadiere forceps (428,055, Intuitive Surgical) were inserted through the curved cannula docked on the first arm for use in traction of the stomach. A needle driver (EndoWrist Needle Driver, 420,117, Intuitive Surgical) was then introduced through the curved cannula docked on the second robotic arm to guide dissection. After the Single-Site system was fully fitted, the third robotic arm could be docked on the patient (Fig. 2C). On the console display, the final locations of the instruments in the abdominal cavity were as follows (Fig. 2D): the Cadiere forceps and needle driver are located on the left and right, respectively. Ultrasonic shears can be seen on the left side of the surgical view. Graspers inserted through the assistant port can be seen on the right.
Dissection and reconstruction procedures were basically the same for both RRDG and CRDG. Details on the surgical procedures for performing CRDG have been described previously [12].
Postoperative management and complication assessment
Management of both patient groups followed the same protocol. After 2 days of postoperative analgesics by continuous intravenous infusion of fentanyl under patient control, administration of the oral pain killer Ultracet ER (Janssen-Ortho, LLC, Gurabo, Puerto Rico) was initiated on postoperative day 3. Postoperative pain assessment was performed in all patients using VAS, with possible scores ranging from 0 to 10. Pain scores were recorded in a post anesthesia care unit (PACU; at 30 min after surgery), and then recorded at 6, 24, and 48 h after surgery. Sips of water and a clear liquid diet were resumed at 2 and 3 days after the operation, respectively. A soft diet was permitted on the evening of the third day after surgery. Thirty-day morbidity and mortality were assessed by Clavien-Dindo classification and monitored on a weekly basis [13, 14].
Outcomes
The primary safety endpoint was defined as the development of major complications of Clavien-Dindo classification grade III or higher [13, 14]. All events were discussed and judged during weekly quality control meetings. The secondary endpoints were operation time, intraoperative blood loss, conversion rate, the number of retrieved lymph nodes, pathologic margin status, recovery of bowel function (days until gas passing), length of hospital stay, postoperative pain (VAS score), and postoperative laboratory results, including WBC counts and CRP levels.
Statistical analysis
The sample size for testing the primary endpoint was calculated using PASS 2000 (NCSS Statistical Software, Kaysville, Utah, USA) on the basis of previous studies reporting complication rates of 4, 4.5, 15.7, and 15.4%, respectively [3, 15–17]. An expected value of 4% and a threshold value of 15% were set using two-sided testing at a 20% significance level and 70% power. The necessary sample size in this trial was deemed to be 19 cases on the basis of a single-stage design to test the null hypothesis (a complication rate ≥15%) versus an alternative hypothesis (complication rate ≤4%). If the number of confirmed complications was two or less, then the null hypothesis was to be rejected. The complication rate estimate and its 80% confidence interval (CI) were calculated. Categorical variables are expressed as numbers and percentages, and continuous variables are reported as medians with minimum and maximum values. All p-values less than 0.05 were regarded as significant, and all statistical tests were two-sided. We analyzed secondary outcomes with the Mann–Whitney U test and dichotomous outcomes with the χ 2 or Fisher exact tests. All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
Table 1 shows details on the characteristics of the patients who participated in the trial. The characteristics of the patients were not significantly different from usual patients, except that the weight of the RRDG group was less than that of the CRDG group (60 kg [46–75] vs. 75 kg [56–100], p < 0.001).
Table 2 list the operative and postoperative parameters. Operation time and docking time for RRDG were 190 min (125–279) and 7 min (2–15), respectively. These times were significantly longer than those for CRDG (operation time: 137 min [117–174], p < 0.001; docking time: 3 min [1–9], p < 0.001). None of the patients required intraoperative or postoperative blood transfusions. All robotic surgeries were successfully completed without converting to open or laparoscopic surgery. Time to recovery of bowel function; days until starting sips of water, a liquid diet, and a soft diet; and hospital stay were 3, 2, 3, 3, and 5 days, respectively. Both surgery groups showed similar postoperative recovery. VAS scores for postoperative pain were highest at 6 h after surgery and gradually decreased at 24 and 48 h. WBC and CRP levels showed peak elevations on the day of the operation and postoperative day 3, respectively. No significant differences were noted in VAS score and WBC levels between the two groups. CRP levels after RRDG were significantly lower at postoperative days 1 and 3, compared to the CRDG group (POD#1: 27 mg/L [8–85] versus 35.8 mg/L [26–155], p = 0.006; POD#3: 43.3 mg/L [15–147] versus 83.7 mg/L [26–155], p = 0.013). At a median of 9 months of follow-up (range 4–13 months), all patients were free of disease. No late major complications or re-admission were observed.
Postoperative complications are summarized in Table 3. Assessed by the Clavien-Dindo system, no major complication, grade III or higher, developed in the RRDG group (80% CI [0.0–11.4%]).
Discussion
To our knowledge, this is the first trial to outline the surgical outcomes of RRDG. Applying the novel technique, we were able to successfully perform RRDG without instances of robot system-related events, insufficient lymph node dissection, major clinical events, or intraoperative difficulties necessitating conversion to laparoscopic or open surgery. The hypothesis of our study was that if the number of confirmed complications was two or fewer during this trial, the new technique could then be regarded as a safe procedure. Since no major complications were identified, our results suggest that RRDG using the Single-Site system and a third robotic arm is feasible and safe for use in early gastric cancer patients requiring D1+ lymphadenectomy.
During the trial, we experienced advantages of RRDG over conventional robot or reduced-port laparoscopic approaches. First, compared with CRDG, RRDG helped reduce surgical trauma and adverse risks associated with multiple ports. Other studies have also made efforts to reduce the number of ports during surgery in an attempt to reduce surgical trauma and subsequent risks of pain, unnecessary scar, and organ damage [18, 19]. To evaluate surgical trauma associated with CRDG and RRDG, we assessed and compared pain scores and inflammatory laboratory results, including WBC counts and CRP levels. According to our results, lower CRP levels for RRDG could be regarded as an indicator of less trauma, highlighting the potential clinical impact of the reduced-port surgery on reducing trauma following gastrectomy. Second, compared with a laparoscopic reduced-port approach, robotic systems provide surgeons with articulated movements and better ergonomics. Moreover, the Single-Site system enabled us to manipulate ipsilateral robotic arms with automatic swapping of the left and right robotic arms. Furthermore, our procedure could be performed without technical difficulty requiring only one assistant. In this regard, RRDG could be preferable to conventional robot or laparoscopic reduced-port approaches. The only unsatisfactory outcome of RRDG in this study was a longer operation time. Notwithstanding, we have performed more than 150 surgeries applying the conventional approach, and our experience with the reduced-port approach is only in the initial learning phase [20, 21]. Once we overcome the necessary learning curve, we expect that operation times for RRDG will be close to that of CRDG in due time.
Limitations to the study design warrant consideration. First, the present study was conducted as a single-arm, phase I/II clinical trial to assess the safety and feasibility of RRDG. To outline the surgical outcomes of the novel procedure, we compared perioperative data to those for CRDG performed during the same period as an internal control. However, larger, multi-institutional, randomized, and controlled studies are required to validate the value of RRDG. Second, this study reported only short-term outcomes. Long-term surgical outcomes and oncologic results should be assessed in the future.
Even though many studies on reduced-port surgery have yet to show clear advantages over conventional minimally invasive surgery [17, 22], moving forward, we still expect to record a clinically meaningful impact for RRDG on surgery-induced trauma after further accumulation of experience with the procedure. We believe that RRDG may lay the foundation for achieving radical, pure single-port gastrectomy in the future. This belief is in line with the thoughts of the pioneer who developed robotic single-site cholecystectomy and proclaimed that a simple single-site surgical procedure can pave the way for more complex surgical procedures that are currently feasible only with the use of multiple ports [23].
In conclusion, our results suggest that RRDG offers acceptable feasibility and safety as a valid alternative to CRDG. Our reduced-port robotic surgery using the Single-Site system and a third arm could also be potentially applicable as a highly advanced, minimally invasive surgery for other solid organ diseases.
Abbreviations
- RRDG:
-
Reduced-port robotic distal gastrectomy
- CI:
-
Confidence interval
References
Deng Y, Zhang Y, Guo TK (2015) Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surg Oncol 24(2):71–77. doi:10.1016/j.suronc.2015.02.003
Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L (2012) Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg 256(1):39–52. doi:10.1097/SLA.0b013e3182583e2e
Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY (2014) Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol 32(7):627–633. doi:10.1200/jco.2013.48.8551
Amin AT, Gabr A, Abbas H (2015) Laparoscopy assisted distal gastrectomy for T1 to T2 stage gastric cancer: a pilot study of three ports technique. Updates Surg 67(1):69–74. doi:10.1007/s13304-015-0279-2
Kashiwagi H, Kumagai K, Monma E, Nozue M (2015) Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 29(6):1321–1326. doi:10.1007/s00464-014-3827-9
Kim SM, Lee JH, Lee SH, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S (2015) Techniques of reduced PRT laparoscopy-assisted distal gastrectomy (duet LADG). Ann Surg Oncol 22(3):793. doi:10.1245/s10434-014-4087-6
Kunisaki C, Ono HA, Oshima T, Makino H, Akiyama H, Endo I (2012) Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg 29(3):261–268. doi:10.1159/000341677
Morelli L, Guadagni S, Di Franco G, Palmeri M, Di Candio G, Mosca F (2015) Da Vinci single site(c) surgical platform in clinical practice: a systematic review. Int J Med Robot Comput Assist Surg. doi:10.1002/rcs.1713
Angus AA, Sahi SL, McIntosh BB (2014) Learning curve and early clinical outcomes for a robotic surgery novice performing robotic single site cholecystectomy. Int J Med Robot Comput Assist Surg 10 (2):203–207. doi:10.1002/rcs.1540
Bae SU, Jeong WK, Bae OS, Baek SK (2015) Reduced-port robotic anterior resection for left-sided colon cancer using the Da Vinci single-site platform. Int J Med Robot Comput Assist Surg. doi:10.1002/rcs.1677
Sendag F, Akdemir A, Zeybek B, Ozdemir A, Gunusen I, Oztekin MK (2014) Single-site robotic total hysterectomy: standardization of technique and surgical outcomes. J Minim Invasive Gynecol 21(4):689–694. doi:10.1016/j.jmig.2014.02.006
Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249(6):927–932. doi:10.1097/01.sla.0000351688.64999.73
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213 pii]
Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH (2012) Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg 99(11):1554–1561. doi:10.1002/bjs.8887
Tokunaga M, Kondo J, Tanizawa Y, Bando E, Kawamura T, Terashima M (2012) Postoperative intra-abdominal complications assessed by the Clavien-Dindo classification following open and laparoscopy-assisted distal gastrectomy for early gastric cancer. J Gastrointest Surg 16(10):1854–1859. doi:10.1007/s11605-012-1981-8
Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH (2015) Comparison of reduced port totally laparoscopic distal gastrectomy (duet TLDG) and conventional laparoscopic-assisted distal gastrectomy. Ann Surg Oncol 22(8):2567–2572. doi:10.1245/s10434-014-4333-y
Park S, Bergs RA, Eberhart R, Baker L, Fernandez R, Cadeddu JA (2007) Trocar-less instrumentation for laparoscopy: magnetic positioning of intra-abdominal camera and retractor. Ann Surg 245(3):379–384. doi:10.1097/01.sla.0000232518.01447.c7
Marcovici I (2001) Significant abdominal wall hematoma from an umbilical port insertion. JSLS 5(3):293–295
Koo EJ, Youn SH, Baek YH, Roh YH, Choi HJ, Kim YH, Jung GJ (2012) Review of 100 cases of single port laparoscopic cholecystectomy. J Korean Surg Soc 82(3):179–184. doi:10.4174/jkss.2012.82.3.179
Ahn SH, Son SY, Jung do H, Park do J, Kim HH (2014) Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 219(5):933–943. doi:10.1016/j.jamcollsurg.2014.07.009
Poon JT, Cheung CW, Fan JK, Lo OS, Law WL (2012) Single-incision versus conventional laparoscopic colectomy for colonic neoplasm: a randomized, controlled trial. Surg Endosc 26(10):2729–2734. doi:10.1007/s00464-012-2262-z
Pietrabissa A, Sbrana F, Morelli L, Badessi F, Pugliese L, Vinci A, Klersy C, Spinoglio G (2012) Overcoming the challenges of single-incision cholecystectomy with robotic single-site technology. Arch Surg 147 (8):709–714. doi:10.1001/archsurg.2012.508
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1007706). The authors would like to thank Anthony Thomas Milliken, ELS (Editing Synthase, Seoul, Korea) for his help with the editing of this manuscript and thank Soyoung Kim, Kyoung Hee Lee, and other nursing staff members for their professional help and enthusiasm.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Mrs. Youn Nam Kim, Mr. Dong-Su Jang, Drs. Seungho Lee, Jin Kyong Kim, Yoo Min Kim, Taeil Son, Woo Jin Hyung, and Hyoung-Il Kim have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Lee, S., Kim, J.K., Kim, Y.N. et al. Safety and feasibility of reduced-port robotic distal gastrectomy for gastric cancer: a phase I/II clinical trial. Surg Endosc 31, 4002–4009 (2017). https://doi.org/10.1007/s00464-017-5435-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5435-y