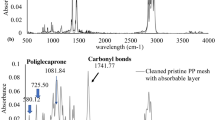
Abstract
Background
Oxidative degradation by reactive oxygen species (ROS) from inflammation initiates cross-linking, depolymerization, and formation of a quasi-crystalline quality in polypropylene (PP) meshes that cause embrittlement (J Urol 188:1052, 2012). Embrittlement leads to change in tensile strength and is associated with post-operative complications that include pain, adhesion, dislodgment, and fragmentation.
Methods
A laboratory environment was constructed to study the relationship between concentration of ROS and change in tensile strength. Samples of Ethicon Ultrapro© PP mesh were exposed to 1 mM, 0.1 M, or 1 M hydrogen peroxide solutions for 6 months and were subjected to load displacement tensile testing (LDTT) and compared to unexposed (0 M) meshes of the same brand.
Results
Load at failure and elongation to failure after LDTT were determined with 95 % confidence interval. For unexposed (0 M) samples, tensile strength was 28.0 ± 2.4 lbf and elongation to failure was 2.0 ± 0.3 in. For samples exposed to 1 mM, tensile strength was 19.2 ± 1.1 lbf and the elongation to failure was 2.0 ± 0.1 in. For samples exposed to 0.1 M, tensile strength was 19.3 ± 1.6 lbf and elongation to failure was 1.9 ± 0.1 in. For samples exposed to 1 M, tensile strength was 20.7 ± 1.2 lbf and elongation to failure was 0.47 ± 0.02 in.
Conclusion
The results demonstrated that a 6-month exposure to a physiologic range of ROS (1 mM) decreased tensile strength of PP mesh by 31 %. 1 mM and 0.1 M samples behaved similarly demonstrating properties of a quasi-crystalline nature. 1 M samples displayed qualities of extreme embrittlement. Scanning electron microscopy (SEM) observed fiber changes. 1 M meshes had features of brittle materials. Knowledge of changes in physical properties of PP meshes is useful for considerations for the development of a more biocompatible surgical mesh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In 1960, polypropylene surgical mesh was introduced by Usher and colleagues for hernia repair. Since this time, many new meshes have been developed in a wide variety of surgical specialties, most repair tissue in the anterior abdominal wall. Meshes are used and desired for their ability to “artificially produce tissue of density and toughness of fascia…” [1]. PP devices also can withstand sterilization while retaining flexibility; can be easily formed into different shapes, sizes, and thicknesses; and are of a relatively low cost [4]. According to the Technical Committee 150 on implants for surgery of the International Organization for Standardization, PP is a permanently implanted biomaterial [4]. A biomaterial is expected to show biocompatibility by being reasonably inert, non-toxic, non-carcinogenic, resistant to degradation, and able to sustain performance in the body. In spite of high expectations of biocompatibility and the extreme importance of knowing the specific physical properties of any biomaterial, suppliers of newer PP meshes are reluctant to publicize findings of the non-inert qualities of implants [2]. Meanwhile, PP surgical meshes have gained much scrutiny and numerous publications on their inability to show reliable biocompatibility and are even the subjects of several class action lawsuits. The devices degrade, shrink, and erode in the body leading to post-operative complications [7]. Recently, these devices have even raised speculation about their potential toxicity from additives and stabilizers added to PP in the manufacturing process that may leach from the material during its modification in the human body [6]. On January 3, 2012, the FDA issued an order requiring all manufacturers of surgical meshes used in transvaginal correction of incontinence to study the risks and effects of their devices by following patient outcomes for at least 3 years. It is of great importance not only to describe possible patient outcomes, but also to understand and measure processes of PP mesh degradation.
Surgical meshes made from modified PP are used in a variety of procedures. It is known that PP has a chemical structure that is highly susceptible to oxidation. Radiation and free radical species become trapped in the crystalline structure of polymeric materials. Free radicals may attack terminal carbons, leading to cross-linking and chain scission, depolymerization, oxidative degradation, and ultimately the generation of more free radicals [7]. Neutrophils produce reactive oxygen species (ROS) such as hydrogen peroxide, hypochlorous acid, and enzymes like myeloperoxidase [9]. These are thought to be significant in inducing the oxidative process that degrades transplanted mesh [10]. The oxidative process in humans is not chronologically linear because the inflammatory state of the individual may be complex. Evidence of the rate and severity of degradation of surgical meshes in humans can therefore be highly variable [3]. Factors observed to have effects include age, smoking, diabetes, and body mass index [3]. The final result is a material with changes in and structural integrity and physical properties such as significant embrittlement that directly contributes to post-operative complications [8]. Measurement of embrittlement in vivo is extremely difficult. In order to investigate the relationship between ROS and embrittlement, a basic laboratory environment was constructed in which PP mesh samples were exposed to various concentrations of ROS. Higher concentrations of ROS were expected to be more positively correlated to measured effect, and significant changes were anticipated to be measurable at low, physiologic concentrations. Load displacement tensile testing (LDTT) was used to determine strength at failure and elongation to failure as means of comparison of mesh samples exposed to various concentrations of ROS for 6 months. These values from exposed samples were compared and plotted against LDTT measurements from new, out-of-package, unexposed samples. In addition, SEM analysis was used to exam fracture surfaces for changes indicative of embrittlement of ROS-exposed samples.
Materials and methods
Ethicon Ultrapro© meshes were used as a prototype for PP meshes in this experiment, and the samples were cut by hand from 6 × 6 inch2 sheets of Ethicon Ultrapro© donated by the manufacturer to the University of New Mexico Hospital Department of General Surgery. This model of mesh was chosen because of its ease of preparation, desirable pore size, symmetric pattern (Fig. 1A), and other features conducive to the testing method and apparatus being used; and simply for its availability. Presumably, Ethicon Ultrapro© has material properties and changes in such properties after exposure to ROS in common with all PP meshes. How this mesh specifically differs from other meshes by the same manufacturer or in the same class of materials is unknown. The mesh specimens were hand-cut into identical dog bone shapes measuring: 5 inch in total length, 0.75 inch in width at the thickest distal ends of the bog bone shape and continue at this same thickness for 0.5 inch in length, and a medial thickness of 0.25 inch in width for 3.5 inch in the central portion of the dog bone shapes (Fig. 1B). The dog bone shape was chosen to reduce slippage and limit breakage at the clamp during tensile testing. Samples were placed in sealed containers to prevent evaporation and filled with 0 M, 1 mM, 0.1 M, or 1 M hydrogen peroxide solutions. Samples were stored at room temperature in dark, sealed boxes for 6 months. This experiment was conducted with the goal of determining whether mesh would show degradation due to free radicals despite lack of exposure to forces, heat, and other physiologic factors. Hydrogen peroxide was used as a reactive oxygen species (ROS) because its role in inflammation and a minimum concentration of 1 mM (100 μM) was chosen because of reports of concentrations in the vicinity of 500 μM of ROS in the serum of human adults [5]. The 6-month exposure period was chosen as the exposure duration to ensure adequate exposure time to ROS. We wanted to generate an exposure duration versus strength curve to examine the effects of exposure time, but were limited by the number of pieces of mesh we had available for study and will do so in future studies. LDTT was done using an Instron 4400R tensile/compression machine. Specimens were placed in 0.75 × 0.5 inch2 metal clamps (Fig. 2) with rubber padding, and the apparatus crosshairs were displaced at a rate of 1 inch/min as data acquisition of load and displacement was obtained 4 times per second until the specimen broke/failed. Displacement nearest the point of failure was denoted as elongation to failure, and load nearest the point of failure was denoted as the tensile strength of the material. Samples that broke at the clamp were disregarded and not included in results. Typically samples broke in central portion of dog bone structure. Breakage at the clamps was presumed from over-tightening the clamps, poor alignment, or malformations resulted from preparation of the samples. Data regarding elongation to failure and tensile strength were collected, analyzed, and plotted with respect to samples of the same concentration. SEM images were obtained of specimen fracture surfaces after LDTT to look for features of the PP mesh, in particular, the fracture surface. This view shows the internal structure of the fiber and its characteristics as it failed. Stress cracking and fiber changes are common to brittle materials. SEM was used to observe samples from environments of the various concentrations or ROS; however, we were only able to observe one sample per concentration. SEM analysis was carried out using a FEI Quanta 3D Field Emission Gun SEM/Focused ion beam instrument. Samples were first sputter coated with a 20-nm-thick layer of gold/palladium using a K950X Turbo Evaporator (Quorum Technologies®) to eliminate imaging artifacts and increase resolution.
Results
LDTT yielded results for tensile strength and elongation to failure that were determined with 95 % confidence interval (CI). Displacement data were truncated so that the initial length measurements were regarded when the samples began to bear load. There was initial lag in the displacement without load as the samples fibers became aligned with the direction of pull in the apparatus. For samples unexposed (0 M), tensile strength was 28.0 ± 2.4 lbf and elongation to failure was 2.0 ± 0.3 in. For samples exposed to 1 mM, tensile strength was 19.2 ± 1.1 lbf and the elongation to failure was 2.0 ± 0.1 inch. For samples exposed to 0.1 M, tensile strength was 19.3 ± 1.6 lbf and elongation to failure was 1.9 ± 0.1 inch. For samples exposed to 1 M, tensile strength was 20.7 ± 1.2 lbf and elongation to failure was 0.47 ± 0.02 inch. Strength at various percentages of failure and deviation in load and elongation were plotted for different concentrations (Fig. 3). A plot of average load displacement curves for each concentration was made to compare concentrations to one another (Fig. 4).
A plot comparing the displacement and load during LDTT of average curves for meshes of various concentration. The plot demonstrates relative similarity between samples at 1 mM and 0.1 M after exposure to ROS for 6 months. 1 M samples demonstrate behavior associated with very rigid materials with little stretch and less displacement per unit load. Final points on the curves indicate average tensile strength and elongation to failure
Images from the fracture surface of a 1 M mesh exposed to ROS demonstrate a rough, sharper fiber end that is more typical of brittle materials (Fig. 5). Stress cracking was difficult to observe due to gold/palladium coating and limitations in resolution. Images of the pulled ends of a 0 M mesh demonstrate a gentle, blunted fracture surface that is typical of non-brittle materials (Fig. 6).
Discussion
This study was limited in the amount of material available to test and resultant small sample size. Specimens were precisely hand-cut, and molds were not used to create dog bone shapes to ensure that each sample was identical in number and patterns of fiber and pores. Misalignment of cuts would alter the number and recruitment of fibers in tensile testing. It was found in this study that waste material was higher than expected due to need for consistency of test samples.
This study was limited in its breadth, resolution, and application. Ethicon Ultrapro© was studied exclusively and chosen primarily for its ease of use and availability. This experiment was possible in part thanks to charitable donation of materials from Ethicon, but the authors of this study do not have any specific ties, investments, or special interests in this manufacturer. It is not known how this mesh specifically differs from other meshes by the same manufacturer or in the same class of materials. Presumably, Ethicon Ultrapro© undergoes a process of degradation and embrittlement similar to all PP meshes in the setting of a human immune response.
This study is not a perfect model for a process that occurs in the human body, but may be interrupted as experimental evidence that ROS plays a significant role in the alteration of material properties that mesh undergo in vitro. The inflammatory reaction that plays a role in mesh degradation is nonlinear and varies depending upon individual characteristics. At this time, there is no mechanistic way to anticipate the exact concentration of ROS introduced by an acute or chronic inflammatory response. Nonetheless, knowledge about changes in tensile strength as result of various ROS concentrations and lengths of exposure could be a useful for surgeons and technical developers, but should be used as an approximation and not an accurate predictor of patient outcome. Other processes, in particular sterilization and chemical refining of meshes, may cause alterations in the molecular structure that play a role in mesh failure [7]. Filament type, weave, pore size, and other surface and fiber characteristics vary among brands of mesh and may vary slightly from individual meshes of the same brand. These differences increase the total surface area exposed to body microenvironments. The characteristics correlated most to optimal performance is an area of interest, but ultimately are unknown at this time [7].
Conclusion
The results demonstrated that over a relatively short time, a 6-month exposure, even a small concentration of ROS (1 mM) could decrease tensile strength of a sample of PP mesh by 31 %. Concentrations on this order have been known to occur physiologically in humans [5]. Exposure to ROS and affect on tensile strength are not well correlated to concentration of the ROS. Results for samples exposed in 1 mM and 0.1 M solutions behaved similarly and yielded similar tensile strength at failure, suggesting that the concentration of ROS does not correlate in a linear fashion to changes in physical property. Samples at these concentrations had similar elongation to failure as unexposed samples, but diminished tensile strength, and are perhaps demonstrating a quasi-crystalline quality described in many polymeric materials as they undergo free radical oxidation. This is contrast to the shortened elongation to failure of the high concentration 1 M samples, which displayed particularly brittle qualities.
This study will be a starting point for future studies that will examine various types of mesh (composition and weaves) in addition to the effects of free radical exposure at various timepoints, both shorter and longer in duration.
References
Amid PK (1997) Classifications of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15–21
Bloch B (1984) International standards for surgical implants. Ann R Coll Surg Engl 66:369–371
Costello CR, Bachman SL, Ramshaw BJ, Grant SA (2007) Materials characterization of explanted polypropylene hernia meshes. J Biomed Mater Res 83:44–49
Davis JR (2003) Handbook of materials for medical devices. ASM International, Materials Park, OH
Korotkova E, Misini B, Dorozhko EV, Bukkel MV, Plotnokov EV, Linert W (2011) Study of OH radicals in human serum blood of healthy individuals and those with pathological schizophrenia. Int J Mol Sci 12:401–410
Sheftel VO (1990) Toxic properties of polymers and additives. Rapra Technology, Shawbury, UK
Sternshuss G, Ostergard D, Patel H (2012) Post-implantation alteration of polypropylene in the human. J Urol 188:1052
Usher FC, Cogan JE, Lowry TI (1960) A new technique for the repair of inguinal and incisional hernias. Arch Surg 81:847–854
Weiss SJ (1989) Tissue destruction by neutrophils. N Engl J Med 320:365–376
Williams DF (1982) Review: biodegradation of surgical polymers. J Mater Sci 17:1233–1246
Acknowledgments
Mesh used in this experiment was donated by Ethicon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jean Kurtz, Ben Rael, Jesus Lerma, Catherine Wright, Tariq Khraishi, and Edward D. Auyang have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Kurtz, J., Rael, B., Lerma, J. et al. Effects of reactive oxygen species on the physical properties of polypropylene surgical mesh at various concentrations: a model for inflammatory reaction as a cause for mesh embrittlement and failure. Surg Endosc 30, 3250–3255 (2016). https://doi.org/10.1007/s00464-015-4646-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4646-3