Abstract
Background
The prevalence of type 2 diabetes is growing in both developed and developing countries and is strongly linked with the prevalence of obesity. Bariatric surgical procedures such as laparoscopic vertical sleeve gastrectomy (LVSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) are increasingly being utilized to manage related comorbid chronic conditions, including type 2 diabetes.
Methods
A systematic review of randomized controlled trials (RCTs) was undertaken using the PRISMA guidelines to investigate the postoperative impact on diabetes resolution following LVSG versus LRYGB.
Results
Seven RCTs involving a total of 732 patients (LVSG n = 365, LRYGB n = 367) met inclusion criteria. Significant diabetes resolution or improvement was reported with both procedures across all time points. Similarly, measures of glycemic control (HbA1C and fasting blood glucose levels) improved with both procedures, with earlier improvements noted in LRYGB that stabilized and did not differ from LVSG at 12 months postoperatively. Early improvements in measures of insulin resistance in both procedures were also noted in the studies that investigated this.
Conclusions
This systematic review of RCTs suggests that both LVSG and LRYGB are effective in resolving or improving preoperative type 2 diabetes in obese patients during the reported 3- to 5-year follow-up periods. However, further studies are required before longer-term outcomes can be elucidated. Areas identified that need to be addressed for future studies on this topic include longer follow-up periods, standardized definitions and time point for reporting, and financial analysis of outcomes obtained between surgical procedures to better inform procedure selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prevalence of type 2 diabetes is growing internationally, in both developed and developing countries. Obesity is an independent risk factor for the development of type 2 diabetes mellitus and other metabolic conditions [1]. Indeed, 55 % of the burden of diabetes has been attributed directly to obesity (as defined by elevated BMI), and this rises to 60 % when considered with inactivity [2]. Alarmingly, the collective disease burden of obesity alone is rapidly approaching that of tobacco, accounting for ~8 % of all disease burden and in some population groups, is already overtaking it [2].
With the rapidly growing rates of type 2 diabetes internationally, there is an increasing impact on individual health and quality of life as well as a subsequent economic burden associated. In Australia, type 2 diabetes accounts for approximately 11 % of the AUD$56 billion healthcare costs attributed directly or indirectly to obesity and overweight [3]. This same analysis has suggested that on an individual basis it costs over AUD$4000 per year to treat a patient with uncomplicated type 2 diabetes in Australia, and this more than doubles (AUD$9645) in patients with micro- and macro-vascular complications [3]. Similarly, a study that collectively reviewed the financial impact of diabetes in France, Germany, Italy, Spain and the UK estimate this to be in the order of EURO90 billion annually [4]. The direct costs associated with treating diabetes reported in this study ranged from EURO1708 to EURO5899 per patient; however, this varied by country, prevalence and methodology used for assessment [4]. In the USA, the total cost of diagnosed diabetes in 2012 was estimated at USD$245 billion (direct medical costs USD$176 billion and USD$69 billion from reduced productivity) [5].
In view of these considerable and potentially avoidable costs associated with type 2 diabetes, there is increasing global interest in identifying and supporting effective population-based prevention strategies along with sustainable individual management approaches to reducing the burden of type 2 diabetes and economic pressure caused by it and the widespread obesity that is associated with its increase. Bariatric surgical procedures, such as laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic vertical sleeve gastrectomy (LVSG), are becoming increasingly accepted as cost-effective and efficacious strategies to manage obesity-related chronic disease and metabolic conditions in the moderately to severely obese individuals [6–9]. LRYGB is a two-step procedure in which the gastric reservoir is significantly reduced and proximal intestine bypassed to induce a level of malabsorption to further facilitate weight loss [10]. Moreover, changes in gastric hormone signaling (such as peptide YY and glucagon-like factor 1) may further reduce appetite and modulate energy expenditure, therefore maintaining weight loss over long period of time [11]. LRYGB is well established as a bariatric and metabolic procedure. LVSG, on the other hand, is a newer procedure that was originally considered as being primarily restrictive, in which 90 % of the stomach is permanently removed to reduce the gastric capacity while maintaining the integrity of the pyloric sphincter. Further studies are suggesting that like LRYGB there may be an element of gastric hormone modification that occurs post-LVSG that may contribute to the outcomes obtained. It should be noted that neither of these procedures are without a degree of risk, and complications or failure to achieve the desired clinical outcomes may lead to further burden on the health system and reduced postoperative quality of life. Nonetheless, these procedures may also offer an additional treatment modality for the management of chronic comorbid conditions arising from or exacerbated by morbid obesity.
This systematic review describes the findings of the peer review literature regarding postoperative diabetes resolution reported from randomized control trials (RCTs) comparing LVSG and LRYGB bariatric procedures. Other clinical outcomes identified from the larger systematic review and meta-analysis of which this forms a part are described elsewhere [12, 13].
Materials and methods
Inclusion and exclusion criteria
The literature on the RCTs comparing postoperative type 2 diabetes resolution following LVSG and LRYGB procedures was reviewed. Additional inclusion criteria included adult subjects (>16 years), elective surgical patients randomized to receive either LVSG or LRYGB, and changes to comorbid disease from baseline.
Search strategies and data collection
Electronic databases (MEDLINE, PubMed, EMBASE, CINAHL, Cochrane Register of Systematic Reviews, Science Citation Index) were cross-searched for RCTs published between January 2000 and November 2015 to capture the studies published since Regan et al.’s [14] report of the LVSG as a stand-alone procedure. Search terms were tailored for each search engine in an attempt to identify all published papers meeting the inclusion criteria. Limits were set to RCTs and adult patients (>16 years) to reflect the inclusion criteria. Search strategies utilized included combinations of “laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopic”[All Fields]), “gastric sleeve”[All Fields] OR “sleeve gastrectomy”[All Fields] AND “Roux en Y”[All Fields] OR "gastric bypass"[All Fields] AND “outcomes”[All Fields]. Reference lists of existing review articles were examined for additional citations. Authors of included papers were contacted by e-mail for clarification or additional information where required. The present work was undertaken according to the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) [15]. Two authors (EO and MAM) individually appraised studies identified to assess for compliance with agreed inclusion criteria. One author (EO) undertook the data extraction. The authors were not blinded to the source of the document or authorship for the purpose of data extraction. The data compiled by both authors were compared, and consensus was achieved through discussion or contact with corresponding authors. The Jadad method for assessment of methodological quality of studies was applied to the included studies [16]. This score produces a number between one and five based on the reporting of randomization, blinding and accounting for all subjects at the end of the follow-up period, with higher scores representing a higher methodological quality [16].
Results
Included studies
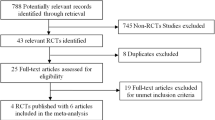
Search outcomes identified 478 citations through literature searches (n = 473) and hand searches of bibliographical information (n = 5). Fifty-eight full-text articles were retrieved and assessed against eligibility criteria following screening of abstracts. Fifty-one studies were excluded, of which 39 were found not to be in conformity with RCT study design, 11 were reviews (including existing systematic reviews or meta-analyses), four studies reported different outcomes or follow-up time frames of otherwise eligible studies, one described outcomes of bariatric procedures in an adolescent population, one reported clinical outcomes of LVSG versus open LRYGB, while another reported LVSG versus mini-gastric bypass. Three otherwise eligible studies did not report on diabetes resolution as an outcome. In addition, two protocols describing studies eligible for inclusion in this systematic review, that currently are in progress, were also located [17, 18]. Seven unique studies reported on type 2 diabetes resolution [19–25], while one paper providing additional information on postoperative glycemic control [26] on a smaller subset of the SM-BOSS study [21] was also included (and for the intents and purposes of the systematic review considered as part of the included Peterli et al. [21] publication). While a meta-analysis of the data obtained from this systematic review was initially intended, this was not possible due to differences in the variations in time intervals and methods of outcome reporting within the selected papers. See PRISMA diagram Fig. 1.
Seven RCTs involving a total of 732 patients (LVSG n = 365, LRYGB n = 367) reported on the resolution or improvement of comorbid disease following bariatric procedures [19–25]. Included studies were of a moderate methodological quality, with an average Jadad score of 3 (range 2–5). Randomization and withdrawals through the follow-up period were described by all studies, while blinding was reported to have occurred in only one study [23]. All included studies were conducted between 2005 and 2015 and published within the last 5 years. Follow-up periods reported ranged from 3 months to 5 years postoperatively, with 32–100 % at the final data collection point (mean 77.5 %). Three included papers [19, 24, 25], as well as a secondary publication from the SM-BOSS study [26], identified postoperative glycemic management as their primary outcome, while the other four included papers reported on changes in postoperative comorbid disease status as secondary outcomes (where weight loss was the primary outcome) [20–23]. Two studies specified using internationally recognized diagnostic criteria for type 2 diabetes definitions [21, 23]. Change in type 2 diabetes was defined by biochemical parameters [19, 24], reduction or cessation of medication requirements [19, 22], assessment by endocrinologist/physician responsible for follow-up [21] or measures were not clearly defined [20, 25]. Table 1 outlines the characteristics of included studies.
Of the included studies, two studies [19, 25] were conducted entirely on subjects with type 2 diabetes, while all other studies included a mix of diabetic and non-diabetic subjects that ranged from 17 [23] to 84 % [24] (average 38 %) of subjects. One study did not define the number of patients at baseline with type 2 diabetes, however, did include the presence of one or more comorbidity associated with obesity including but not limited to type 2 diabetes [22]. Time periods reported vary from 3 months to 5 years, and few reported at the same time intervals.
Improvement or resolution
Improvement in or full resolution of type 2 diabetes was reported by or able to be computed for all included studies. The methods for defining improvement or resolution were not clearly reported or varied between studies: Changes to oral hypoglycemic agents or insulin requirements [19–25] and/or biochemical measures [19, 21, 23–25] were the most frequently utilized methods.
Early diabetes improvement and resolution varied between the two studies reporting at 3 months postoperatively. Helmio et al. [22] reported 45 and 42 % improvement and 37 and 44 % resolution in type 2 diabetes with LVSG versus LRYGB, respectively, at 3 months, based on changes to unspecified diabetic medication requirements. Both procedures were reported to provide equivocal improvement or resolution of diabetes (LVSG 84.3 %, LRYGB 93.3 %, p = 0.6) [22]. de Barros et al. [24] on the other hand reported a 28 and 83 % resolution of type 2 diabetes with LVSG versus LRYGB, respectively, over the same time period based on measures of glycemic control (p = 0.023).
Helmio et al. [22] was the only study to have reported diabetes improvement or resolution at 6 months postoperatively. Further to their 3-month results, an additional 4 % of patients who received LVSG experienced complete resolution of their preoperative type 2 diabetes (41 %), assumedly accounting for the slightly lower proportion of patients at 6 months with diabetes improvement (43 % at 6 months vs. 45 % at 3 months) [22]. Similarly, in those who received LRYGB a further 7 % reported resolution of their preexisting diabetes compared to the results observed at 3 months (51 %), while numbers of those with diabetes improvement remained stable between the 3 and 6 months’ time points (42 %) [22].
Peterli et al. [21] reported both diabetes resolution and improvement at 12 months postoperatively. The authors identified 57.7 % of LVSG patients and 67.9 % of LRYGB patients with diabetes preoperatively ceased taking their diabetic medications. Additionally, they reported improvement in diabetes control in a further approximately 42 % of patients post-LVSG and 28 % post-LRYGB [21]. One patient in the LRYGB group had no improvement in their type 2 diabetes management at 1 year following the procedure [27]. Keidar et al. [25] reported no difference in remission rate of diabetes between surgical interventions at 12 months.
Two studies [19, 23] reported diabetes resolution at 3 years postoperatively. Kehagias et al. [23] reported relatively low diabetes prevalence at baseline (17 % in each intervention arm) and reported resolution of diabetes in 80 % of patients at 3 years postoperatively, with no difference between groups. Yang et al. [19] required poorly controlled type 2 diabetes (defined as HbA1C ≥7 % after 6 months of medical management) for inclusion in their study. At 36 months postoperatively, they report 78.6 % of patients post-LVSG and 85.2 % of patients post-LRYGB had achieved full remission of their type 2 diabetes as defined as HbA1C levels of ≤6 mmol/L and without further need for either oral hypoglycemic agents or insulin [19]. When considered in terms of HbA1C of ≤6.5 mmol/L, this increased to 89.3 and 92.6 % for LVSG and LRYGB, respectively [19].
Zhang et al. [20] is the only study to report beyond 3 years and include follow-up outcomes at 5 years postoperatively. Of the approximately 30 % of patients with type 2 diabetes preoperatively, 88.9 and 87.5 % of those undergoing LVSG and LRYGB, respectively, were reported to have resolution or improvement at 5 years [20]. No statistically significant difference was observed between interventions.
Glycosylated hemoglobin (HbA1C) and fasting blood glucose (FBG)
Four studies [19, 24–26] reported additional detail regarding changes to the biochemical parameters underpinning diabetes management. Baseline FBG measures varied widely between studies reflecting the differing inclusion criteria, but were not significantly different between groups within studies. Yang et al. [19] and Keidar et al. [25] reported baseline FBG of ~10 mmol/L (185 mg/dL) in their diabetic population, while de Barros et al. [24] and Peterli et al. [26] reported FBG around 6 mmol/L (108 mg/dL).
FBG within the first week and at 3 months postoperatively was described for a small subset [26] (LVSG n = 14, LRYGB n = 13) of the larger SM-BOSS study [21]. FBG improved between baseline, 1 week and 3 months by 2 and ~10 % post-LRYGB and 12 and ~15 % post-LVSG, respectively [26].
de Barros et al. [24] reported statistically significant postoperative changes in FBG at 3 months, with LRYGB affecting greater positive change than LVSG (p = 0.034). However, on average the FBG levels reduced by ~12 % in both groups from baseline to 3 months and remained within non-diabetic levels for both time points [24]. Likewise, HbA1C reduced by ~10 % in both groups at 3 months postoperatively, with no statistically significant difference observed between groups [24].
Keidar et al. [25] reported on FBG and HbA1C at 3 and 12 months postoperatively. Significant reductions in FBG from baseline to both data collection points, with an initial 62 and 51 % drop from baseline to 3 months in LVSG and LRYGB, respectively, and further 9 and 10 % reduction, respectively, between 3 and 12 months [25]. A statistically and clinically significant reduction in HbA1C was seen at 3 months (p < 0.001) and maintained at 12 months for both surgical procedures compared to baseline. No difference was seen at 12 months with regard to the degree of HbA1C reduction between procedures (LVSG 2.37 ± 2.22 %, LRYGB 1.57 ± 1.35 %, p = 0.34) [25].
Yang et al. [19] reported substantial and sustained improvements in FBG and HbA1C compared to baseline throughout the 3-year follow-up period in both procedures. FBG was seen to reduce from diabetic levels (~10 mmol/L [180 mg/dL]) to ~7.0 mmol/L (126 mg/dL) following both interventions at 3 months and progressively reduced throughout the follow-up period until stabilizing at 12 months postoperatively [19]. FBG at 3-year follow-up remained comparable at <6 mmol/L (<108 mg/dL) irrespective of intervention. HbA1C followed a similar pattern to that of FBG: A significant reduction was observed from baseline to 3 months (p < 0.05) and continued to decrease throughout the follow-up period until stabilizing at the 12 months [19]. Reduction in HbA1C appears to have occurred more rapidly between 3 and 9 months in those who received LRYGB than LVSG; however, by 9 months postoperatively HbA1C levels were comparable between groups and these lower levels continued to be sustained to the final data collection at 3 years postoperatively. No statistical differences were observed between procedures at 3 years (p = 0.3). When considering change in HbA1C from baseline, LRYGB appears to demonstrate a greater percentage of reduction than LVSG at each time point; however, this was not statistically significant at 3 years (LVSG 2.7 ± 1.1 %, LRYGB 3.1 ± 1.3 %; p = 0.1) [19] (Table 2).
Glucose tolerance
Keidar et al. [25] was the only study to investigate the postoperative effect on glucose tolerance as measured by oral glucose tolerance test (OGTT). LVSG demonstrated a reduction in 2-h glucose levels from 15.66 mmol/L at baseline to 9.39 ± 2.55 and 6.66 ± 3.27 mmol/L at 3 and 12 months, respectively (p < 0.001 for both time points from baseline, and p = 0.003 from 3 to 12 months), while LRYGB demonstrated a reduction from 15.72 ± 5.61 mmol/L at baseline to 7.22 ± 4.50 mmol/L at 3 months and 7.11 ± 3.33 mmol/L at 12 months (p < 0.001 for both from baseline) [25].
Measures of insulin secretion and resistance
The four studies [19, 24–26] that investigated glycemic outcomes as their studies’ primary outcome also reported measures of insulin secretion and/or resistance in addition to those of glycemic control. de Barros et al. [24] reported on both serum insulin and homeostasis model assessment of insulin resistance (HOMA-IR) scores (defined as FBG × 0.0555 × insulin/22.5, with normal values ≤3.40). Serum insulin was observed to reduce from baseline to 3 months postoperatively by ~30 % following LVSG and ~44 % following LRYGB; however, changes in this parameter were not assessed for statistical differences. No statistically significant difference was found between interventions with regard to serum insulin at baseline or at 3 months [24]. No significant changes were observed in HOMA-IR scores between groups pre- and postoperatively (p = 0.3) [24].
Peterli et al. [26] also reported significant reductions in postoperative insulin resistance associated with both procedures. From elevated but comparable insulin levels at baseline, these were seen to reduce by ~45 and ~50 % at 3 months in LVSG and LRYGB, respectively [26]. Similarly, elevated baseline HOMA index (defined as ≥3.8) indicating insulin resistance in both groups reduced by ~65 % in both arms postoperatively to near-normal levels at 3 months, with a statistically significant reduction from baseline (LRYGB mean 3.4 ± 0.3; LVSG mean 4.0 ± 0.6, p < 0.001) [26]. Interestingly, statistically significant changes in HOMA index (p = 0.018) at 1 week post-surgery were observed for both procedures [26].
Keidar et al. [25], on the other hand, measured incremental area under the curve at 30 min (AUC30) for both insulin and C-peptide and demonstrated a significant increase in both insulin and C-peptide AUC30 at both 3 and 12 months compared to baseline. They also computed insulinogenic index (change in insulin0–30/change in glucose0–30) which was shown to be comparable at baseline between groups and improve significantly at 3 months [LVSG 4.50 ± 5.23–6.51 ± 6.82 (p = 0.03); LRYGB 5.79 ± 7.70–8.43 ± 6.04, p = 0.01] [25]. Although these improvements were maintained at 12 months [LVSG 6.76 ± 5.28 (p = 0.02 from baseline); LRYGB 8.55 ± 7.6 (p = 0.01 from baseline)], no further improvements were observed between 3 and 12 months [25].
Finally, Yang et al. [19] reported on C-peptide levels as a measure of insulin secretion. C-peptide levels approached significant difference between groups at baseline (LVSG 2.2 ± 0.7 ng/mL; LRYGB 2.6 ± 1.0 ng/mL, p = 0.062) but were comparable at 3-year follow-up (1.7 ± 1.5 ng/mL, 1.8 ± 0.6 ng/mL, p = 0.285) [19]. Change in C-peptide from baseline levels to 3 months approached being significantly different (LVSG 0.5 ± 0.5 ng/mL, LRYGB 0.7 ± 0.4 ng/mL, p = 0.06) [19].
Reduction in anti-diabetic medication
Yang et al. [19] and Keidar et al. [25] report specifically on the changes to diabetic medication—both oral hypoglycemic agents (OHAs) and insulin—usage between baseline and final data collection point.
Keidar et al. [25] report the number of patients using OHAs, insulin and dietary modification to manage their glycemic control throughout their follow-up periods. Forty-seven percent (n = 9) of those who received LVSG were reliant on OHAs, 21 % (n = 4) on insulin and 26 % (n = 5) on dietary management at baseline. This reduced to 21 % (n = 4) on OHAs and no one on insulin and an increase to 74 % (n = 14) on dietary management alone at 3 months [25]. Further reductions in OHAs were noted at 12 months (15 %, n = 3), while one person (5 %) had resumed the use of insulin, and the number of patients remaining on dietary management was maintained [25]. In contrast, of those who received LRYGB, 54 % (n = 12) were managing their diabetes with OHAs, 18 % (n = 4) with insulin and a further 18 % (n = 4) with dietary management at baseline [25]. The use of OHAs reduced to 22 % (n = 5) at 3 months, but increased to 36 % (n = 8) at 12 months, while the requirement for insulin remained consistent between 3- and 12-month follow-up periods (9 %, n = 2). Dietary management which at 3 months was the primary management strategy for 59 % (n = 13) of patients post-LRYGB reduced to 41 % (n = 9) at 12 months [25].
From a baseline level of 96.9 % in the LVSG group, Yang et al. [19] report oral hypoglycemic use reduced to just 14.3 % at 3 years postoperatively. Similarly, from 93.8 % in the LRYGB group on oral hypoglycemic at baseline, only 7.4 % required these at 3 years postoperatively [19]. Looking at postoperative changes to insulin requirements, 47 and 56.2 % of those undergoing LVSG and LRYGB, respectively, at baseline were requiring insulin to manage their diabetes: At 3 years, this had reduced to only 2 patients (7.1 %) in the LVSG group postoperatively and no patients post-LRYGB with insulin requirements [19]. There was no difference between medication use at each time point irrespective of procedure performed [19].
Discussion
This systematic review demonstrates the ability of both LVSG and LRYGB to produce improvements in or complete resolution in type 2 diabetes. In studies that have continued to monitor comorbid disease for 3–5 years postoperatively, the improvements and disease remissions reported within and by the first postoperative year appear to be maintained. Though not specifically correlated in this review, individual examination of the included RCTs suggests this has remained the case even when some level of weight recidivism is concurrently reported.
With the exception of one study [24] reporting early improvement in glycemic control achieved with LRYGB (p = 0.023), all other included RCTs reported equivocal outcomes at the final data collection period between procedures. This suggests both LVSG and LRYGB are equally effective at bringing about resolution of this common comorbid condition in the bariatric surgery population. The one existing meta-analysis on the topic that similarly limits inclusion of RCTs, specifically in diabetic patients, provides comparable results. Wang et al. [28] determined no difference between the outcomes of LVSG versus LRYGB when assessed according to change in HbA1C, FBG or the use of anti-diabetic medication. However, in other systematic reviews and meta-analyses that have incorporated studies with varying research methodologies, considerable variations in results are reported. Cho et al. [29] reporting on 11 studies and Li et al. [30] reporting on 18 studies comprised of a mix of RCTs, prospective and retrospective study designs incorporating a range of surgical approaches, both suggest improved remission in type 2 diabetes is achieved following LVSG when compared to LRYGB in a treatment effect that approaches statistical significance (p = 0.07 and p = 0.03, respectively). Yip et al. [31], on the other hand, included 33 studies of unspecified methodology, reviewed the proportion of remission of type 2 diabetes in 1177 patients (LRYGB n = 998, LVSG n = 179) and determined that 59 % of patients post-gastric bypass procedure and 51.2 % patients post-sleeve gastrectomy were free of diabetes by using a definition of diabetes as “HbA1C < 6 %” at the end of the data collection period. Furthermore, they reported that diabetes remission rates had stabilized by 12 months postoperatively [31]. However, there remains concern regarding the reliability and validity of these results, due to the fact that various levels of evidence, i.e., from level I to level IV were included in their analysis.
In reviews that did not limit the inclusion to diabetic subjects, results varied further. Zhang et al. [32] reported on the 2-year outcomes following LRYGB and LVSG in studies of varying methodology and reported analogous results in terms of type 2 diabetes resolution between both procedures (OR 1.05, 95 % CI 0.90, 1.23, p = 0.55). Zhang et al. [33], using similar inclusion criteria, reported a statistically significant reduction in type 2 diabetes in favor of LVSG (OR 3.29, 95 % CI 0.19, 19.56, p < 0.00001). Finally, the most recent systematic review and meta-analysis on the topic by Li et al. [34] likewise included a wide range of study methodologies in an adult population receiving LRYGB versus LVSG, however, reported a reduction in type 2 diabetes associated with LRYGB and did not achieve statistical significance (OR 1.27, 95 % CI 0.95, 1.69, p = 0.1). It is likely that heterogeneity introduced by non-controlled study designs and inclusion of surgical variations such as mini-gastric bypass and open as well as laparoscopic approaches to RYGB and VGS contribute considerably to the differences in these findings reported. For this reason, by virtue of the homogenous inclusion criteria applied to methodology and surgical considerations, the present review may be considered to provide the most robust, reliable and valid evidence presently available on the postoperative diabetic outcomes of LRYGB versus LVSG in an obese population.
One notable element missing from peer review literature currently is a financial analysis, based on the systematic reviews and meta-analyses comparing various bariatric procedures. Presently, broader modeling and systematic reviews of studies that combine multiple bariatric procedures and compare these to conservative or non-surgical management have been conducted [9, 35]. These suggest that in the long term (10 years or a lifetime) providing an obese patient with bariatric surgery will result in healthcare cost savings, while it is assumed that with the reduction in chronic and comorbid disease such as those described in this review associated with LRYGB and LVSG, subsequent reductions in both non-health and healthcare-associated costs should also be evident. Studies or subsequent analyses that investigate and compare the relative cost–benefit of commonly performed bariatric procedures such as LRYGB and LVSG are now required, particularly as bariatric surgery becomes a more accepted and common practice, to provide an additional layer of information to inform decision making regarding procedure selection in this patient population.
Similarly, with improvements in comorbid disease resolution, subsequent improvement in patient-reported quality of life (QoL) and functional measures should also be evident, yet are rarely investigated as part of RCTs looking at other clinical outcomes and comorbid disease improvement. Only one of the included studies in the present work incorporated QoL assessment into their study design and reported outcomes [20]. Although a few studies investigate QoL as their primary outcome in studies comparing a variety of bariatric surgical procedures [36–38], much of the works on this aspect of postoperative outcomes do not include a surgical comparator [39–41] or, like the cost-effectiveness studies, are focused on surgical versus non-surgical interventions for obesity [42–44].
While weight loss has traditionally been considered as the mechanism by which bariatric surgery facilitates improvements in diabetes and glycemic control, early improvements in metabolic features reported in some of the included RCTs before significant weight loss is reported add weight to the counter-theories that other metabolic mechanisms contribute. In the present review, rapid early improvements in glycemic control in both procedures were noted, with LRYGB demonstrating more pronounced improvements than LVSG seen as early as 1 week postoperatively in FBG [26] and continuing with comparatively greater reductions in FBG and HbA1C until around 9 months postoperatively. By 12 months, these differences in procedures had disappeared and produced comparable results [19]. In addition to postoperative caloric restriction, modifications to the hormones influencing glucose metabolism are theorized to play a key role in mediating these early changes [45]. Pancreatic beta-cell function has been shown to be improved following both LVSG and LRYGB through enhanced secretion of glucagon-like peptide 1 (GLP-1) [46]. Further improvements in glucose metabolism are of particular interest following LRYGB where in addition to a degree of malabsorption being induced by the anatomical changes following the surgery, the neuroendocrine handling of nutrients prematurely delivered to the distal small bowel leads to increased secretion of the glucagon-like peptide (GLP-1) which most likely contributes to improved glucose regulation and subsequent enhanced insuling secretion [45]. Furthermore, anorexic hormones such as peptide YY and oxyntomodulin may also be enhanced, and glucotrophic glucose-dependent insulinotropic polypeptide secretion decreases following LRYGB [45]. The role of modifications to gastric and/or intestinal hormonal secretion is supported by the lack of impact on hepatic or peripheral insulin sensitivity demonstrated following 600 kcal/day caloric restriction in the absence of surgery or gastric banding that is clearly evident following RYGB [47]. Furthermore, early changes to hepatic insulin sensitivity that are followed later by changes to peripheral insulin sensitivity have been described after RYGB [48]. Other mechanisms theorized that may contribute to the improvement in glucose metabolism include changes in bile salt metabolism following both LVSG and LRYGB [49], and changes to the gut microflora following RYGB [49].
Limitations
There are a number of limitations that need to be considered with regard to this systematic review.
First, the limited availability of long-term follow-up data is a major limitation in the literature on this topic at the present time. Half of all included studies in this review reported data on 12-month postoperative follow-up or less, which restricts the ability to draw conclusions about the long-term impact of the surgical procedures investigated. Whether improvement and/or resolution of comorbid disease continues beyond the presently reported follow-up period, and how this may be affected by weight recidivism, postoperative hormonal changes or revisional surgeries remain unclear. Further to this point are a number of patients lost to follow-up in the studies that extend beyond 1 year postoperatively. Peterli et al. [21] reported follow-up of only 32 % of the original participants at the final data collection point of 3 years and reported data only to 1 year postoperatively, when retention of patients remained 100 %. Intention-to-treat analyses are used by all three studies [19, 20, 23] that reported outcomes beyond the first postoperative year, while their actual retention of patients ranged from 81 [20] to 96 % [23] (average 88.2 %). Embedding strategies into research protocols that optimize retention and/or understanding patient dropout is an important requirement to ensure the efficacy and benefit in these bariatric procedures over a longer period of time can be determined. This may also contribute to managing loss to follow-up in standard clinical practice in this patient group. Furthermore, studies extending beyond an intermediate duration of postoperative follow-up are essential for an objective assessment and cost–benefit analyses on the impact of bariatric surgery over the long term.
Second, there are multiple definitions of disease resolution, which range from accepted international standards to more subjective measures such as changes to usage of relevant medication classes. For this reason, it is unclear how comparable broad categories such as disease “improvement” or “resolution” are between studies and therefore the appropriateness of combining results in a systematic review. This also highlights the need for adopting standardized definitions to form an important part of the protocol development for future publications on this topic.
Third is the variation between reporting intervals in the studies that were included. Of the included studies, only the time points of 3 months and 3 years were reported on by more than one study. This limits the ability to make direct comparison to outcomes between studies and procedures. Similar to the need for standardized definitions of disease resolution, an agreement for controlled trials to report on set data points at established time points would significantly assist in future reviews of this type, particularly where meta-analysis is possible.
Fourth, the present review has made no attempt to correlate or compare weight loss outcomes to the effect on diabetic resolution/improvement during the documented follow-up periods. Given that weight loss is likely, a significant factor driving the resolution of diabetes and some other comorbidities such as joint and musculoskeletal conditions and obstructive sleep apnea, consideration of these clinical outcomes in the absence of an attempt to correlate with weight outcomes perhaps does not describe the outcome fully. Similarly, if the present findings are intended to be used to assist in clinical decision making regarding procedure selection, additional consideration should be given to the complications associated with each procedure. There is therefore the risk of oversimplifying a series of complex results by describing various clinical outcomes separately.
Fifth is the moderate methodological quality of the included studies. Of the six included studies, only one obtained a score of greater than three (of the possible five) according to the Jadad score [16] by virtue of its inclusion of blinding, an element omitted in all other included studies. Traditional measures of methodological quality such as those assessed by Jadad score are often difficult to apply to surgical studies where blinding may not be logistically possible or ethical. The usefulness of methodological assessments within systematic reviews and meta-analysis remains a source of contention, and recommendations to individually assess studies against predetermined methodological qualities relevant to the given study context are gaining favor. When considered in this light, the methodological quality of the included papers may perform better than their Jadad score implies.
Finally, at the present time there remains a relatively small number of RCTs investigating this topic, and this in conjunction with the limitations outlined above reduces the statistical power of the analyses performed. Despite this, we believe that by utilizing RCTs exclusively for inclusion in this systematic review, the present work represents a synthesis of the strongest evidence presently available in the literature.
Conclusions
In conclusion, this systematic review of RCTs suggests that both LVSG and LRYGB are effective in resolving or improving preoperative type 2 diabetes in obese patients during the reported 3- to 5-year follow-up periods. Further studies are required before longer-term outcomes can be elucidated. Areas identified that need to be addressed for future studies on this topic include longer follow-up periods, standardized definitions and time point for reporting, and financial analysis of outcomes obtained between surgical procedures to better inform procedure selection.
References
Preventative Health Taskforce (2009) Australia: the healthiest country by 2020. In: Obesity Working Group ed. Technical report 1: Obesity in Australia an need for urgent action, including addendum for October 2008 to June 2009. Australian Government, Canberra
Sassi F, Devaux M, Checchini M, Rusticelli E (2009) The obesity epidemic: analysis of past and projected future trends in selected OECD countries. In: OECD Health working papers no 45. OECD, Paris
Colagiuri S, Lee CM, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, Caterson ID (2010) The cost of overweight and obesity in Australia. Med J Aust 192:260–264
Kanavos P, van den Aardweg S, Schurer W (2012) Diabetes expenditure, burden of disease and management in 5 EU countries. In: LSE Health, London School of Economics, 113
American Diabetes Association (2013) Economic costs of diabetes in the U.S. in 2012. Diabetes Care 36:1033–1046
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev 8:CD003641
Organisation for Economic and Cooperation Development (2014) OBESITY update. In June 2014: OECD Directorate for Employment, Labour and Social Affairs [cited 2016 27 January 2016]. http://www.worldobesity.org/resources/aboutobesity/
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13:1–190, 215–357, iii–iv
Borisenko O, Adam D, Funch-Jensen P, Ahmed AR, Zhang R, Colpan Z, Hedenbro J (2015) Bariatric surgery can lead to net cost savings to health care systems: results from a comprehensive European decision analytic model. Obes Surg 25:1559–1568
Suter M, Donadini A, Romy S, Demartines N, Giusti V (2011) Laparoscopic Roux-en-Y gastric bypass: significant long-term weight loss, improvement of obesity-related comorbidities and quality of life. Ann Surg 254:267–273
Miras AD, le Roux CW (2013) Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 10:575–584
Osland E, Yunus RM, Khan S, Memon B, Memon MA (2016) Late postoperative complications in laparoscopic sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-y gastric bypass (LRYGB): meta-analysis and systematic review. Surg Laparosc Endosc Percutaneous Tech 26:193–201
Osland E, Yunus RM, Khan S, Alodat T, Memon B, Memon MA (2016) Postoperative early major and minor complications in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review. Obes Surg 26:2273–2284. doi:10.1007/s11695-016-2101-8
Regan JP, Inabnet WB, Gagner M, Pomp A (2003) Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 13:861–864
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 339:b2535
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Biter LU, Gadiot RP, Grotenhuis BA, Dunkelgrun M, van Mil SR, Zengerink HJ, Smulders JF, Mannaerts GH (2015) The Sleeve Bypass Trial: a multicentre randomized controlled trial comparing the long term outcome of laparoscopic sleeve gastrectomy and gastric bypass for morbid obesity in terms of excess BMI loss percentage and quality of life. BMC Obes 2:30
Fischer L, Wekerle AL, Bruckner T, Wegener I, Diener MK, Frankenberg MV, Gartner D, Schon MR, Raggi MC, Tanay E, Brydniak R, Runkel N, Attenberger C, Son MS, Turler A, Weiner R, Buchler MW, Muller-Stich BP (2015) BariSurg trial: sleeve gastrectomy versus Roux-en-Y gastric bypass in obese patients with BMI 35–60 kg/m2—a multi-centre randomized patient and observer blind non-inferiority trial. BMC Surg 15:87
Yang J, Wang C, Cao G, Yang W, Yu S, Zhai H, Pan Y (2015) Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m2. BMC Surg 15:88
Zhang Y, Zhao H, Cao Z, Sun X, Zhang C, Cai W, Liu R, Hu S, Qin M (2014) A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg 24:1617–1624
Peterli R, Borbely Y, Kern B, Gass M, Peters T, Thurnheer M, Schultes B, Laederach K, Bueter M, Schiesser M (2013) Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 258:690–694 (discussion 695)
Helmio M, Victorzon M, Ovaska J, Leivonen M, Juuti A, Peromaa-Haavisto P, Nuutila P, Vahlberg T, Salminen P (2014) Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow up. SJS 103:175–181
Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F (2011) Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2. Obes Surg 21:1650–1656
de Barros F, Setubal S, Martinho JM, Monteiro AB (2015) Early endocrine and metabolic changes after bariatric surgery in grade III morbidly obese patients: a randomized clinical trial comparing sleeve gastrectomy and gastric bypass. Metab Syndr Relat Disord 13:264–271
Keidar A, Heschkop KJ, Marko L, Schweiger C, Hecht L, Bartov N, Kedar A, Weiss R (2013) Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetalogica 56:1914–1918
Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C (2009) Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250:234–241
Peterli R. Personal communication regarding diabetes resolution/improvement from SM-BOSS paper (Ann Surg 2013). Via email communication with E Osland 16 June 2016
Wang MC, Guo XH, Zhang YW, Zhang YL, Zhang HH, Zhang YC (2015) Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for obese patients with Type 2 diabetes: a meta-analysis of randomized controlled trials. Am Surg 81:166–171
Cho JM, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ (2015) Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis 11:1273–1280
Li JF, Lai DD, Lin ZH, Jiang TY, Zhang AM, Dai JF (2014) Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutaneous Tech 24:1–11
Yip S, Plank LD, Murphy R (2013) Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta-analysis of outcomes. Obes Surg 23:1994–2003
Zhang C, Yuan Y, Qiu C, Zhang W (2014) A meta-analysis of 2-year effect after surgery: laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus. Obes Surg 24:1528–1535
Zhang Y, Wang J, Sun X, Cao Z, Xu X, Liu D, Xin X, Qin M (2015) Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass for morbid obesity and related comorbidities: a meta-analysis of 21 studies. Obes Surg 25:19–26
Li J, Lai D, Wu D (2016) Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg 26:429–442
Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, Sharma AM, Manns B, Tonelli M (2011) Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med 26:1183–1194
Major P, Matlok M, Pedziwiatr M, Migaczewski M, Budzynski P, Stanek M, Kisielewski M, Natkaniec M, Budzynski A (2015) Quality of life after bariatric surgery. Obes Surg 25:1703–1710
Nadalini L, Zenti MG, Masotto L, Indelicato L, Fainelli G, Bonora F, Battistoni M, Romani B, Genna M, Zoppini G, Bonora E (2014) Improved quality of life after bariatric surgery in morbidly obese patients. Interdisciplinary group of bariatric surgery of Verona (G.I.C.O.V.). Il Giornale di chirurgia 35:161–164
Campos GM, Rabl C, Roll GR, Peeva S, Prado K, Smith J, Vittinghoff E (2011) Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg 146:149–155
Laurino Neto RM, Herbella FA (2013) Changes in quality of life after short and long term follow up of Roux-en-Y gastric bypass for morbid obesity. Arq Gastroenterol 50:186–190
Julia C, Ciangura C, Capuron L, Bouillot JL, Basdevant A, Poitou C, Oppert JM (2013) Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes Metab 39:148–154
Bennett JC, Wang H, Schirmer BD, Northup CJ (2007) Quality of life and resolution of co-morbidities in super-obese patients remaining morbidly obese after Roux-en-Y gastric bypass. Surg Obes Relat Dis 3:387–391
Oh SH, Song HJ, Kwon JW, Park DJ, Lee YJ, Chun H, Kim S, Shim KW (2013) The improvement of quality of life in patients treated with bariatric surgery in Korea. J Korean Surg Soc 84:131–139
O’Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, Strauss B, Marks S, Schachter L, Chapman L, Anderson M (2006) Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 144:625–633
Karlsen TI, Lund RS, Roislien J, Tonstad S, Natvig GK, Sandbu R, Hjelmesaeth J (2013) Health related quality of life after gastric bypass or intensive lifestyle intervention: a controlled clinical study. Health Qual Life Outcomes 11:17
Knop FK, Taylor R (2013) Mechanism of metabolic advantages after bariatric surgery: it’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care 36(Suppl 2):S287–S291
Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E (2013) Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab 98:4391–4399
Ioconelli A, Gaggini M, Gastaldelli A, Mingrone G (2012) Short term effects of laparoscopic adjustable gastric banding (LAGB) and Roux-en-Y gastric bypass (RYGB) vs very low calorie diet (VLCD). Diabetologia 55(supp 1):584
Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S (2014) Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63:1725–1737
Cho YM (2014) A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J 38:406–415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Emma Osland, Rossita Mohamad Yunus, Shahjahan Khan, Breda Memon and Muhammed Ashraf Memon have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Osland, E., Yunus, R.M., Khan, S. et al. Diabetes improvement and resolution following laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a systematic review of randomized controlled trials. Surg Endosc 31, 1952–1963 (2017). https://doi.org/10.1007/s00464-016-5202-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5202-5