Abstract
Background
The role of laparoscopic liver resection (LLR) for large or multiple intrahepatic cholangiocarcinomas (ICCs) remains equivocal. The main concerns are potential risks of inadequate resection margin, tumor rupture, uncontrollable bleeding, tumor seeding, and inadequate lymph node sampling. In this study, we aimed to determine the safety, feasibility, and oncological efficacy of LLR for large (≥5 cm) or multiple (≥2) ICCs.
Methods
Among 50 patients receiving liver resection for ICC between May 2004 and January 2016, 12 patients who had undergone LLR for large or multiple ICCs (Group A, n = 12) were compared with 18 patients who had undergone LLR for small solitary ICCs (Group B, n = 18), as well were compared with 20 patients who had undergone open liver resection for large or multiple ICCs (Group C, n = 20). Perioperative and long-term outcomes were analyzed.
Results
Compared with Group B, Group A had fewer patients with T1 tumors (58.3 vs. 100%; P = 0.006) and a longer hospital stay (14 vs. 9 days; P = 0.039); operating time, blood loss, surgical margin, cases receiving lymph node dissection, conversion rates, and morbidity were comparable. There were no life-threatening complications and no mortality. No tumor rupture or dissemination occurred, nor did port-site recurrence follow surgery. After a median follow-up of 22 months, no difference was noted in 3-year overall survival (56.3 vs. 59.5%; P > 0.05) and recurrence-free survival (43.8 vs. 50%; P > 0.05) between the two groups. Similarly, perioperative and long-term outcomes were comparable between Group A and Group C.
Conclusion
LLR for large or multiple ICCs is technically safe, feasible, and oncologically effective in select patients. It provides a favorable option for patients seeking curative treatment. The minimally invasive nature will benefit these patients without compromising the oncological efficacy. Future larger-scale studies and well-designed randomized trials are warranted to evaluate this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intrahepatic cholangiocarcinoma (ICC), which originates from cholangiocytes, is a fatal malignant disease that represents the second most common primary liver cancer [1]. It has been reported that the incidence of ICC is steadily rising [2, 3]. In the USA, this rate is increasing with an annual percentage change of 2.30%, from 0.44 per 100,000 in 1973 to 1.18 per 100,000 in 2012 [4]. Liver resection remains the definitive treatment for patients with ICC. Nevertheless, more than half of ICC patients [5] develop recurrence despite complete surgical extirpation of the disease, and prognosis remains unsatisfactory such that the 5-year overall survival (OS) after surgery was recently reported to be between 20 and 45% [6–9].
In recent decades, laparoscopic liver resection (LLR) has gained widespread acceptance [10, 11] and has been widely adopted for the treatment of malignant liver disease, given its minimally invasive nature and well-known advantages including less blood loss and operating time, shorter hospital stay, lower complication rates, and survival outcomes comparable to open liver resection (OLR) [12–14]. Although a growing number of studies [15–19] have also demonstrated safe execution of LLR for large or multiple hepatocellular carcinomas or colorectal liver metastases, few reports [20–22] on LLR for ICC exist and these focus mainly on small solitary tumors. Data on the feasibility and safety of LLR for large or multiple ICCs remain unclear. The main concerns for such surgery are potential risks of inadequate resection margin, tumor rupture, uncontrollable bleeding, tumor seeding, and failure of lymph node dissection (LND) under laparoscopy. Therefore, whether the minimally invasive nature will benefit patients with large or multiple ICCs without compromising the oncological efficacy thus remains an interesting topic.
In 2013, Takahashi and colleagues [23] reported one case of LLR for a patient with a large ICC of 77 × 50 mm and successfully achieved laparoscopic LND. However, this case report lacks long-term oncological evaluation, and more data with larger series are warranted to provide guidance for surgeons wishing to perform LLR for large or multiple ICCs. We report herein our preliminary experience on perioperative and long-term outcomes of LLR for the treatment of large (≥5 cm) or multiple (≥2) ICCs and also compare the results with LLR for small solitary ICCs, as well with OLR for large or multiple ICCs. To the best of our knowledge, this is the first study to specifically focus on the safety, feasibility, and oncological efficacy of LLR for large or multiple ICCs, and also the first to include both comparison between LLR for ICC regarding different tumor size and number, and between different surgery options for large or multiple ICCs.
Methods
Patients
Data from patients who underwent liver resection for ICC between May 2004 and January 2016 were retrospectively reviewed. Three major surgeons participated over this time period in this study. Patients were selected after excluding the following cases: (1) concurrent other cancer; (2) palliative resections; (3) periductal infiltrative ICC; and (4) procedures requiring vascular or biliary reconstruction. Of a total of 50 cases of liver resections that met this criteria, 30 underwent LLR for large or multiple ICCs (Group A, n = 12) or for small solitary ICCs (Group B, n = 18), while 20 underwent OLR for large or multiple ICCs (Group C, n = 20). A main comparison was conducted between Group A and Group B. A comparison analysis of perioperative and long-term outcomes was also made between Group A and Group C. Cancer stage was established according to the Tumor Node Metastasis (TNM) classification based on the American Joint Committee on Cancer Staging Manual, 7th edition [24]. LND was performed in patients who had enlargement of lymph nodes detected by intraoperative ultrasonography or preoperative imaging materials. Complications were graded according to the Clavien–Dindo classification [25]. Major resection was defined as resecting more than three Couinaud’s segments or resecting the right posterior lobe according to the 2015 Morika recommendations for LLR [10]. This study was approved by the Institutional Review Board of the Sir Run Run Shaw Hospital, Hangzhou, China.
Surgical technique
The basic technique of LLR was described in our previous reports [26, 27] and remained unchanged during our study. In brief, the patient was placed in the supine position under general anesthesia. A carbon dioxide pneumoperitoneum maintained between 10 and 14 mm Hg was established. Four to five ports were generally used based on the surgical extent. The location of the tumor was examined by direct vision or via intraoperative ultrasonography. A transection line was usually marked along the surface of the liver using diathermy. In major hepatectomy, liver hilar dissection was performed and selective inflow occlusion was achieved to minimize the risk of bleeding. Liver parenchymal transection was performed by Laparoscopic Multifunctional Dissector (LPMOD) using the technique of aspiration and curettage [26, 27]. The resected specimen was removed through a remote suprapubic incision. A drainage tube was usually placed in the resection area to drain intra-abdominal fluid.
Follow-up plan
Patients who received resection for ICC underwent a routine follow-up plan. All patients were checked in the outpatient clinic every 3 months for the first 2 years and every 6 months thereafter. The maximum follow-up for each patient in this study was 60 months after surgery. Laboratory tests including biochemistry liver function test, serum cancer antigen 19-9, and routine blood tests were performed. Patients with suspected recurrence also underwent contrast-enhanced computed tomography or magnetic resonance imaging for further detection.
Statistical analysis
Continuous variables were reported as medians with ranges. The Mann–Whitney U test was used to compare significant differences of the continuous variables, and Fisher’s exact test was used to compare the categorical variables between groups. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. All analyses of data were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Significance was defined as a P value of less than 0.05.
Results
Baseline characteristics
Baseline characteristics are summarized in Table 1. No significant difference was observed between Groups A and B in terms of baseline characteristics.
Intraoperative data
Intraoperative data are listed in Table 2. Thirteen patients (43.3%) underwent major hepatectomy and 17 (56.7%) minor hepatectomy. Major hepatectomy tended to be more frequently applied in Group A (58.3 vs. 33.3%), but the difference was not significant (P = 0.264). The median operating time and median blood loss were shorter and less in Group B [170 (80–445) min and 200 (20–1200) ml, respectively] than in Group A [212.5 (60–500) min and 350 (30–2000) ml, respectively], but the difference was also not statistically significant (both P > 0.05). Five patients (16.7%) underwent conversions intraoperatively because of exposure problems (n = 2), tight adhesion (n = 2), and uncontrollable bleeding (n = 1). Five patients (16.7%) received intraoperative blood transfusions. LND was performed in six cases (20%). No significant difference was noted in the rates of patients receiving conversions, transfusions, or LND between the two groups (all P > 0.05).
Pathology and postoperative outcomes
Pathology and postoperative outcomes are presented in Table 3. The patients in Group A had a larger median tumor size (5.25 vs. 2.75 cm, P < 0.001). Nine patients (30%) had a maximum tumor size larger than 5 cm (8 with a solitary lesion and 1 with multiple lesions), whereas 21 (70%) had a maximum tumor size less than 5 cm (18 with a solitary lesion, 3 with multiple lesions). Group A had fewer patients with T1 tumors (58.3 vs. 100%, P = 0.006) and was more likely to have positive lymph nodes (25% vs. 0, P = 0.054). The number of patients with vascular invasion, histological grade of ICC, number of R0 resections, and depth of surgical margin was comparable between the two groups (all P > 0.05).
The morbidity of complications was similar between the two groups (25 vs. 27.8%, P = 1). Severe complications (graded IIIa or more according to the Clavien–Dindo classification) occurred in two cases (16.7%) in Group A and one case (5.6%) in Group B. These three complications included two cases of pleural effusion requiring thoracentesis (graded IIIa, one in Group A and another in Group B) that recovered soon after the intervention, and one case of gastroparesis receiving nasogastric intubation (graded IIIa, in Group A) that resolved gradually within 2 weeks postoperatively. The median length of postoperative hospital stay was significantly longer in Group A [14 (6–23) days] than in Group B [9 (4–46) days, P = 0.039]. No deaths were noted during hospitalization.
Long-term outcomes
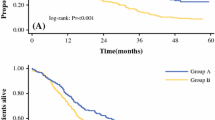
After a median follow-up of 22 (range 3–60) months, the OS and recurrence-free survival (RFS) outcomes were analyzed by the Kaplan–Meier method. The median follow-up of Groups A and B was 17.5 (range 3–60) and 24 (range 5–60) months, respectively. No significant difference was noted in the median follow-up between the two groups (P = 0.518). The estimated 3-year OS and RFS rates in all patients were 56.7 and 46.7%, respectively. There was no significant difference in the 3-year OS rate (56.3% vs. 59.5%, P = 0.576) nor in the 3-year RFS rate (43.8 vs. 50%, P = 0.359) between the two groups (Fig. 1). Of the total 30 patients, 13 (43.3%) experienced ICC recurrence (six in Group A and seven in Group B), intrahepatic recurrence being the most frequent. There was no significant difference in recurrence rates between the two groups (50 vs. 38.9%, P = 0.711). Eight patients died, all of disease progression (four in Group A and four in Group B), with no significant difference in mortality between the two groups (33.3 vs. 22.2%, P = 0.678).
Survival curves of laparoscopic liver resection for large or multiple ICCs (Group A) and laparoscopic liver resection for small solitary ICCs (Group B). A Comparison of estimated 3-year overall survival between Group A (56.3%) and Group B (59.5%). B Comparison of estimated 3-year recurrence-free survival between Group A (43.8%) and Group B (50%)
Comparison with OLR for large or multiple ICCs
To further confirm the feasibility, safety, and oncological efficacy of LLR for large or multiple ICCs, we also compared these 12 cases of LLR (Group A) with 20 cases of OLR for large or multiple ICCs (Group C) occurring in the same period. There was no statistically significant difference in perioperative data between Groups A and C (Table 4). Long-term outcomes were also comparable between the two groups with regard to the estimated 3-year OS rates (56.3 vs. 32.7%, P = 0.282) and RFS rates (43.8 vs. 27.9%, P = 0.940) (Table 4). These data showed that LLR was as feasible and safe as OLR, and not inferior to OLR in terms of oncological efficacy for large or multiple ICCs.
Discussion
In recent decades, LLR has progressively been applied for the treatment of many malignant liver diseases [11, 28]. According to a recent review [11], among a total of 9527 cases of LLR performed worldwide, 6190 (65%) were for malignancy. Hepatocellular carcinomas (n = 3072, 49.6%) and colorectal liver metastases (n = 1582, 25.6%) were the most common indications for LLR, whereas cholangiocarcinomas constitute a small proportion (n = 116, 1.87%). Although the developed techniques, advanced equipment, and accumulated expertise in LLR have widened its indications to include large or multiple hepatocellular carcinomas or colorectal liver metastases [15, 17–19, 29], thus far, no study available to date has focused specifically on the safety and feasibility of LLR for large or multiple ICCs, nor has its long-term oncological outcomes been reported.
In fact, large or multiple ICCs could be regarded as a contraindication for LLR owing to concerns regarding difficulty in achieving R0 resection and LND and the potential risks of tumor rupture, massive bleeding, and tumor seeding. Although traditional liver resection was reported as feasible [30] in resecting large or multiple ICCs, laparoscopic surgery is more technically challenging because it cannot avoid loss of tactile feedback and there is limited space available for manipulation. Large or multiple ICCs seem to represent a restricted zone for many surgeons wishing to attempt LLR.
Therefore, whether this minimally invasive approach is feasible and safe for patients with large or multiple ICCs is worth being explored. Actually, the primary purpose of this study was to determine the safety and feasibility of LLR for large or multiple ICCs.
Improvements in laparoscopic technique and patient selection have made the gradual adoption of LLR for ICC feasible [20–22]. However, these studies mainly focused on small solitary ICCs (tumor size always less than 5 cm). In the current study, all 12 cases of LLR for large (≥5 cm) or multiple (≥2) ICCs (Group A) were safely and successfully performed. Three cases (25%) required conversions intraoperatively that did not occur in conditions of emergency. R0 resection was achieved in all 12 patients (100%) with a median margin of 10 mm. Four cases (33.3%) underwent LND totally laparoscopically. There was no uncontrollable bleeding or other life-threatening complications, and no mortality. The comparison analysis of Groups B and C further confirmed the safety and feasibility of LLR for large or multiple ICCs.
However, patients receiving LLR for large or multiple ICCs (Group A) experienced a longer length of postoperative hospital stay (14 vs. 9 days; P = 0.039) than those with small solitary lesions (Group B), one possible reason being that Group A received more major resections (58.3 vs. 33.3%), which might have led to more injury and prolonged time for postoperative recovery; moreover, more major complications occurred in Group A (16.7 vs. 5.6%), which has been reported to correlate with a longer hospital stay [31], although no significant difference was noted in both aspects (both P > 0.05). Group A also tended to have a longer operating time and more blood loss than Group B. It seems that large tumor size and multiple lesions increase the complexity of the procedures and cause more injuries during resection. A further possible influential factor is the learning curve [32, 33] of LLR required for major or complicated procedures to treat large or multiple ICCs. For treating large or multiple ICCs, although LLR appears to be a complicated procedure, it remains a safe and feasible treatment.
Moreover, whether this minimally invasive approach can benefit those patients without compromising oncological efficacy remains to be solved. Therefore, another purpose of the current study was to evaluate the long-term results of LLR for large or multiple ICCs.
It was reported [34, 35] that prognostic factors including large tumor size, multiple lesions, lymph node metastasis (LNM), and vascular invasion were associated with worse long-term survival of ICC after surgery. Group A had a larger median tumor size (5.25 vs. 2.75 cm, P < 0.001) and fewer patients with T1 tumors (58.3 vs. 100%, P = 0.006) than Group B. In fact, five (41.7%) patients in Group A had a T2 stage tumor (four with multiple lesions, one with vascular invasion), and Group A was more likely to have positive lymph nodes (25% vs. 0, P = 0.054) than Group B.
Though prone to have poorer prognostic factors, patients receiving LLR for large or multiple ICCs (Group A) still had OS and RFS comparable to those with small solitary ICCs (Group B). The 3-year OS and RFS for Group A were 56.3 and 43.8%, respectively, and for Group B were 59.5 and 50%, respectively (both P > 0.05). In addition, when comparing different surgical methods for resecting large or multiple ICCs, there is no significant difference in the 3-year OS and RFS between the laparoscopic approach (Group A) and the open approach (Group C). Collectively, it seems that LLR has a good curative effect in resecting large or multiple ICCs without compromising the oncological efficacy.
Interestingly, there are some possible explanations for these findings. R0 resection is associated with a better survival outcome for ICC patients [6, 36]. All patients (100%) who underwent LLR for large or multiple ICCs received R0 resections with an adequate surgical margin, which may largely contribute to a survival comparable to that for those receiving LLR for small solitary ICCs, and with its open counterpart. The R0 resection rate of LLR for large or multiple ICCs achieved in our study was comparable to other reported rates of 92.5–95.6% [18, 19] of LLR for other types of large malignancy.
Indeed, R0 resection was previously considered a major concern in LLR for large or multiple ICCs. Large or multiple ICCs may require extensive resections, which unquestionably make the procedures more complicated under laparoscopy, thus increasing the difficulty in guaranteeing a negative resection margin. Of note, the laparoscopic approach provides a comprehensive and magnified view for surgeons [37], allowing the anatomic structures to be carefully examined and lesions to be resected completely.
Laparoscopic intraoperative ultrasonography facilitates the safe and radical resection of large or multiple ICCs because it helps to better stage and locate tumors, access potential intrahepatic metastasis and vascular invasion, avoid major vascular injury, and increase the safety of the procedures [38, 39]. Another key is the use of the LPMOD [26, 27], which carries out various functions including dissection, coagulation, and cutting and aspiration simultaneously, contributing to better control of bleeding and visualization of the manipulating field. Furthermore, the “no-touch” principle was strictly obeyed and intraoperative tumor biopsy or compression was prohibited. A remote suprapubic incision was preferred to extract the specimen. As a result, no tumor rupture or dissemination occurred, nor did port-site recurrence follow surgery.
An additional concern in the LLR procedure for treating large or multiple ICCs is the difficulty in performing LND under a laparoscope. Indeed, the role of LND for ICC still remains controversial. Most previous studies urged surgeons to adopt LND as a routine procedure to provide accurate staging [9, 40, 41] for ICC as well as improve survival [42, 43]. However, other scholars [44, 45] argued against this because routine LND did not provide survival benefits. A recent study [46] also reported similar findings that among 51 patients without LNM, the OS rate was comparable between patients undergoing LND (n = 31) and those without (n = 18). In laparoscopic surgery, performing LND is more technically challenging because there is only a limited area available for manipulation under laparoscopy. Current data are scarce regarding laparoscopic LND for ICC. Ratti et al. [21] have reported 10 patients who successfully underwent laparoscopic LND and achieved adequate lymph nodes. Their further comparison analysis with another 10 patients who did not undergo laparoscopic LND demonstrated no difference in survival between the groups. In this study, we avoided extensive LND and performed laparoscopic LND in 6 patients (20%) who were suspected LNM by intraoperative ultrasonography or preoperative imaging. This rate is comparable to the recent reported rates ranging from 9% to 50% in laparoscopic ICC studies [20–22]. However, large or multiple ICCs increase the difficulty in achieving laparoscopic LND. Indeed, ICCs always correlate with LNM in the presence of large-sized tumors. It was also reported [42] that multiple ICC lesions were associated with an increased number of involved nodes (≥3). Nevertheless, in the current study, laparoscopic LND was also successfully performed in Group A, perhaps partly owing to the long-term accumulation of experience of surgeons performing minimally invasive surgery and hepatobiliary surgery at our center [27]. Further studies are warranted to evaluate the role of LND in LLR and its oncologic efficacy for large or multiple ICCs.
In sum, a large-sized tumor or multiple lesions of ICC did not lower the possibility or increase the risks of patients receiving LLR; the surgical technique also had no influence on survival outcomes of patients with large (≥5 cm) or multiple (≥2) ICCs. LLR for large or multiple ICCs is feasible, safe, and oncologically effective, and provides a favorable option for patients seeking curative treatment. The minimally invasive nature will benefit these patients without compromising the oncological efficacy.
This study does have limitations. The relatively small samples and inadequate follow-up time may deter surgeons from drawing a definitive conclusion from the survival results. Moreover, the retrospective non-randomized design also introduces bias. Larger-scale studies with more samples of patients and well-designed randomized trials are warranted to confirm the findings of this study in future procedures.
References
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289
Patel T (2001) Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 33(6):1353–1357
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24(2):115–125
Saha SK, Zhu AX, Fuchs CS, Brooks GA (2016) Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 21(5):594–599
Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Groeschl R, Gamblin TC, Marsh JW, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Choti MA, Gigot JF, Mentha G, Pawlik TM (2013) Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 153(6):811–818
Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM (2015) The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 22(12):4020–4028
Doussot A, Groot-Koerkamp B, Wiggers JK, Chou J, Gonen M, DeMatteo RP, Allen PJ, Kingham TP, D’Angelica MI, Jarnagin WR (2015) Outcomes after resection of intrahepatic cholangiocarcinoma: external validation and comparison of prognostic models. J Am Coll Surg 221(2):452–461
Vitale A, Spolverato G, Bagante F, Gani F, Popescu I, Marques HP, Aldrighetti L, Gamblin TC, Maithel SK, Sandroussi C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM (2016) A multi-institutional analysis of elderly patients undergoing a liver resection for intrahepatic cholangiocarcinoma. J Surg Oncol 113(4):420–426
Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, Wolf RF, Hansen PD (2016) Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 18(1):79–87
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261(4):619–629
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263(4):761–777
Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102(1):82–86
Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA (2015) Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery 157(2):211–222
Yoon SY, Kim KH, Jung DH, Yu A, Lee SG (2015) Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 29(9):2628–2634
Yoon YS, Han HS, Cho JY, Yoon CJ, Kim JH (2012) Laparoscopic approach for treatment of multiple hepatocellular carcinomas. Surg Endosc 26(11):3133–3140
Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG (2013) Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS ONE 8(8):e72328
Kwon Y, Han HS, Yoon YS, Cho JY (2015) Are large hepatocellular carcinomas still a contraindication for laparoscopic liver resection? J Laparoendosc Adv Surg Tech A 25(2):98–102
Shelat VG, Cipriani F, Basseres T, Armstrong TH, Takhar AS, Pearce NW, AbuHilal M (2015) Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 22(4):1288–1293
Nomi T, Fuks D, Louvet C, Nakajima Y, Gayet B (2016) Outcomes of laparoscopic liver resection for patients with large colorectal liver metastases: a case-matched analysis. World J Surg 40(7):1702–1708
Uy BJ, Han HS, Yoon YS, Cho JY (2015) Laparoscopic liver resection for intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 25(4):272–277
Ratti F, Cipriani F, Ariotti R, Gagliano A, Paganelli M, Catena M, Aldrighetti L (2015) Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 30(5):1999–2010
Lee W, Park JH, Kim JY, Kwag SJ, Park T, Jeong SH, Ju YT, Jung EJ, Lee YJ, Hong SC, Choi SK, Jeong CY (2016) Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 30(11):4835–4840
Takahashi M, Wakabayashi G, Nitta H, Takeda D, Hasegawa Y, Takahara T, Ito N (2013) Pure laparoscopic right hepatectomy by anterior approach with hanging maneuver for large intrahepatic cholangiocarcinoma. Surg Endosc 27(12):4732–4733
Nathan H, Pawlik TM (2010) Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 26(3):269–273
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Cai X, Li Z, Zhang Y, Yu H, Liang X, Jin R, Luo F (2014) Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc 28(4):1334–1341
Cai X, Duan L, Wang Y, Jiang W, Liang X, Yu H, Cai L (2016) Laparoscopic hepatectomy by curettage and aspiration: a report of 855 cases. Surg Endosc 30(7):2904–2913
Akyuz M, Yazici P, Yigitbas H, Dural C, Okoh A, Aliyev S, Aucejo F, Quintini C, Fung J, Berber E (2016) Oncologic results of laparoscopic liver resection for malignant liver tumors. J Surg Oncol 113(2):127–129
Cai L, Wei F, Yu Y, Yu H, Liang X, Cai X (2016) Laparoscopic right hepatectomy by the caudal approach versus conventional approach: a comparative study. J Laparoendosc Adv Surg Tech A 26(7):540–547
Spolverato G, Kim Y, Alexandrescu S, Popescu I, Marques HP, Aldrighetti L, Clark Gamblin T, Miura J, Maithel SK, Squires MH, Pulitano C, Sandroussi C, Mentha G, Bauer TW, Newhook T, Shen F, Poultsides GA, Wallis Marsh J, Pawlik TM (2015) Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaboration. Ann Surg Oncol 22(7):2218–2225
Doussot A, Lim C, Gomez Gavara C, Fuks D, Farges O, Regimbeau JM, Azoulay D (2016) Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. Br J Surg 103(13):1887–1894
Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B (2015) Learning curve for laparoscopic major hepatectomy. Br J Surg 102(7):796–804
Brown KM, Geller DA (2016) What is the learning curve for laparoscopic major hepatectomy? J Gastrointest Surg 20(5):1065–1071
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149(5):432–438
Spolverato G, Kim Y, Ejaz A, Alexandrescu S, Marques H, Aldrighetti L, Gamblin TC, Pulitano C, Bauer TW, Shen F, Sandroussi C, Poultsides G, Maithel SK, Pawlik TM (2015) Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg 150(6):538–545
Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L, Nuzzo G, Regimbeau JM (2011) Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 254(5):824–829
Ogiso S, Nomi T, Araki K, Conrad C, Hatano E, Uemoto S, Fuks D, Gayet B (2015) Laparoscopy-specific surgical concepts for hepatectomy based on the laparoscopic caudal view: a key to reboot surgeons’ minds. Ann Surg Oncol 22(Suppl 3):S327–S333
Vigano L, Ferrero A, Amisano M, Russolillo N, Capussotti L (2013) Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg 100(4):535–542
Araki K, Conrad C, Ogiso S, Kuwano H, Gayet B (2014) Intraoperative ultrasonography of laparoscopic hepatectomy: key technique for safe liver transection. J Am Coll Surg 218(2):e37–e41
Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM (2015) Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 17(8):669–680
Bagante F, Gani F, Spolverato G, Xu L, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Marsh JW, Pawlik TM (2015) Intrahepatic cholangiocarcinoma: prognosis of patients who did not undergo lymphadenectomy. J Am Coll Surg 221(6):1031–1040.e1–e4
Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S (2005) Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 29(6):728–733
de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM (2011) Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 29(23):3140–3145
Li DY, Zhang HB, Yang N, Quan Y, Yang GS (2013) Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol 19(47):9084–9091
Kim DH, Choi DW, Choi SH, Heo JS, Kow AW (2015) Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 157(4):666–675
Yamamoto Y, Turkoglu MA, Aramaki T, Sugiura T, Okamura Y, Ito T, Ashida R, Uemura S, Miyata T, Kato Y, Kakuta Y, Nakanuma Y, Uesaka K (2016) Vascularity of intrahepatic cholangiocarcinoma on computed tomography is predictive of lymph node metastasis. Ann Surg Oncol 23(Suppl 4):485–493
Acknowledgements
This study was supported by Grants from the Public Welfare Project of Technology Application Research of Zhejiang Province (No. 2013C33141).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
All authors have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Wei, F., Lu, C., Cai, L. et al. Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas?. Surg Endosc 31, 3646–3655 (2017). https://doi.org/10.1007/s00464-016-5399-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5399-3