Abstract
Background
Peptic ulcer represents the most common cause of upper gastrointestinal bleeding. Endoscopic therapy can reduce the risks of rebleeding, continued bleeding, need for surgery, and mortality. The objective of this review is to compare the different modalities of endoscopic therapy.
Methods
Studies were identified by searching electronic databases MEDLINE, Embase, Cochrane, LILACS, DARE, and CINAHL. We selected randomized clinical trials that assessed contemporary endoscopic hemostatic techniques. The outcomes evaluated were: initial hemostasis, rebleeding rate, need for surgery, and mortality. The possibility of publication bias was evaluated by funnel plots. An additional analysis was made, including only the higher-quality trials.
Results
Twenty-eight trials involving 2988 patients were evaluated. Injection therapy alone was inferior to injection therapy with hemoclip and with thermal coagulation when evaluating rebleeding and the need for emergency surgery. Hemoclip was superior to injection therapy in terms of rebleeding; there were no statistically significant differences between hemoclip alone and hemoclip with injection therapy. There was considerable heterogeneity in the comparisons between hemoclip and thermal coagulation. There were no statistically significant differences between thermal coagulation and injection therapy, though their combination was superior, in terms of rebleeding, to thermal coagulation alone.
Conclusions
Injection therapy should not be used alone. Hemoclip is superior to injection therapy, and combining hemoclip with an injectate does not improve hemostatic efficacy above hemoclip alone. Thermal coagulation has similar efficacy as injection therapy; combining these appears to be superior to thermal coagulation alone. Therefore, we recommend the application of hemoclips or the combined use of injection therapy with thermal coagulation for the treatment of peptic ulcer bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute upper gastrointestinal bleeding (UGIB) can manifest as hematemesis, “coffee ground” emesis, the return of red blood via a nasogastric tube, hematochezia, and/or melena with or without hemodynamic compromise [1, 2]. UGIB results in over 300,000 hospital admissions annually in the USA, with a mortality of 7–10 % [3]. Peptic ulcer disease (PUD) represents the most common cause of UGIB, accounting for a third to half of all episodes [4]. The most frequent causes of PUD are non-steroidal anti-inflammatory drugs and Helicobacter pylori infection, although a variety of other clinical settings can predispose patients to the disease [4]. Despite improvements in the understanding of the etiology of PUD, the incidence of its most common complication, bleeding, has not changed and occurs in 20–30 % of patients [5].
Endoscopic therapy for UGIB can dramatically reduce the risks of rebleeding, continued bleeding, the need for surgery, the number of units of packed erythrocytes required for transfusion, the length of hospital stay, and mortality [6–9]. Until recently, the reported mortality from UGIB had remained unchanged, despite the advances in therapeutic and endoscopic modalities, probably due to the increased use of aspirin and non-steroidal anti-inflammatory drugs in conjunction with the increasing number of multiple comorbidities in an aging population in many countries [10]. Rebleeding is considered the most important risk factor for mortality and occurs in 10–30 % of patients [11].
Endoscopic therapy for PUD bleeding is indicated for patients with active bleeding and for those with a non-bleeding visible vessel in an ulcer [1]. Adherent clot has been controversial with regard to the need for endoscopic treatment, but recent data have shown benefits to endoscopic clot removal and treatment of an underlying lesion instead of observation alone [12]. Endoscopic treatment for ulcer bleeding has come a long way from injections of epinephrine and other solutions and the use of thermal coagulation to the application of mechanical devices such as hemoclips [13]. Recently, a few new technologies have emerged, including endoscopic topical hemostatic powders [14–16].
The treatment for peptic ulcer bleeding is widely studied. It is assumed that endoscopic management of this condition is superior to pharmacotherapy alone; this statement is supported by a previous meta-analysis of this topic [17]. The last review on this question only studied whether a second procedure after epinephrine injection could improve the patients’ outcome [18]. The authors concluded that an additional endoscopic treatment could reduce the rebleeding rates and the need for emergency surgery. However, it is unclear which endoscopic modality (or combination of modalities) presents the best results in terms of hemostasis. Therefore, the objective of this systematic review is to compare, using randomized clinical trials, the different modalities of endoscopic treatment for peptic ulcer bleeding.
Methods
Protocol and registration
This systematic review was conducted in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) recommendations [19]. The review was registered on the PROSPERO international database (CRD42014015131) [20].
Eligibility criteria
-
(a)
Types of studies—Randomized clinical trials comparing different endoscopic modalities for the treatment of patients presenting with acute UGIB caused by PUD. No language or publication date restriction was imposed.
-
(b)
Types of participants—Patients with signs of UGIB whose endoscopic examinations showed gastric or duodenal ulcers with active bleeding, non-bleeding visible vessels, or adherent clots.
-
(c)
Types of intervention—We included trials that assessed contemporary endoscopic hemostatic techniques [21]: injection therapy (all injectates, single or multiple), thermal coagulation (heater probe, argon plasma, microwave coagulation, and monopolar, bipolar, and multipolar electrocoagulations), hemoclip placement, and combinations of treatments. Trials comparing different methods of the same modality (for example, heater probe vs argon plasma coagulation) or varying volumes of the injectate (for example, 10 vs 30 ml of epinephrine injection) were excluded because those are the subjects of different clinical questions. We also excluded studies that compared an endoscopic technique versus placebo or pharmacotherapy alone, as it is already evident that the endoscopic treatment is superior.
-
(d)
Types of outcome measures—The outcomes we measured were the rates of initial hemostasis, rebleeding, emergency surgery, and overall mortality.
Information sources
Studies were identified by searching electronic databases (MEDLINE, Embase, Cochrane, LILACS, DARE, and CINAHL) and scanning reference lists of articles.
Search
The search strategy used for MEDLINE and Embase databases is stated in Supplementary Search. Aiming to select high-quality studies, the filter “AND Random*” was added to the search strategy at MEDLINE database. For Cochrane, LILACS, DARE, and CINAHL databases, the search strategy was: “Gastrointestinal bleeding AND Endoscopy.”
Study selection
Eligibility assessment and the selection of screened records were performed independently in an unblinded, standardized manner by two reviewers (Baracat FI and Bernardo WM). Disagreements between the reviewers were resolved by consensus.
Data collection process
After the paper was read, we used a checklist based on the CONSORT recommendations for reporting a randomized clinical trial [22]. One review author (Baracat FI) extracted the data from each included study using a standardized form (Supplementary Information Sheet), and the second author (Bernardo WM) checked the extracted data.
Data items
Information was extracted from each trial on: (1) the characteristics of the trial participants and the trial’s inclusion and exclusion criteria; (2) type of intervention and control groups (considering different modalities or combinations of techniques in endoscopic hemostatic treatment: injection therapy, thermal coagulation, and hemoclip); and (3) type of outcome measure.
Risk of bias in individual studies
To ascertain the validity of eligible trials, two reviewers worked together in the first three studies (functioning as calibration exercises among the reviewers) and then independently determined: the adequacy of randomization and concealment of allocation; the blinding of patients, healthcare providers, data collectors, and outcome assessors; and the correct report and extent of loss to follow-up. These items meet the criteria applied by the Jadad scale for the assessment of the risk of bias of randomized clinical trials (Supplementary Jadad Scale) [23]; Jadad scores vary from 0 to 5 (scores less than 3 indicate poor methodological quality) and were calculated for each study.
At the study level, we also evaluated whether the endoscopic hemostatic techniques were properly described (for example, volume, concentration, and number of applications of an injectate) or whether they were poorly stated or not described well enough to be reproduced.
Moreover, initial hemostasis and rebleeding were also determined at the outcome level, if they were appropriately defined in each study. The rebleeding suspicion should contemplate clinical and laboratory aspects (new hematemesis, aspirate of fresh blood via nasogastric tube after initial hemostasis, and/or instability of vital signs or reduction in hemoglobin levels). We did not expect those outcomes to be equally defined across the studies, but should have been clearly stated and at least endoscopically confirmed, so the heterogeneity between the definitions does not interfere with the results.
We did not intend to exclude any article from this review based on a higher risk of bias that it presented; however, we intended to produce a subgroup analysis of the trials with higher methodological quality.
Summary measures and planned methods of analysis
Meta-analyses were performed by computing risk differences (RD), using a fixed-effects model. Quantitative analyses were performed on an intention-to-treat basis. RD, absolute risk reduction/increase (ARR/ARI), number needed to treat/harm (NNT/NNH), and 95 % confidence intervals (CI) for each outcome were calculated on each trial for applicability by using the critically appraised topic (CATmaker) software [24].
Meta-analyses were conducted using the Review Manager (RevMan) 5.3 software, obtained from the Web site of the Cochrane Informatics & Knowledge Management Department [25]. We employed risk differences of dichotomous variables using a fixed-effects model to provide the forest and funnel plots for each comparison. Data on risk differences and 95 % CI for each outcome were calculated using the Mantel–Haenszel test, and inconsistency (heterogeneity) was tested by the Chi-square (Chi2) and the Higgins method (I2) [26].
Risk of bias across studies and additional analyses
We assessed the possibility of publication bias by evaluating a funnel plot of the trials’ mean differences for asymmetry, which can result from the non-publication of small trials with negative results (that support the null hypothesis) or the missing data for from the included studies (selective reporting bias). If the heterogeneity of the results of a meta-analysis (I2) was over 50 %, we excluded the report(s) located outside the funnel (outliers) and then performed another meta-analysis without the given report. If we could not detect outliers, true heterogeneity was presumed and discussed. We acknowledge that other factors could produce asymmetry in funnel plots leading to a high heterogeneity (true study heterogeneity), such as differences in trial quality, differences in the population studied, or even different techniques studied under the same endoscopic modality (for example, heater probe and argon plasma coagulation are studied under thermal coagulation).
We considered that the gathering of different trials presenting with discrepant methodological quality in the same analysis could bring a risk of bias across the studies for the result. For this reason, we performed an additional analysis for each comparison, including only the trials of higher methodological quality.
Results
Study selection
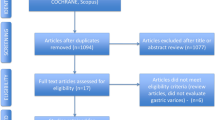
Nine thousand, seven hundred and thirty-eight (9738) studies were screened, and the articles were assessed for eligibility after the title and abstract were read. The following flowchart, an adapted PRISMA flow diagram, illustrates the study selection process (Fig. 1). One trial was excluded from the meta-analyses [31] because it measured different outcomes that could not be adapted to the outcomes reviewed in this paper.
Study characteristics
-
a.
Methods—All twenty-eight studies selected for this review were randomized controlled trials published in English. Almost half of the authors calculated a sample size of patients for their trials.
-
b.
Participants—A total of 2988 patients were involved in this review. Although all patients suffered from acute UGIB caused by PUD, the exclusion criteria were distinct between the trials; some studies were very broad and did not present any clinical exclusion criteria (except for unwillingness to participate in the trial), and others preferred to exclude patients with pregnancy, advanced malignancy, or a coagulopathy or receiving anticoagulant therapy. Regarding the inclusion criteria, most studies only gave the specification that the bleeding should have originated from a peptic ulcer; however, a few trials studied only patients that presented with actively bleeding ulcers.
-
c.
Interventions—Each trial was placed under a specific comparison group according to the endoscopic techniques that were employed. The same trial could be placed under more than one comparison group, if it used three arms in the study (for example, injection therapy × thermal coagulation × combined technique). In that case, each comparison was analyzed separately. After the proper organization of the trials, we framed seven comparison groups, as shown in Table 1. Each comparison group was meta-analyzed separately.
Table 1 Characteristics of the included trials, organized into comparison groups -
d.
Outcomes—Most of the trials assessed, at least, the same outcomes that we intended to evaluate (initial hemostasis, rebleeding, emergency surgery, and overall mortality rates). Nonetheless, some studies expressed their results in other outcomes; fortunately, most of those different outcomes could be adapted to the outcomes measured in this review by collecting some other information within the report. Initial hemostasis and rebleeding outcomes had subtle heterogeneity between the studies (for example, some trials described blood in the stomach after 24 h of the therapy as a rebleeding sign); however, all the trials stated the necessity for endoscopic confirmation of those outcomes. One study [52] only evaluated initial hemostasis and rebleeding rates, but we decided to keep this study in the quantitative analyses.
A summary of the characteristics of the included trials is shown in Table 1, organized into specific comparison groups.
Risk of bias within studies
The risk of bias within the studies was assessed by applying the Jadad scale and evaluating whether the endoscopic hemostatic techniques and the definitions of the outcomes were properly described.
Although some studies reported that they were single-blinded, no trial was double-blinded (as expected for this sort of trial), and this is considered a source of bias. Therefore, the maximum Jadad score of the articles was 3 points, which was achieved by almost two-thirds of the trials. Most of the studies suitably reported the endoscopic techniques and the definitions of the outcomes measured. The data of each selected study are summarized in the Supplementary Risk of Bias within Studies and Supplementary Table 1.
Results of individual studies
The results for each outcome measured in the included studies are shown in Supplementary Table 2 as a proportion of the number of events over the total amount of patients involved in an intention-to-treat basis.
We calculated the event rates and absolute risk reduction (ARR) or increase (ARI) with their respective 95 % CI and the number needed to treat (NNT) or harm (NNH) for each study using the critically appraised topic (CATmaker) software. However, we believe that to show all those numbers would be confusing, considering the great amount of studies and the different comparisons included in this review. These measurements will be graphically expressed along with the meta-analyses.
Synthesis of results and risk of bias across studies
In the following figures, the risk differences and their confidence intervals and the results of the meta-analyses and their respective heterogeneity measures are graphically exhibited; this was performed for every comparison group, according to the sequence presented in the tables. We developed one forest plot and one funnel plot for each outcome, and an additional forest plot excluding the outliers, if necessary. Due to the great amount of graphics, only the most important ones will be shown here; all the others are published in the Supplementary Synthesis of Results. The results of the additional analyses of the trials with higher methodological quality will be expressed in the next topic.
Hemoclip versus injection therapy
This comparison group contains five trials to be meta-analyzed, of which three studies [27, 29, 30] can be classified in the higher methodological quality subgroup.
Initial hemostasis
There were no differences between hemoclip and injection therapy in achieving initial hemostasis (risk difference [RD] 0.01, 95 % confidence interval [CI] −0.02 to 0.04), with the heterogeneity as low as zero (Supplementary Figures [SF] 1 and 2).
Rebleeding
As shown in Fig. 2, the results of the meta-analysis favored the use of hemoclip, resulting in the NNT of 7 (RD −0.13, 95 % CI −0.19 to −0.08), with a low heterogeneity (I 2 = 1 %) (SF 3).
Emergency surgery
When evaluating emergency surgery rates, once again the hemoclip was superior to injection therapy, with a NNT of 20 (RD −0.05, 95 % CI −0.09 to −0.01). The trial conducted by Shimoda was responsible for some heterogeneity; however, the study should not be considered an important outlier, as its removal does not significantly alter the results (SF 4 and 5).
Overall mortality
Concerning the overall mortality rate, there was no statistical difference between the hemoclip and injection therapy groups (RD 0.02, 95 % CI −0.01 to 0.06), with no heterogeneity (SF 6 and 7).
Hemoclip versus hemoclip and injection therapy
In this comparison group, only one trial [29] had higher methodological quality; therefore, we did not conduct a separate analysis for this comparison. Moreover, the results of this trial are similar to the combined results of all the included trials, eliminating the need for a separate analysis.
Initial hemostasis
When evaluating the achievement of initial hemostasis, there was no difference between the hemoclip group and the group that combined hemoclip and injection therapy (RD −0.01, 95 % CI −0.05 to 0.03), with no heterogeneity between the trials (SF 8 and 9).
Rebleeding
There were no differences in rebleeding rates between the hemoclip group and the hemoclip–injection therapy combination group (RD −0.01, 95 % CI −0.07 to 0.06), with low heterogeneity between the trials (I 2 = 10 %) (SF 10 and 11).
Emergency surgery
There were no statistically significant differences in emergency surgery rates between the two groups (RD 0.01, 95 % CI −0.03 to 0.05), with heterogeneity equal to zero (SF 12 and 13).
Overall mortality
There were also no differences concerning the overall mortality rates between the hemoclip group and the group that combined hemoclip and injection therapy (RD 0.03, 95 % CI −0.02 to 0.07), with no heterogeneity (SF 14 and 15).
Hemoclip and injection therapy versus injection therapy
This comparison group contains three trials to calculate the meta-analyses. Only one study [32] did not meet the criteria for entering into the high methodological quality subgroup.
Initial hemostasis
Considering all the trials, there were no differences between the combination of hemoclip with injection therapy and injection therapy alone in achieving initial hemostasis (RD 0.03, 95 % CI −0.01 to 0.07). The analysis presented a low heterogeneity (I 2 = 3 %) (SF 16 and 17).
Rebleeding
Figure 3 shows the results of the meta-analysis favoring the use of the combination of hemoclip and injection therapy rather than injection therapy alone; there was a NNT of 10 (RD −0.10, 95 % CI −0.18 to −0.03) and no heterogeneity (SF 18).
Emergency surgery
To interpret this graphic, we should first explain the cause for the high heterogeneity value (I 2 > 80 %) presented by the meta-analysis of all included trials (SF 19). The trial conducted by Shimoda et al. allowed the endoscopist to perform a different endoscopic hemostatic technique after the patient experienced a rebleeding episode; this may explain why the results presented by this study are disparate from the other trials, allowing us to consider that paper an outlier (clearly shown by the funnel plot—SF 20). Therefore, we should only consider the results of the meta-analysis after excluding this article from this outcome (Fig. 4), which led us to affirm that, for emergency surgery rates, the combination group was superior to injection therapy alone, with a NNT of 9 (RD −0.11, 95 % CI −0.18 to −0.04).
Overall mortality
Concerning the overall mortality rate, there were no differences between the combination and injection therapy groups (RD 0.01, 95 % CI −0.03 to 0.04) with the heterogeneity equal to zero (SF 21 and 22).
Hemoclip versus thermal coagulation
In this comparison group, all five trials included for the meta-analyses are considered to be of a high methodological quality, dismissing the need for a subgroup analysis; however, the thermal coagulation group involves three different types of techniques (heater probe was used by Cipolleta and by Lin in both of his trials, Taghavi applied argon plasma coagulation, whereas Arima studied the soft coagulation mode). Furthermore, Lin et al. [37] combined epinephrine and heater probe in the thermal coagulation group; Taghavi et al. [36] applied epinephrine injection into both groups. These facts may cause some heterogeneity between the results of the trials and will be pointed throughout the outcome analyses.
Initial hemostasis
This outcome should be interpreted with caution, due to the high heterogeneity in both analyses (SF 23 and Fig. 5); as mentioned above, this heterogeneity may be because of the different techniques applied by the authors, and even after excluding the outlier [36] identified by the funnel plot (SF 24), the heterogeneity remains considerable (I 2 = 77 and 55 %, respectively). Curiously, Cipolletta found disparate results from both of Lin’s studies, considering that these three trials applied heater probe coagulation in the thermal coagulation group. These findings may be due to subtle particularities in the population participating in the trials (for instance, Lin et al. [37] excluded five patients from the hemoclip group because of “inability to apply” the clip in ulcers located in difficult positions; it should not be considered a methodological error, once the author clearly stated this exclusion criteria in his methods, but we included those patients in our analyses, considering the intention-to-treat basis). Nevertheless, Fig. 5 shows that the thermal coagulation group is superior to the hemoclip group, with a NNT of 14 (RD 0.07, 95 % CI −0.14 to −0.01), with considerable heterogeneity (I 2 = 55 %). This result should be carefully interpreted and will be discussed later in this review.
Rebleeding
The meta-analysis of all included trials demonstrated a high heterogeneity (I 2 = 76 %). After performing sensitivity analysis through the funnel plot, two studies were identified as outliers [35, 39]. After removing the outliers from the analysis, no statistical difference was detected between the hemoclip and thermal coagulation groups for rebleeding rate (RD 0.02, 95 % CI −0.08 to 0.04); the heterogeneity was low after excluding the outliers (I 2 = 19 %) (SF 25, 26 and 27).
Emergency surgery
For the emergency surgery outcome, there was no statistical difference in the comparison between the hemoclip group and the thermal coagulation group (RD −0.02, 95 % CI −0.05 to 0.01), with heterogeneity equal to zero (SF 28 and 29).
Overall mortality
Concerning the overall mortality rate, analysis of the trials showed no difference between the hemoclip group and the thermal coagulation group (RD 0, 95 % CI −0.03 to 0.03), with no heterogeneity (SF 30 and 31).
Thermal coagulation versus injection therapy
This is the comparison group that contained the largest amount of trials, with a total of 13 included studies. A significant amount of techniques are involved in each group (different injectates, such as epinephrine, alcohol, polidocanol, and combinations of these, and varied coagulation techniques, such as bipolar and multipolar electrocoagulation, heater probe, microwave, and argon plasma coagulation); therefore, we tried to reach the lowest heterogeneity possible in each outcome measured for greater validity of the results. As for the subgroup analyses, seven trials met the criteria to be considered as high-quality studies.
Initial hemostasis
When evaluating the achievement of initial hemostasis, the meta-analysis containing all the included trials resulted in high heterogeneity (I 2 = 71 %) between the studies (SF 32). After applying the sensitivity test through the funnel plot (SF 33), we identified three outliers [48, 51, 52]. Curiously, Lin et al. [52] and Chung studied only patients presenting with actively bleeding ulcers, despite the opposite results encountered. The heterogeneity of the analysis dropped to zero after removing the outliers, showing no difference between the thermal coagulation group and the injection therapy group (RD 0.01, 95 % CI −0.02 to 0.03) (SF 34).
Rebleeding
The analysis of rebleeding rate did not count with high heterogeneity between the studies (I 2 = 25 %); however, the trial conducted by Laine et al. [41] was considered as an outlier in the funnel plot (SF 35 and 36), possibly because the author applied saline solution in the injection therapy group. After removal of this trial (Fig. 6), the heterogeneity of the analysis dropped to zero, showing no statistical differences between the thermal coagulation and injection therapy groups (RD 0.02, 95 % CI −0.02 to 0.06).
Emergency surgery
In the evaluation of the emergency surgery rate, there were no differences between the thermal coagulation group and the injection therapy group (RD 0, 95 % CI −0.03 to 0.04), with heterogeneity equal to zero (SF 37 and 38).
Overall mortality
Concerning the overall mortality rate, there were no statistical differences between the thermal coagulation and injection therapy groups (RD 0.01, 95 % CI −0.02 to 0.04), with no heterogeneity between the studies (SF 39 and 40).
Thermal coagulation versus thermal coagulation and injection therapy
There are only two trials included in this comparison group [42, 53], which could decrease the reliability of the results of the meta-analyses. Both trials are considered to have a high methodological quality, dismissing the need for subgroup analyses.
Initial hemostasis
When combining the results of the trials, the difference found by Bianco, favoring the combined therapy group over the thermal coagulation group in the achievement of initial hemostasis, was not matched by Lin’s results (who encountered no differences at all between the groups), leading to no statistical differences between both groups (RD 0.07, 95 % CI −0.00 to 0.14) with considerable heterogeneity, as expected (I 2 = 51 %) (SF 41 and 42).
Rebleeding
Once again, both authors reached different results in their trials when evaluating the rebleeding rate. However, this time Lin appointed to a difference between the groups, favoring the combination therapy; Bianco found a small difference in her trial, favoring the same group (although it was not statistically significant). As a result, the meta-analysis shows that the combination therapy (thermal coagulation and injection therapy group) is superior to thermal coagulation alone in preventing rebleeding, with a NNT of 9 (RD −0.11, 95 % CI −0.21 to −0.02) and considerable heterogeneity, as expected (I 2 = 56 %) (SF 43 and 44).
Emergency surgery
There was no heterogeneity between the trials, and the meta-analysis shows no statistical differences between the thermal coagulation group and the thermal coagulation and injection therapy group in emergency surgery rate (RD −0.05, 95 % CI −0.11 to 0.01) (SF 45 and 46).
Overall mortality
Concerning the overall mortality rate, the analysis of the trials showed no difference between the thermal coagulation group and the thermal coagulation and injection therapy group (RD −0.02, 95 % CI −0.08 to 0.03), with heterogeneity equal to zero (SF 47 and 48).
Thermal coagulation and injection therapy versus injection therapy
Over again, this comparison group contains only two trials, and both studies are high-quality trials, dismissing the subgroup analyses. As the trials involved a very different amount of patients (Lin et al. [42] evaluated 96 patients, whereas Chung et al. [54] studied 276 patients), the different results between the trials will lead to a high heterogeneity, and sensitivity tests through a funnel plot will probably consider Lin as an outlier. It is appropriate, however, to remember that the authors studied different thermal coagulation techniques: Lin applied bipolar electrocoagulation and Chung used the heater probe.
Initial hemostasis
When evaluating the achievement of initial hemostasis, there was no difference between the injection therapy group and the group that combined thermal coagulation and injection therapy (RD 0.01, 95 % CI −0.02 to 0.04), with no heterogeneity between the trials (SF 49 and 50).
Rebleeding
As hypothesized before, the differences between the studies’ results led to a high heterogeneity (I 2 = 85 %) and the meta-analysis showed the superiority of the combination therapy over the injection therapy alone in preventing rebleeding, with a NNT of 12 (RD −0.08, 95 % CI −0.14 to −0.02) (SF 51). However, if we consider the funnel plot (SF 52) and exclude Lin’s trail (as an outlier), we would remain with Chung’s results that showed no statistical differences in the comparison between the groups (RD −0.03, 95 % CI −0.10 to 0.03).
Emergency surgery
For the emergency surgery rate, the meta-analysis showed a statistical difference in the comparison between the thermal coagulation and injection therapy group and injection therapy group, favoring the combination therapy with a NNT of 16 (RD −0.06, 95 % CI −0.12 to −0.00) and low heterogeneity (I 2 = 2 %) (SF 53 and 54).
Overall mortality
Concerning the overall mortality rate, the meta-analysis showed no difference between the injection therapy group and the thermal coagulation and injection therapy group (RD −0.01, 95 % CI −0.06 to 0.04), with no heterogeneity between the studies (SF 55 and 56).
Summary of the results
Table 2 shows a summary of the results of the meta-analyses of all the comparison groups. There were no differences in the mortality rate in all comparisons.
Additional analyses
We performed additional analyses for each outcome, considering only the trials with higher methodological quality, in the following comparison groups: hemoclip versus injection therapy; hemoclip and injection therapy versus injection therapy; and thermal coagulation versus injection therapy. The other comparison groups dismissed the need for an additional analysis mostly because all the included trials were high-quality studies, except for the comparison between hemoclip versus hemoclip and injection therapy, in which only one trial would enter the subgroup analyses.
When the results from the subgroup analyses were compared to the results from the analyses that included all the trials, there was only one outcome where the subgroup analyses showed a statistically different result. In the first comparison group (hemoclip vs injection therapy), when evaluating the emergency surgery rates, there was a tendency favoring the hemoclip group, but it was not statistically significant (RD −0.05, 95 % CI −0.10 to 0.00). In the analysis including all the trials, the hemoclip was superior to the injection therapy in emergency surgery rates. In the other two comparison groups, the subgroup analyses did not significantly alter any result found in the analyses of all included trials.
Discussion
Summary of evidence and limitations
Despite the considerable number of trials included in this review, most of which were considered as having high methodological quality, a limitation of the meta-analyses was the relatively low number of studies included in each of the comparison groups (not more than five trials), except for the comparison of the injection therapy group versus the thermal coagulation group. However, some very interesting results were found and deserve to be discussed.
Injection therapy was often considered the control group, as it is widely performed, most likely because of its ease of use, availability, and the extensive experience with its application by most endoscopists [55]. When this technique is compared with combination therapy (either with hemoclip or with thermal coagulation), the meta-analyses statistically favored the combination therapy. Interestingly, both combinations presented better results in the same outcomes: the rebleeding rate and the emergency surgery rate. This fact could lead to the assumption that the injection therapy alone is probably as effective as its combination with another modality in terms of the initial hemostasis; however, due to its ephemeral effect, patients treated with this single modality present higher rebleeding rates and a higher need for emergency surgery. Despite the low numbers of trials included in those comparison groups, the results encountered by the meta-analyses are vastly supported by previous reviews and many consensuses [18, 21, 55–57] and they should be considered by the endoscopists when facing this emergency situation.
The comparison between the hemoclip group and the injection therapy group (both as single-modality treatments) also generated an interesting result. Hemoclip was superior over the injection therapy when evaluating the rebleeding rate, with a NNT of 7 (if accounting with all the included trials) or 6 (in the subgroup analysis of the higher methodological quality studies), meaning that for each six or seven patients presenting with UGIB caused by a peptic ulcer, one would benefit in not developing rebleeding during the evolution of the treatment (should all patients be treated with hemoclip instead of injection of epinephrine or other injectate). This is a very representative result that carries a high reliability and clinical relevance. The hemoclip group was also statistically favored over the injection therapy group in the need for emergency surgery in the analysis containing all the included trials. As pointed before, those results may be due to the transient effect of the injection therapy when it is applied alone.
Despite the absence of statistical differences in the comparison between the hemoclip group and the group that combined injection therapy and hemoclip application, we consider those results very meaningful and applicable. Only three trials were involved in this comparison group (of which only one was considered to have high methodological quality); however, the heterogeneity in every outcome analysis was very low (varying between 0 and 10 %), which means that the results are probably reliable. In every outcome analyzed, strictly no difference was encountered between the hemoclip group and hemoclip and injection therapy group. In daily practice, the meaning of those results is that the combined application of an injectate does not improve the hemostatic efficacy of the use of hemoclip alone.
The results generated from the meta-analyses of the hemoclip group versus the thermal coagulation group should be carefully interpreted, for many reasons. At first, five trials were included in this group, of which all are considered to have high methodological quality; however, there are three different techniques evaluated under the thermal coagulation group (heater probe was used by Cipolleta and by Lin in both of his trials, Taghavi applied argon plasma coagulation, whereas Arima studied the soft coagulation mode). Another variance regarding the techniques is that Lin et al. [37] combined epinephrine and heater probe in the thermal coagulation group and Taghavi et al. [36] applied epinephrine injection in both groups (in combination with argon plasma coagulation and with hemoclip); these facts may have been responsible for the high heterogeneity levels present in the evaluation of initial hemostasis and rebleeding rates. In the analysis of the achievement of initial hemostasis, the heterogeneity was considerable, even after excluding the outlier [36] pointed by the funnel plot and this analysis favors the thermal coagulation group over the hemoclip group. The analyses of rebleeding rate (which presented with an acceptable heterogeneity after performing the sensitivity analysis, through the funnel plot, and excluding the two outlier trials from the analysis: Cipolletta et al. [39] and Arima et al. [35]), need for emergency surgery, and overall mortality rate showed no statistical differences between the hemoclip group and the thermal coagulation group. As previously mentioned, the results of this comparison group should be considered with caution. Perhaps a solution for this issue is the publication of a greater number of trials studying this comparison; so the meta-analyses could be performed separately, according to the technique employed in the thermal coagulation group (instead of clustering different techniques under the same group).
The comparison group involving the thermal coagulation group versus the injection therapy group was the one that had the highest number of trials (a total of 13 studies). On the one hand, this could lead to more solid results; on the other, the significant amount of techniques involved in each group (i.e., different injectates, such as epinephrine, alcohol, polidocanol, and combinations of these, and varied coagulation techniques, such as bipolar and multipolar electrocoagulation, heater probe, microwave, and argon plasma coagulation) could compromise the results with high heterogeneity rates between the studies. Fortunately, the sensitivity tests performed through funnel plots were able to find the outliers (which were excluded), dropping the heterogeneity to zero in all of the analyses, validating the results reached in every outcome. The trial conducted by Laine et al. [41] applied saline solution in the injection therapy group and was considered as an outlier in the evaluation of rebleeding. We believe that this injectate should not be considered as a good option for an injection therapy, because its effect may be temporary (as corroborated by the results presented by Laine et al. [41]). No differences were found in every comparison made between the thermal coagulation group and the injection therapy group, indicating similar hemostatic results when performed as single techniques.
The comparison between the combination of thermal and injection therapy versus the thermal coagulation group had only two trials (although both reports present high methodological quality). Moreover, those studies showed some considerably heterogeneous results concerning the assessment of initial hemostasis and rebleeding rates (I 2 = 51 % and I 2 = 56 %, respectively). The meta-analyses favored the combined technique when evaluating the rebleeding rate, with a NNT of 9. There were no statistical differences in the appraisal of the other outcomes. The results encountered in these analyses lack greater reliability (for the reasons previously stated), as more studies assessing this comparison are needed for more solid evidence.
Conclusions
The first conclusion of this systematic review is that injection therapy should not be used as a single modality in the treatment of UGIB caused by a peptic ulcer, since its combination with either hemoclip or thermal coagulation produces better results.
The application of hemoclip is superior to injection therapy as a single modality, and the combined application of hemoclip and an injectate does not improve the hemostatic efficacy above the use of hemoclip alone.
As a single modality, thermal coagulation has a similar hemostatic efficacy to the use of injection therapy, and these combined modalities appear to be superior to the use of a thermal coagulation technique alone.
Based on what has been presented by this systematic review, we recommend the application of hemoclips or the combined use of an injection therapy with a thermal coagulation method for the treatment of patients presenting with acute peptic ulcer bleeding.
Future perspectives
Regarding emerging technology, endoscopic topical hemostatic powders are currently being studied in randomized trials and future reviews shall include this modality in its analyses as the trials’ results are published.
References
American Society for Gastrointestinal Endoscopy (2004) ASGE Guide line: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc 60:497–504
Wilcox CM, Alexander LN, Cotsonis G (1997) A prospective characterization of upper gastrointestinal hemorrhage presenting with hematochezia. Am J Gastroenterol 92:231–235
Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE (1995) Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol 90:568–573
Rockall TA, Logan RF, Devlin HB, Northfield TC (1995) Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 311:222–226
Blocksom JM, Tokioka S, Sugawa C (2004) Current therapy for nonvariceal upper gastrointestinal bleeding. Surg Endosc 18:186–192
Cappell MS, Friedel D (2008) Initial management of acute upper gastrointestinal bleeding: from initial evaluation up to gastrointestinal endoscopy. Med Clin North Am 92:491–509
Kovacs TO (2008) Management of upper gastrointestinal bleeding. Curr Gastroenterol Rep 10:535–542
Gralnek M, Jensen DM, Gorbein J, Kovacs TO, Jutabha R, Freeman ML, King J, Jensen ME, Cheng S, Machicado GA, Smith JA, Randall GM, Sue M (1998) Clinical and economic outcomes of individuals with severe peptic ulcer hemorrhage and nonbleeding visible vessel: an analysis of two prospective clinical trials. Am J Gastroenterol 93:2047–2053
Pedroto I, Dinis-Ribeiro M, Ponchon T (2012) Is timely endoscopy the answer for cost-effective management of acute upper gastrointestinal bleeding? Endoscopy 44:721–722
Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J (1997) Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ 315:510–514
Simoens M, Rutgeerts P (2001) Non-variceal upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 15:121–133
Bleau BL, Gostout CJ, Sherman KE, Shaw MJ, Harford WV, Keate RF, Bracy WP, Fleischer DE (2002) Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc 56:1–6
Sung J (2006) Best endoscopic hemostasis for ulcer bleeding: Is there such a treatment? Gastrointest Endosc 63:774–775
Sung JJ, Luo D, Wu JC, Ching JY, Chan FK, Lau JY, Mack S, Ducharme R, Okolo P, Canto M, Kalloo A, Giday SA (2011) Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy 43:291–295
Giday SA, Kim Y, Krishnamurty DM et al (2011) Long–term randomized controlled trial of a novel nanopowder hemostatic agent (TC–325) for control of severe arterial upper gastrointestinal bleeding in a porcine model. Endoscopy 43:296–299
Smith LA, Stanley AJ, Bergman JJ, Kiesslich R, Hoffman A, Tjwa ET, Kuipers EJ, von Holstein CS, Oberg S, Brullet E, Schmidt PN, Igbal T, Mangiavillano B, Masci E, Prat F, Morris AJ (2014) Hemospray Application in Nonvariceal Upper Gastrointestinal Bleeding; results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol 48:89–92
Barkun AN, Toubouti Y, Rahme E, Bardou M (2009) Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc 69:786–799
Vergara M, Bennett C, Calvet X, Gisbert JP (2014) Epinephrine injection versus epinephrine injection and a second endoscopic method in high-risk bleeding ulcers. Cochrane Database Syst Rev. doi:10.1002/14651858.CD005584.pub3
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions : explanation and elaboration. J Clin Epidemiol 62(10):1–34
PROSPERO Center for Revews and Dissemination, University of York. “Guidance notes for registering a systematic review with PROSPERO”. http://www.crd.york.ac.uk/PROSPERO/
Barkun A, Bardou M, Marshall JK (2003) Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 139:843–857
Consolidated Standards of reporting Trials – CONSORT; http://www.consort-statement.org/consort-statement/
Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ et al (1996) Assessing the quality of reports on randomized clinical trials: Is blinding necessary? Controlled Clin Trials 17:1–12
Center for Evidence-Based Medicine, Headington, Oxford. Critically Appraised Topics (CAT). http://www.cebm.net/catmaker-ebm-calculators/
(RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaborations
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Ljubicic N, Budimir I, Biscanin A, Nikolic M, Supanc V, Hrabar D, Pavic T (2012) Endoclips vs large or small-volume epinephrine in peptic ulcer recurrent bleeding. World J Gastroenterol 18:2219–2224
Jubicic L, Supanc V, Vrsalovic M (2004) Efficacy of endoscopic clipping for actively bleeding peptic ulcer: comparison with polidocanol injection therapy. Hepatogastroenterology 51(56):408–412
Shimoda R, Iwakiri R, Sakata H, Ogata S, Kikkawa A, Ootani H, Oda K, Ootani A, Tsunada S, Fujimoto K (2003) Evaluation of endoscopic hemostasis with metallic hemoclips for bleeding gastric ulcer: comparison with endoscopic injection of absolute ethanol in a prospective, randomized study. Am J Gastroenterol 98(10):2198–2202
Chou YC, Hsu PI, Lai KH, Lo CC, Chan HH, Lin CP, Chen WC, Shie CB, Wang EM, Chou NH, Chen W, Lo GH (2003) A prospective, randomized trial of endoscopic hemoclip placement and distilled water injection for treatment of high-risk bleeding ulcers. Gastrointest Endosc 57(3):324–328
Gevers AM, De Goede E, Simoens M, Hiele M, Rutgeerts P (2002) A randomized trial comparing injection therapy with hemoclip and with injection combined with hemoclip for bleeding ulcers. Gastrointest Endosc 55(4):466–469
Chung IK, Ham JS, Kim HS, Park SH, Lee MH, Kim SJ (1999) Comparison of the hemostatic efficacy of the endoscopic hemoclip method with hypertonic saline-epinephrine injection and a combination of the two for the management of bleeding peptic ulcers. Gastrointest Endosc 49(1):13–18
Grgov S, Radovanović-Dinić B, Tasić T (2013) Could application of epinephrine improve hemostatic efficacy of hemoclips for bleeding peptic ulcers? A prospective randomized study. Vojnosanit Pregl 70(9):824–829
Lo CC, Hsu PI, Lo GH, Lin CK, Chan HH, Tsai WL, Chen WC, Wu CJ, Yu HC, Cheng JS, Lai KH (2006) Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc 63(6):767–773
Arima S, Sakata Y, Ogata S, Tominaga N, Tsuruoka N, Mannen K, Shiraishi R, Shimoda R, Tsunada S, Sakata H, Iwakiri R, Fujimoto K (2010) Evaluation of hemostasis with soft coagulation using endoscopic hemostatic forceps in comparison with metallic hemoclips for bleeding gastric ulcers: a prospective, randomized trial. J Gastroenterol 45(5):501–505
Taghavi SA, Soleimani SM, Hosseini-Asl SM, Eshraghian A, Eghbali H, Dehghani SM, Ahmadpour B, Saberifiroozi M (2009) Adrenaline injection plus argon plasma coagulation versus adrenaline injection plus hemoclips for treating high-risk bleeding peptic ulcers: a prospective, randomized trial. Can J Gastroenterol 23(10):699–704
Lin HJ, Perng CL, Sun IC, Tseng GY (2003) Endoscopic haemoclip versus heater probe thermocoagulation plus hypertonic saline-epinephrine injection for peptic ulcer bleeding. Dig Liver Dis 35(12):898–902
Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD (2002) A prospective, randomized trial of endoscopic hemoclip versus heater probe thermocoagulation for peptic ulcer bleeding. Am J Gastroenterol 97(9):2250–2254
Cipolletta L, Bianco MA, Marmo R, Rotondano G, Piscopo R, Vingiani AM, Meucci C (2001) Endoclips versus heater probe in preventing early recurrent bleeding from peptic ulcer: a prospective and randomized trial. Gastrointest Endosc 53(2):147–151
Skok P, Krizman I, Skok M (2004) Argon plasma coagulation versus injection sclerotherapy in peptic ulcer hemorrhage - a prospective, controlled study. Hepatogastroenterology 51(55):165–170
Laine L, Estrada R (2002) Randomized trial of normal saline solution injection versus bipolar electrocoagulation for treatment of patients with high-risk bleeding ulcers: is local tamponade enough? Gastrointest Endosc 55(1):6–10
Lin HJ, Tseng GY, Perng CL, Lee FY, Chang FY, Lee SD (1999) Comparison of adrenaline injection and bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gut 44(5):715–719
Gralnek IM, Jensen DM, Kovacs TO, Jutabha R, Jensen ME, Cheng S, Gornbein J, Freeman ML, Machicado GA, Smith J, Sue M, Kominski G (1997) An economic analysis of patients with active arterial peptic ulcer hemorrhage treated with endoscopic heater probe, injection sclerosis, or surgery in a prospective, randomized trial. Gastrointest Endosc 46(2):105–112
Llach J, Bordas JM, Salmerón JM, Panés J, García-Pagán JC, Feu F, Navasa M, Mondelo F, Piqué JM, Mas A, Terés J, Rodés J (1996) A prospective randomized trial of heater probe thermocoagulation versus injection therapy in peptic ulcer hemorrhage. Gastrointest Endosc 43:117–120
Choudari CP, Rajgopal C, Palmer KR (1992) Comparison of endoscopic injection therapy versus the heater probe in major peptic ulcer haemorrhage. Gut 33(9):1159–1161
Panés J, Viver J, Forné M (1991) Randomized comparison of endoscopic microwave coagulation and endoscopic sclerosis in the treatment of bleeding peptic ulcers. Gastrointest Endosc 37(6):611–616
Waring JP, Sanowski RA, Sawyer RL, Woods CA, Foutch PG (1991) A randomized comparison of multipolar electrocoagulation and injection sclerosis for the treatment of bleeding peptic ulcer. Gastrointest Endosc 37(3):295–298
Chung SC, Leung JW, Sung JY, Lo KK, Li AK (1991) Injection or heat probe for bleeding ulcer. Gastroenterology 100(1):33–37
Sollano JD, Ang VN, Moreno JA (1991) Endoscopic hemostasis of bleeding peptic ulcers: 1:10000 adrenalin injection vs. 1:10000 adrenalin +1% aethoxysclerol injection vs. heater probe. Gastroenterol Jpn 26(3):83–85
Laine L (1990) Multipolar electrocoagulation versus injection therapy in the treatment of bleeding peptic ulcers. A prospective, randomized trial. Gastroenterology 99(5):1303–1306
Lin HJ, Lee FY, Kang WM, Tsai YT, Lee SD, Lee CH (1990) Heat probe thermocoagulation and pure alcohol injection in massive peptic ulcer haemorrhage: a prospective, randomised controlled trial. Gut 31(7):753–757
Lin HJ, Tsai YT, Lee SD, Lai KH, Lee FY, Lin CY, Lee CH (1988) A prospectively randomized trial of heat probe thermocoagulation versus pure alcohol injection in nonvariceal peptic ulcer hemorrhage. Am J Gastroenterol 83(3):283–286
Bianco MA, Rotondano G, Marmo R, Piscopo R, Orsini L, Cipolletta L (2004) Combined epinephrine and bipolar probe coagulation vs. bipolar probe coagulation alone for bleeding peptic ulcer: a randomized, controlled trial. Gastrointest Endosc 60(6):910–915
Chung SS, Lau JY, Sung JJ, Chan AC, Lai CW, Ng EK, Chan FK, Yung MY, Li AK (1997) Randomised comparison between adrenaline injection alone and adrenaline injection plus heat probe treatment for actively bleeding ulcers. BMJ 314(7090):1307–1311
Lu Y, Chen YI, Barkun A (2014) Endoscopic Management of Acute Peptic Ulcer Bleeding. Gastroenterol Clin North Am 43(4):677–705
Laine L, McQuaid KR (2009) Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol 7(1):33–47
Calvet X, Vergara M, Brullet E (2004) Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology 126(2):441–450
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses, The PRISMA Statement
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs Felipe Baracat, Eduardo Moura, Wanderley Bernardo, Leonardo Zorron Pu, Ernesto Mendonça, Diogo Moura, Renato Baracat, and Edson Ide have no conflicts of interest or financial ties to disclosure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baracat, F., Moura, E., Bernardo, W. et al. Endoscopic hemostasis for peptic ulcer bleeding: systematic review and meta-analyses of randomized controlled trials. Surg Endosc 30, 2155–2168 (2016). https://doi.org/10.1007/s00464-015-4542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4542-x