Abstract
Background
Intracorporeal Billroth I (ICBI) (delta-shaped) anastomosis is being increasingly used for laparoscopic distal gastrectomy. However, few studies have focused on the safety and feasibility of adopting this new technique. The present study aimed to review the surgical outcomes after the initial experience of performing ICBI anastomosis and to evaluate whether this technique can be safely adopted without increasing operative risk during the early learning process.
Methods
Forty-two consecutive patients who underwent ICBI anastomosis with laparoscopic distal gastrectomy by a single surgeon were enrolled, and their operative outcomes and hospital course were compared with those of 179 patients who underwent conventional extracorporeal Billroth I (ECBI) anastomosis by the same operator. The learning curve was assessed by evaluating the moving average of anastomosis time.
Results
The operating time in the ICBI group was significantly longer than that in the ECBI group (142 vs. 116 min, p < 0.001). However, there were no significant differences in the postoperative hospital course such as gas passage, diet initiation, postoperative fever, and hospital stay between the two groups. Postoperative morbidity did not significantly differ between the ICBI and ECBI groups (7.1 vs. 12.3 %, p = 0.428). No anastomosis-related complications occurred in the ICBI group. The mean anastomosis time for ICBI anastomosis was 24 ± 5 min, and the anastomosis average time curve showed that it reached a plateau approximately after the 14th case.
Conclusions
ICBI anastomosis has a steep learning curve without increasing operative risk in the early learning process, when performed by experienced laparoscopic surgeons. The technical feasibility and clinical advantages of intracorporeal anastomosis need to be proven in future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In Korea and Japan, where the gastric cancer incidence is very high, laparoscopic gastrectomy is widely accepted as a good alternative to open surgery for the treatment of early gastric cancer [1, 2]. Although the long-term outcome has yet to be proven, several studies have demonstrated that short-term surgical outcomes of laparoscopic gastrectomy were comparable to or even better than those of conventional open surgery [3–5]. Moreover, advances in surgical technique and laparoscopic instruments have led to advanced laparoscopic procedures such as laparoscopic total gastrectomy, extended lymph node dissection, or totally laparoscopic gastrectomy with intracorporeal anastomosis [6].
The conventional anastomosis technique for laparoscopic distal gastrectomy was extracorporeal anastomosis through minilaparotomy. In this method, gastric resection and anastomosis are performed with a direct view through a minilaparotomy incision on the epigastrium. An advantage of extracorporeal anastomosis is that surgeons can perform anastomosis as they would in open surgery. However, it leaves a minilaparotomy wound, and performing anastomosis in the narrow and restricted space is often difficult, leading to possible subsequent complications.
More recently, several techniques of intracorporeal anastomosis have been developed for laparoscopic distal gastrectomy [7]. Billroth I anastomosis using a circular stapler has been the most preferred reconstruction technique for laparoscopic as well as open gastrectomy because of its technical feasibility and possible benefits from preserving gastroduodenal food passage. However, it has been seldom used for intracorporeal anastomosis because of the technical difficulties encountered in using a circular stapler intracorporeally. Intracorporeal Billroth I anastomosis (ICBI), known as delta-shaped anastomosis, was first introduced by Kanaya et al. in [8], and in this technique, endoscopic linear staplers are used for gastroduodenostomy. Since then, ICBI has become one of the most commonly used intracorporeal anastomosis techniques for laparoscopic distal gastrectomy. Although several studies have reported the technical feasibility of this procedure [9–15], it remains a demanding surgical technique, especially for a novice surgeon because of unfamiliarity with using linear staplers for gastroduodenostomy.
Few studies have focused on the safety and feasibility in the process of adopting this new technique. Therefore, the present study aimed to review the surgical outcomes after the initial experience of performing ICBI anastomosis by a single surgeon, and to evaluate whether this technique can be safely adopted without increasing operative risk in the early learning process.
Materials and methods
Patients
This study was approved by the institutional review board of Chonnam National University Hospital, South Korea, which waved the informed consent of patients. From the beginning of January 2013 to January 2014, a single surgeon (O Jeong) performed laparoscopic distal gastrectomy with ICBI anastomosis in 42 patients; these patients were included in the present study. Before using this technique, the surgeon had experience performing more than 300 laparoscopic gastrectomies. To compare the surgical outcomes with an established operative procedure, 179 patients who underwent extracorporeal Billroth I (ECBI) anastomosis with laparoscopic distal gastrectomy (between January 2010 and January 2014) by the same operator were selected as a control.
All patients underwent endoscopy with biopsy and abdominal computed tomography scanning for preoperative staging. Endoscopic ultrasonography, liver magnetic resonance imaging, or chest computed tomography was performed in selected patients. The indication of laparoscopic gastrectomy was mucosal or submucosal tumor without lymph node metastasis (cT1N0M0) that was not suitable for endoscopic treatment. D1+ lymph node dissection was performed in most cases, and patients who underwent D2 lymph node dissection were not included in the present study. The decision about intracorporeal or extracorporeal anastomosis was mostly based on the patients’ choice.
Patient data regarding demographics, operative results, pathologic reports, hospital courses, morbidity, and mortality were prospectively obtained using the electronic gastric cancer database system. Pathologic stages were based on the seventh edition of the Union for International Cancer Control tumor-node-metastasis (TNM) system [16]. Intraoperative complications were defined as any events (such as vessel injury, organ injury, or technical failure) that required open conversion or an additional procedure. Postoperative morbidity and mortality were defined as complications or death within 30 days after surgery. Each complication was recorded based on the guideline of definition and severity of complications issued by Jung et al. [17].
Operative technique
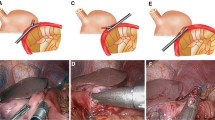
Delta-shaped gastroduodenostomy using endoscopic linear staplers was performed as described by Kanaya et al. [8]. The patient was placed in the reverse Trendelenburg position with the two legs apart. Five abdominal ports were used: two right operator ports (5-mm upper and 12-mm lower port), two left 12-mm assistant ports, and one umbilical port for laparoscope insertion. The liver was retracted upward using a simple suture technique as illustrated in Fig. 1A. Under a 12- to 14-mmHg pneumoperitoneum, gastric dissection was started by dividing the greater omentum 3–4 cm apart from the gastroepiploic vessel, and then by moving toward to the left gastroepiploic area. All gastric and lymph node dissection was performed using an electrocautery or LigaSure™ (LigaSure Advance™, Covidien, Colorado, USA).
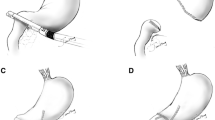
After completing gastric dissection, the stomach was transected using endoscopic linear staplers through the left lower assistant port (Fig. 1B). The distal two-thirds of the stomach was resected after marking the imaginary resection line at the lesser and greater curvatures. We do not routinely perform tumor localization, such as endoscopic clipping, or intraoperative esophagogastroduodenoscopy, when the tumors are located in the distal part of the stomach (such as distal to the gastric angle). Instead, we selectively perform intraoperative esophagogastroduodenoscopy for tumors that are located near the middle part of the stomach and are thought to require localization at the discretion of surgeons. Then, the duodenum was transected from the posterior to anterior side through the left upper 12-mm assistant port (Fig. 1C). The left upper assistant port is more appropriate to transect the duodenum from the posterior to anterior side. After making small entry holes at the posterior tip of the duodenum stapler line and at the greater curvature side of the gastric resection line, anastomosis was performed using a 45-mm endoscopic linear stapler (PSE 45A; Ethicon Endo-Surgery; Cincinnati, OH, USA) between the posterior wall of the stomach and the posterior to superior wall of the duodenum (Fig. 1D). At this point, a linear stapler was introduced through the left lower assistant port, and the stomach and the duodenum should be adequately retracted to perform the anastomosis in a proper position. After anastomosis, three anchoring sutures were placed at the common entry hole, and the hole was closed using a 60-mm linear stapler through the left lower assistant port (Fig. 1E). The techniques of anastomosis and common hole closure are described in more detail with illustrations in Fig. 2.
Illustrations of the techniques of A anastomosis and B common hole closure. After making small entry holes at the tip of the duodenum and gastric transection line, anastomosis is created between the posterior wall of the stomach and the posterior to superior wall of the duodenum through the left lower assistant port (A). Three anchoring sutures were placed at the common entry hole, and the hole was closed using a 60-mm linear stapler through the left lower assistant port (B)
For ECBI anastomosis, a 5- to 6-cm minilaparotomy incision was made on the epigastrium. Through this minilaparotomy, a purse-string suture was placed at the duodenal stump and the anvil head was fixed. Gastroduodenostomy was performed using a 28- or 29-mm circular stapler through the minilaparotomy incision, similar to that performed in the conventional open technique [18]. After the anastomosis was performed, the stomach was resected using two linear staplers.
Postoperative care
Patients were managed using the same clinical pathway protocol, regardless of the anastomosis procedure. Briefly, no preoperative bowel preparation or nasogastric tube was used. Preoperative fasting was avoided until the night before surgery. A carbohydrate-rich drink was administered to patients 2–3 h before surgery. Prophylactic antibiotics were only used during the operation without additional postoperative use. During the operation, intraoperative normothermia was maintained using a warm air blanket, and an intermittent pneumatic compression device was used for thromboprophylaxis. An abdominal drain was not routinely inserted except for selected cases. An abdominal drain was only indicated after difficult operations, such as those involving excessive operative bleeding or fragile and fatty abdominal tissue. Moreover, when there was a risk of anastomosis complications due to technical errors occurring during the anastomosis procedure, an abdominal drain was inserted for early detection of anastomosis problems. Postoperatively, patients were allowed to have an oral diet starting on postoperative day (POD) 1 or 2. Postoperative pain was managed using epidural anesthesia for 3–4 days after surgery. Intravenous fluid was administered in restrictive amount until POD 3. Patients were discharged from the hospital on POD 6–8, based on the fulfillment of discharge criteria.
Statistical analysis
Data are expressed as mean ± SD or n (%). Student’s t test was used to compare continuous variables, and the Chi-square test or Fisher’s exact test was used to compare categorical data as appropriate. To assess the learning curve of the anastomosis procedure, the moving average of anastomosis time in a group of four patients was plotted, and the changes in the average anastomosis time were evaluated. The statistical analyses were performed by SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA), and two-sided p values of less than 0.05 were considered statistically significant.
Results
Characteristics of patients
Clinicopathological characteristics of the two anastomosis groups are summarized in Table 1. The ICBI group consisted of 22 men and 20 women with a mean age of 58.4 ± 10.9 years. The mean body mass index (BMI) was 24.8 ± 3.4 kg/m2, and 19 (45.2 %) patients had an underlying comorbidity. According to TNM staging, 39 (92.9 %), two, and one patient(s) had stage IA, stage IB, and stage IIB gastric cancer, respectively.
There were no significant differences in sex, BMI, comorbidity, tumor location, tumor size, histological classification, and TNM stage between the ICBI group and ECBI group. However, the ICBI group was significantly younger than the ECBI group (58.4 vs. 62.7 years, p = 0.027).
Operative results and hospital courses
Table 2 shows the operative results of the two groups. The mean operating time in the ICBI group was significantly longer than that of the ECBI group (142 vs. 116 min, p < 0.001). The amount of intraoperative blood loss was significantly lower in the ICBI group (50 vs. 105 mL, p < 0.001). The proximal margin lengths were 42 ± 23 mm in the ICBI group and 61 ± 49 mm in the ECBI group (p = 0.016). There was no intraoperative complication or open conversion in the ICBI group. No significant intergroup differences were found with respect to intraoperative complication, open conversion, and the number of harvested lymph nodes.
Postoperative hospital courses are summarized in Table 3. There were no significant differences in gas passage or diet start time between the two groups. The incidence of postoperative fever and blood transfusion was also similar between the two groups. The mean hospital stay of the ICBI and ECBI groups was 6.9 ± 1.3 days and 9.3 ± 13.7 days, respectively (p = 0.365). There were no significant differences in morbidity (7.1 vs. 12.3 %, p = 0.428) and mortality (0 vs. 1.1 %, p = 1.000) between the ICBI and ECBI group.
Table 4 details the postoperative complications in the two groups. In the ICBI group, three patients presented local complications (one gastric stasis, one wound complication, and one abdominal bleeding). No anastomosis-related complications occurred in the ICBI group. Meanwhile, 20 local complications and four systemic complications occurred in the ECBI group. The most common local complication was gastric stasis (n = 5), followed by wound complication (n = 4) and gastrointestinal bleeding (n = 4). Four patients required reoperation and two patients died of postoperative complication (one gastrointestinal bleeding and one postoperative pancreatitis) in the ECBI group.
Learning curve of intracorporeal anastomosis
The anastomosis time in 42 consecutive cases of ICBI anastomosis is shown in Fig. 3. Anastomosis time was measured in every patient from the cutting of the stomach to anastomosis completion. The moving average of anastomosis time showed that anastomosis time reached a plateau approximately after the fourteenth case, which can be regarded as overcoming the learning curve. After the fourteenth case, the mean anastomosis time significantly reduced from 30 ± 4 to 22 ± 2 min (p < 0.001).
Discussion
With advances in surgical technique and laparoscopic instruments, many gastric surgeons are currently attempting to perform totally laparoscopic gastrectomy, in which all the procedure, including gastric resection and anastomosis, are performed intracorporeally without additional abdominal incision [6]. Several techniques of intracorporeal anastomosis have been developed for totally laparoscopic gastrectomy [7]. When selecting a proper anastomosis technique, technical feasibility and safety would be the most essential because of the severe nature of anastomosis-related complications. However, acquiring a new surgical skill is often challenging because of longer operating time and the risk of increasing operative morbidity before overcoming the learning curve. The feasibility and safety in the early learning process is the main concern for surgeons who are trying to adopt a new anastomosis technique.
Unlike other anastomosis techniques, ICBI (delta-shaped) anastomosis has been relatively less widely accepted by gastric surgeons because of the concerns about technical difficulties in using linear staplers for gastroduodenostomy. In the present study, we have focused on the feasibility and safety of this new technique during the early learning process. Short-term surgical outcomes, including postoperative recovery and complications, after the initial experience of performing ICBI anastomosis were comparable to those of the established ECBI procedure. Similar to our study, Kanaji et al. [19] evaluated surgical outcomes in the newly introduced phase of intracorporeal anastomosis and showed that the surgical outcomes were comparable to those of established extracorporeal anastomosis. Because we verified the technical feasibility and acceptable surgical outcomes of the ICBI technique, we are currently performing ICBI anastomosis as the primary choice of intracorporeal reconstruction after laparoscopic distal gastrectomies in our institution.
Despite the technical feasibility of ICBI anastomosis, the clinical advantages of this procedure have yet to be proven. Besides the cosmetic benefit, some studies have suggested faster bowel recovery [11, 14], less postoperative pain [15], or reduced hospital stay [9, 13] with intracorporeal anastomosis. In one large retrospective study comparing ICBI and ECBI anastomosis, obese patients were found to benefit more from intracorporeal anastomosis with reduced postoperative complications [10]. This may be explained by the fact that extracorporeal anastomosis through a narrow minilaparotomy space would be more difficult especially in obese patients. On the other hand, a recent systemic review showed no clinically significant advantages of intracorporeal anastomosis with respect to operative time, bowel recovery, hospital stay, or anastomosis-related complications [12]. Our study also showed a similar result with no significant differences in gas passage, diet start, hospital stay, and postoperative morbidity between the ICBI and ECBI group. Instead, the operating time was significantly longer in the ICBI group. Because of the inherent limitation of retrospective studies and inconsistent results, we cannot confirm the superiority of either of the intra- or extracorporeal anastomosis as of now. Therefore, the anastomosis method should be selected according to the patient’s condition and the surgeon’s preference.
It has been demonstrated that 30–60 cases are required to achieve competence in laparoscopic distal gastrectomy [20, 21]. As for ICBI anastomosis, it is expected that a relatively small number of cases will be required to overcome the learning curve. Surgeons usually introduced to the intracorporeal technique after they are convinced of technical proficiency at laparoscopic gastrectomy. Kanaya et al. [22] suggested that the anastomosis time for delta-shaped anastomosis reached a plateau at approximately 10 min, after a few experiences performing the technique. In our study, the changes in the average anastomosis time showed that anastomosis time began to decrease approximately after the tenth case and reached a plateau approximately after the fourteenth case. The mean anastomosis time after reaching a plateau was 22 ± 2 min. Because of the differences in personal experiences with laparoscopic gastrectomy and technical proficiency, the number of cases for overcoming the learning curve will differ according to each surgeon. However, we believe that ICBI anastomosis has a steep learning curve for experienced laparoscopic surgeons.
Besides delta-shaped anastomosis, several other techniques for ICBI anastomosis have been reported. Tanimura et al. [23] described a triangular stapling technique with linear staplers. In their method, the posterior walls of the stomach and duodenum are stapled in an inverted manner, and the anterior walls are stapled in an everted manner, making the shape of the anastomosis triangular. Kim et al. [24] introduced an intracorporeal gastroduodenostomy technique using a circular stapler, in which the circular stapler was introduced through a 3-cm extension of the left lower port. Omori et al. [25] developed an intracorporeal circular stapling Billroth I anastomosis technique for single-incision laparoscopic gastrectomy. They obviated the need for purse-string sutures for anvil insertion into the duodenal bulb. These innovations are all expected to contribute to a shift from “laparoscopy assisted” to “totally laparoscopic” procedures, and long-term outcomes for these procedures are awaited.
Interestingly, the present study found that the proximal margin lengths were significantly different between the two groups. During the extracorporeal anastomosis, we can directly identify a tumor location and decide on the location of the gastric resection line by opening the stomach. This might result in a relatively longer proximal resection margin in the ECBI group than the ICBI group. However, despite the shorter proximal margin length, no patients in the ICBI group had residual tumors at the proximal resection margin. Nevertheless, tumors that are located near the middle third of the stomach may require proper tumor localization before performing the intracorporeal anastomosis.
Anastomosis bleeding occasionally occurs after ECBI anastomosis, because the anastomosis-stapling line cannot be examined inside of the lumen after the anastomosis is completed. Despite no significant difference in overall morbidity, we found that anastomosis bleeding only occurred in the ECBI group. During ICBI anastomosis, bleeding of the anastomosis line can be directly examined before closing the common hole, and can be easily controlled during the operation. This may contribute to the lower incidence of anastomosis bleeding after the ICBI technique.
In the present study, the length of hospital stay in the ICBI group was found to be relatively shorter than that of the ECBI group. Although the difference was not statistically significant, the data may ultimately show that intracorporeal anastomosis provides significantly shorter length of hospital stay with a larger cohort (underpowered). The possible explanation for the shorter length of hospital stay is that the ECBI group had more patients having complications requiring operation or longer hospital stay, while most of the morbidity cases in the ICBI group were treated conservatively. However, a large randomized study will be required to investigate whether intracorporeal anastomosis can reduce hospital stay in gastric cancer patients.
The present study has some limitations. Although the choice of anastomosis technique was determined mainly based on the patient’s choice during the study period, selection bias could not be avoided in this retrospective study. The ICBI group was significantly younger than the ECBI group because younger patients prefer intracorporeal anastomosis. This may have influenced the surgical outcomes in the ICBI group. Second, this study is limited by the fact that it was based on the experience of a single surgeon who has substantial experience performing laparoscopic gastrectomy. The technical feasibility and especially the learning curve may depend on the extent of the surgeon’s experience performing laparoscopic gastrectomy.
In conclusion, the present study showed that ICBI (delta-shaped) anastomosis can be safely adopted by experienced laparoscopic surgeons without increasing the operative risk during the initial learning period. Totally laparoscopic gastrectomy with intracorporeal anastomosis may have several advantages over laparoscopy-assisted gastrectomy, but these advantages have yet to be proven in future clinical trials. Finally, we emphasize that substantial experience performing laparoscopic gastrectomy would be essential for successful initial outcomes when adopting a new anastomosis technique.
References
Jeong O, Park YK (2011) Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 11:69–77
Kitano S, Shiraishi N (2004) Current status of laparoscopic gastrectomy for cancer in Japan. Surg Endosc 18:182–185
Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM (2008) Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 248:721–727
Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L (2012) Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg 256:39–52
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251:417–420
Kim HH, Ahn SH (2011) The current status and future perspectives of laparoscopic surgery for gastric cancer. J Korean Surg Soc 81:151–162
Hosogi H, Kanaya S (2012) Intracorporeal anastomosis in laparoscopic gastric cancer surgery. J Gastric Cancer 12:133–139
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 195:284–287
Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R (2011) Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc 25:1395–1401
Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH (2011) A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc 25:1076–1082
Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, Chin HM, Hur H (2008) Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg 12:1015–1021
Kim DG, Choi YY, An JY, Kwon IG, Cho I, Kim YM, Bae JM, Song MG, Noh SH (2013) Comparing the short-term outcomes of totally intracorporeal gastroduodenostomy with extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer: a single surgeon’s experience and a rapid systematic review with meta-analysis. Surg Endosc 27:3153–3161
Lee SW, Tanigawa N, Nomura E, Tokuhara T, Kawai M, Yokoyama K, Hiramatsu M, Okuda J, Uchiyama K (2012) Benefits of intracorporeal gastrointestinal anastomosis following laparoscopic distal gastrectomy. World J Surg Oncol 10:267
Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, Ohga T, Adachi E, Toh Y, Okamura T, Baba H (2009) Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc 23:2374–2379
Kim BS, Yook JH, Choi YB, Kim KC, Kim MG, Kim TH, Kawada H, Kim BS (2011) Comparison of early outcomes of intracorporeal and extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A 21:387–391
Sobin LH, Gospodarowicz MK, Wittekind Ch (eds) (2009) International union against cancer (UICC) TNM classification of malignant tumors. 7th edn. Wiley, Oxford
Jung MR, Park YK, Seon JW, Kim KY, Cheong O, Ryu SY (2012) Definition and classification of complications of gastrectomy for gastric cancer based on the accordion severity grading system. World J Surg 36:2400–2411
Kitano S, Yang HK (eds) (2012) Laparoscopic gastrectomy for cancer—standard techniques and clinical evidences (Chapter 20), 1st edn. Springer, Tokyo
Kanaji S, Harada H, Nakayama S, Yasuda T, Oshikiri T, Kawasaki K, Yamamoto M, Imanishi T, Nakamura T, Suzuki S (2013) Surgical outcomes in the newly introduced phase of intracorporeal anastomosis following laparoscopic distal gastrectomy is safe and feasible compared with established procedures of extracorporeal anastomosis. Surg Endosc. doi:10.1007/s00464-013-3315-7
Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, Han SU (2007) Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 21:28–33
Kim MC, Jung GJ, Kim HH (2005) Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol 11:7508–7511
Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I (2011) The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer 14:365–371
Tanimura S, Higashino M, Fukunaga Y, Takemura M, Nishikawa T, Tanaka Y, Fujiwara Y, Osugi H (2008) Intracorporeal Billroth 1 reconstruction by triangulating stapling technique after laparoscopic distal gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 18:54–58
Kim HI, Woo Y, Hyung WJ (2012) Laparoscopic distal gastrectomy with an intracorporeal gastroduodenostomy using a circular stapler. J Am Coll Surg 214:e7–e13
Omori T, Tanaka K, Tori M, Ueshima S, Akamatsu H, Nishida T (2012) Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc 26:1490–1494
Disclosure
Drs. Oh Jeong, Mi-Ran Jung, Young-Kyu Park, and Seong-Yeop Ryu have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, O., Jung, M.R., Park, Y.K. et al. Safety and feasibility during the initial learning process of intracorporeal Billroth I (delta-shaped) anastomosis for laparoscopic distal gastrectomy. Surg Endosc 29, 1522–1529 (2015). https://doi.org/10.1007/s00464-014-3836-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3836-8