Abstract
Background
Laparoscopic surgery and enhanced recovery after surgery (ERAS) programs were two major improvements for the management of colorectal diseases. The purpose of this systemic review was to examine whether laparoscopic colorectal surgery still improved short-term postoperative outcomes in comparison with open surgery when both groups of patients received ERAS programs.
Methods
PubMed, Embase, the Cochrane Central Register of Controlled Trials, and reference lists of the identified studies were searched to identify randomized clinical trials that compared laparoscopic with open surgery in patients undergoing colorectal resection in the context of ERAS programs. The outcome measures were analyzed, and the quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Results
Five randomized clinical trials encompassing 598 patients were included in the final analysis. Two of them were multicenter trials. The ERAS programs implemented in the five included trials cannot be classified as optimal ERAS programs, but suboptimal ERAS programs. Laparoscopic colorectal surgery significantly reduced total hospital stay (weighted mean difference (WMD) −1.92 days; 95 % confidence interval (CI) −2.61–−1.23 days; P < 0.00001) and number of complications (relative risk (RR) 0.78; 95 % CI 0.66–0.94; P = 0.007) compared with open surgery in the setting of ERAS programs. No significant differences were found between groups for primary hospital stay, number of patients with complications, readmission rates, and mortality. The quality of evidence for all outcomes was low-to-moderate on the GRADE scale, and none had high quality.
Conclusions
Laparoscopic colorectal resection significantly reduced total hospital stay and number of complications when compared with open surgery in the setting of suboptimal ERAS programs, but the benefits of laparoscopic colorectal resection remain to be proved within optimal ERAS programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Recovery from colorectal resection has traditionally been a prolonged and complicated affair, with a hospital stay of 6–12 days and an overall morbidity of 20–30 % [1, 2]. Over the past two decades, there have been two major improvements in the field of colorectal surgery; the introduction of laparoscopic surgery and the implementation of enhanced recovery after surgery (ERAS) programs, also referred to as “fast track” surgery, both focusing on minimizing the surgical stress to improve short-term outcomes [3, 4].
Since its introduction in 1991 [3], laparoscopic colorectal surgery has become increasingly popular. Evidence from randomized controlled trials and meta-analyses showed that laparoscopic colorectal surgery was associated with shorter hospital stay, less postoperative complications, and pain in comparison with open surgery [5–8]. The long-term oncological results were equivalent between laparoscopic and open surgery [6]. In parallel with the laparoscopic development, the ERAS programs, pioneered by Kehlet and coworkers in the mid-1990s [9], have been shown to improve markedly postoperative recovery of open and laparoscopic colorectal surgery [10]. The ERAS programs have been successfully adopted all over the world. These programs combine a number of evidence-based elements such as optimal postoperative analgesia, early oral feeding, and early mobilization [11]. Studies of ERAS programs have reported hospital stay of 2–3 days following open colorectal surgery which is comparable to any of the best laparoscopic trials in the literature [12, 13].
Although the feasibility and efficacy of laparoscopic colorectal surgery have been demonstrated, the technique is still not widely used and 68.6 % of cases are still performed open in the United States [14]. The laparoscopic colorectal resection procedure is technically demanding. The significant learning curve and prolonged operative times have made laparoscopic surgery for colorectal cancer more challenging [15, 16]. Moreover, a recent study reported that an operative duration >3 h was an independent risk factor for infectious complications in patients undergoing a laparoscopic right colectomy [17].
Therefore, if improvement in short-term postoperative results can be achieved using ERAS programs alone, then the perceived advantages of laparoscopic over open surgery may be less clear. The purpose of the present meta-analysis was to examine whether laparoscopic colorectal surgery still improved short-term postoperative outcomes in comparison with open surgery when both groups of patients received ERAS programs. The present meta-analysis was performed consistent with the recommendations of the Preferred Reporting items for systematic Reviews and meta-analyses (PRISMA) statement [18].
Materials and methods
Literature search
We searched MEDLINE via PubMed, EMBASE, and Cochrane Central Register of Controlled Trials for randomized controlled trials (RCTs) comparing laparoscopic with open surgery in patients undergoing colorectal resection in the context of ERAS programs. Sources were searched up to May 2014. No language restrictions were applied. To ensure that no clinical trials were overlooked, the reference lists of identified articles, previous review articles were manually searched to identify additional studies. The International Clinical Trials Registry Platform of the World Health Organization (www.who.int/trialsearch/) was also searched for any additional relevant registered trials. The full search strategy for PubMed is presented in Table 1.
Inclusion and exclusion criteria
RCTs comparing laparoscopic with open surgery in adult patients (aged >18 years) undergoing colorectal resection for malignant or benign disease in the context of ERAS programs were eligible for inclusion. According to the guidelines of the ERAS group, there are more than 20 ERAS items in the ERAS programs [19, 20]. Because some items might have been implemented in modern traditional care, we made an arbitrary decision that ERAS programs study should include at least seven items. Studies were required to report at least one of the outcome measures mentioned below. When more than one version of the same study was found, only the most recent version was included. When there was overlap between the results of studies reported by the same institution or authors, the larger, higher-quality study was included. Excluded studies (1) were not randomized controlled trials; (2) had <7 items applied; (3) had no documentation of individual items of the ERAS programs; (4) had no data available for the present meta-analysis; or (5) involved emergency surgery; (6) had only abstracts. Article titles and abstracts were screened, and full texts were reviewed independently by two reviewers (C.L.Z. and D.D.H.); discrepancies were resolved by discussion between the reviewers.
Data extraction and outcome measures
All eligible studies were reviewed, and all relevant data were extracted independently by two reviewers (C.L.Z. and D.D.H.) using a specifically designed data extraction form. Discrepancies were resolved by discussion between the reviewers and review of the original articles. Extracted information from each eligible study included (1) study information, including the name of the first author, year of publication, number of patients in each group, and number of ERAS items applied; (2) patient information including age, gender, and site of surgery; (3) follow-up time and outcome measures.
Primary outcome measures included (1) primary hospital stay (defined as the number of days in hospital after surgery until discharge); (2) total hospital stay (defined as primary hospital stay plus the additional hospital days for patients who were readmitted within 30 days after surgery); (3) number of complications; (4) number of patients with complications; (5) readmission rates; and (6) mortality. Secondary outcome measures included (1) operation time; (2) hospital costs; and (3) quality of life.
Considering that some ERAS items might be implemented but not reported in the final publication, investigators of all included trials were contacted to obtain the original protocols of ERAS programs. In addition, if the compliance of ERAS items was not reported, we also tried to contact the investigators to obtain the level of compliance.
Assessment of risk of bias
The quality of methodology of the included RCTs was assessed independently by two reviewers (C.L.Z. and D.D.H.) using the Cochrane Collaboration’s risk of bias tool [21]. Discrepancies were resolved by discussion between the reviewers. The seven domains assessed were (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. The risk of bias for each domain was rated as high (seriously weakens confidence in the results), low (unlikely to seriously alter the results), or unclear.
Statistical analysis
Meta-analyses were performed using relative risk (RR) for dichotomous outcomes and weighted mean difference (WMD) for continuous outcomes. Pooled estimates were presented with 95 % confidence interval (CI). Data reported as medians and interquartile ranges were converted to means and SDs [21]. The presence and amount of heterogeneity were assessed with Q test and I 2 index, and P < 0.1 was considered statistically significant [22, 23]. A random-effects model was used for pooling when there was evidence of heterogeneity; otherwise, a fixed-effects model was used. Funnel plots were created to determine the presence of publication bias, and asymmetry of each funnel plot was evaluated with Egger weighted linear regression test, with P < 0.1 considered significant [24]. For all other comparisons, P < 0.05 was used to determined statistical significance, and all tests were two-sided. The data analysis was performed with Review Manager software version 5.2 from the Cochrane Collaboration and STATA version 12.0 (StataCorp, College Station, TX). Some outcomes were not analyzed but were presented in a descriptive way.
Assessing quality of evidence
The quality of evidence for each outcome measure was rated with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [25], as recommended by the Cochrane Collaboration. The quality of evidence for each outcome measure was rated as high (further research is very unlikely to change our confidence in the estimate of effect), moderate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate), or very low (any estimate of effect is very uncertain). The analyses were performed with GRADEpro software version 3.6 (http://ims.cochrane.org/revman/gradepro).
Results
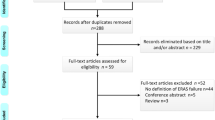
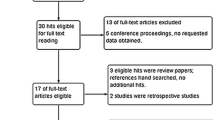
The initial literature search yielded 290 potentially relevant studies. Five RCTs [26–30] (total, 598 patients) published between 2005 and 2014 were included in the meta-analysis (Fig. 1). There were two studies [26, 28] that included patients treated in multiple centers, and the others were single-center studies.
Table 2 shows the characteristics of the included studies. The number of ERAS items applied in the five included studies contained a median of 16 (range 12–18). Some ERAS items which were actually used but not reported were obtained from authors of four included RCTs [26–28, 30]. The exact items used in each study are listed in Table 3. Figure 2 shows evaluation of risk of bias for the included trials. Three trials [26, 28, 29] used adequate methods for generating allocation sequence. In the other two trials [27, 30], the allocations were described as “randomized,” but the detailed method was not specified. Adequate methods for allocating concealment were used in four trials [26–29], but concealment of the allocation sequence was not sufficiently described in one trial [30]. Patients and outcome assessors were effectively blinded in two trials [26, 30]. All included trials were at low risk of bias for incomplete outcome data and selective reporting. None of the included trials were completely free from other bias. Two large trials [26, 28] were at high risk for other bias because of the low compliance with ERAS items. Three other small trials did not report the compliance in the final publication, but we managed to obtain the original compliance level of two trials by contacting the investigators. According to their replies, the compliance level of these two trials [27, 30] was good. Thus, these two trials were at low risk of other bias. The risk of other bias in one trial [29] was unclear because the compliance with ERAS items was not successfully obtained from the investigator.
Primary outcome measures
Laparoscopic colorectal surgery significantly reduced total hospital stay (WMD −1.92 days; 95 % CI −2.61–−1.23 days (Fig. 3); P < 0.00001; I 2 = 0 %; Fig. 4) and number of complications (RR 0.78; 95 % CI 0.66–0.94; P = 0.007; I 2 = 15 %; Fig. 5) compared with open surgery in the setting of ERAS programs. No significant differences were found between groups for primary hospital stay (WMD −1.01 days; 95 % CI −2.14–0.12 days; P = 0.08; I 2 = 77 %; Fig. 3), number of patients with complications (RR 0.81; 95 % CI 0.64–1.04; P = 0.10; I 2 = 0 %; Fig. 6), readmission rates (RR 0.73; 95 % CI 0.39–1.36; P = 0.32; I 2 = 33 %; Fig. 7), and mortality (RR 0.53; 95 % CI 0.19–1.44; P = 0.21; I 2 = 0 %; Fig. 8).
Secondary outcome measures
Operation time was assessed in four studies. Two of the studies did not report the mean and SD for this outcome. Thus, the meta-analysis was not done for operation time. However, all these four studies showed that operation time was significantly increased for the laparoscopic group.
Only two trials [28, 29] assessed the financial impact of laparoscopic colorectal surgery and consistently showed that hospital costs were similar between groups. The meta-analysis was not done for hospital costs due to the limited data available.
Quality of life was assessed in three studies [26, 28, 29] and consistently showed that the quality of life were similar in the two groups, although different quality of life questionnaires were used in these studies.
Publication bias
We used the Egger weighted linear regression test [24] to examine the asymmetry of funnel plots for all six meta-analysis outcomes and found that the funnel plots for primary hospital stay, total hospital stay, number of complications, number of patients with complications, operation time, and mortality were symmetrical (P = 0.779, 0.967, 0.778, 0.801 and 0.659). The funnel plots were asymmetrical for readmission rates (P = 0.076).
Discussion
The present systematic review and meta-analysis showed that laparoscopic colorectal surgery is associated with a significant reduction in total hospital stay and number of complications when compared with open surgery in the setting of ERAS programs. However, laparoscopic surgery significantly increased operation time. There were no significant differences in primary hospital stay, number of patients with complications, readmission rates, mortality, hospital costs, and quality of life between the groups. The quality of evidence was assessed using the GRADE approach (Fig. 9) [25].
The quality of evidence for all outcomes was low-to-moderate on the GRADE scale, and none had high quality. The reasons for the downgraded quality of evidence for each outcome are noted in Fig. 9. A high statistical heterogeneity was identified in the meta-analysis of primary hospital stay and readmission rates, which may have resulted in inconsistency. However, we did not downgrade the quality of evidence because of the heterogeneity, which could be explained by the variation between studies in the experience of surgeons, the number of ERAS items incorporated, compliance with ERAS items, and definition of discharge criteria.
Despite the reduction in total hospital stay associated with laparoscopy, no significant benefit was identified in primary hospital stay. There are several possible explanations. Only the study by Basse et al. [30] showed no significant reduction in primary hospital stay, which was significantly shorter than that of the other studies. The results of this study are likely to be the most favorable because ERAS programs have been well developed in their hospital. However, the highest readmission rates (26.7 and 20 % for open and laparoscopic groups respectively) were reported in this study. In addition, results displayed a tendency toward lower readmission rates for the laparoscopic group, although pooled analysis failed to reach conventional levels of statistical significance. Moreover, the longer operative time in the included RCTs may offset the potential advantages of laparoscopy [15, 17].
It has been demonstrated that improved adherence to the ERAS programs was significantly associated with improved clinical outcomes following major colorectal cancer surgery [31, 32]. However, the compliance of ERAS items in the two included large-scale multicenter trials was low. For example, the items of early oral feeding, early mobilization, and optimal analgesia were not well implemented in the EnROL trial [26]. Early oral feeding has been proved to reduce length of hospital stay and total postoperative complications [33]. Failure to mobilize was associated with prolonged length of hospital stay [34], while optimized pain relief, allowing early mobilization and early return of gut function, is a prerequisite for enhanced recovery [11]. Thus, the ERAS programs implemented in the five included RCTs cannot be classified as optimal ERAS programs, but suboptimal ERAS programs, and the role of laparoscopic colorectal surgery in the context of suboptimal ERAS programs is still uncertain in the present meta-analysis.
Apart from the above concerns, several additional limitations are associated with the included RCTs that warrant caution in the interpretation of the results of this meta-analysis. First, only two trials performed adequate blinding of assessors and patients; two of the included studies were unclear in randomization sequence generations; and concealment of the allocation sequence was not sufficiently described in one study; all of which may introduce biases. Second, the number of studies included in this meta-analysis was small. However, two of the included studies were large-scale multicenter trials, and the present meta-analysis was based on 598 patients. Third, the funnel plots were asymmetrical for readmission rates, which indicated the existence of publication bias. Finally, the meta-analysis was not done for hospital costs due to the limited data available. Thus, the effect of laparoscopic colorectal resection on hospital costs cannot be concluded in this meta-analysis.
In conclusion, laparoscopic colorectal resection significantly reduced total hospital stay and number of complications when compared with open surgery in the setting of suboptimal ERAS programs. However, the benefits of laparoscopic colorectal resection remain to be proved within optimal ERAS programs.
References
Kehlet H (2008) Fast-track colorectal surgery. Lancet 371:791–793
Wick EC, Shore AD, Hirose K, Ibrahim AM, Gearhart SL, Efron J, Weiner JP, Makary MA (2011) Readmission rates and cost following colorectal surgery. Dis Colon Rectum 54:1475–1479
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1:144–150
Wilmore DW, Kehlet H (2001) Management of patients in fast track surgery. BMJ 322:473–476
Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V (2002) Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg 236:759–766 disscussion 767
Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J (2006) Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg 93:921–928
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Bardram L, Funch-Jensen P, Jensen P, Crawford ME, Kehlet H (1995) Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 345:763–764
Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z (2013) Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 56:667–678
Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 248:189–198
Basse L, Hjort Jakobsen D, Billesbolle P, Werner M, Kehlet H (2000) A clinical pathway to accelerate recovery after colonic resection. Ann Surg 232:51–57
Kehlet H, Mogensen T (1999) Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 86:227–230
Bardakcioglu O, Khan A, Aldridge C, Chen J (2013) Growth of laparoscopic colectomy in the United States: analysis of regional and socioeconomic factors over time. Ann Surg 258:270–274
Braga M, Frasson M, Vignali A, Zuliani W, Di Carlo V (2007) Open right colectomy is still effective compared to laparoscopy: results of a randomized trial. Ann Surg 246:1010–1014 discussion 1014–1015
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242:83–91
Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP (2014) Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum 57:616–622
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, Ljungqvist O, Soop M, Ramirez J (2012) Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 31:801–816
Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O (2012) Guidelines for perioperative care in elective colonic surgery: enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 31:783–800
Higgins JPT, S G (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11:193–206
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP, Dutton SJ (2014) Multicenter Randomized Controlled Trial of Conventional Versus Laparoscopic Surgery for Colorectal Cancer Within an Enhanced Recovery Programme: EnROL. J Clin Oncol 32(17):1804–1811
Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, Li J (2012) Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg 16:1379–1388
Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, Sprangers MA, Cuesta MA, Bemelman WA (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254:868–875
King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH (2006) Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg 93:300–308
Basse L, Jakobsen DH, Bardram L, Billesbolle P, Lund C, Mogensen T, Rosenberg J, Kehlet H (2005) Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg 241:416–423
Cakir H, van Stijn MF, Lopes Cardozo AM, Langenhorst BL, Schreurs WH, van der Ploeg TJ, Bemelman WA, Houdijk AP (2013) Adherence to enhanced recovery after surgery and length of stay after colonic resection. Colorectal Dis 15:1019–1025
Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146:571–577
Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC, Yu Z (2013) Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg 30:225–232
Smart NJ, White P, Allison AS, Ockrim JB, Kennedy RH, Francis NK (2012) Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis 14:e727–e734
Acknowledgments
This study was supported by the clinical nutriology of medical supporting discipline of Zhejiang Province (No. 11-ZC24).
Disclosures
Drs. Cheng-Le Zhuang, Dong-Dong Huang, Fan-Feng Chen, Chong-Jun Zhou, Bei-Shi Zheng, Bi-Cheng Chen, Xian Shen and Zhen Yu have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cheng-Le Zhuang and Dong-Dong Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhuang, CL., Huang, DD., Chen, FF. et al. Laparoscopic versus open colorectal surgery within enhanced recovery after surgery programs: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc 29, 2091–2100 (2015). https://doi.org/10.1007/s00464-014-3922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3922-y