Abstract
Multiple sclerosis (MS) refers to chronic inflammation of the central nervous system including the brain and spinal cord. Assessing for the presence of dysphagia in subjects with MS represents a challenge for neurologists in clinical practice. The aim of the present study was to verify the relationship between DYMUS scores, a patient-reported scale, and objective symptoms using the Dysphagia Outcome Severity Score (DOSS), based on fiber-optic endoscopy. Data were collected in a multicenter study. Two hundred and fifteen MS patients were enrolled, irrespective of self-reported dysphagia. DOSS revealed dysphagia in 122 subjects (56.7%). Compared with non-dysphagic subjects, the presence of dysphagia was related to more severe disability, longer disease duration, and a progressive form of the disease. A DYMUS score of 0 strongly correlated with a DOSS of 6 (sensitivity 100%) while DYMUS score of > 2 correlated with a DOSS < 7 (specificity 82%) of the self-reported scale. The DYMUS questionnaire can be a useful clinical tool for red-flagging patients who should undergo objective testing and referral to a otorhinolaryngologist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing problems are relatively frequent in patients with multiple sclerosis (MS) ranging from 33 to 43% [1]. The range, in prevalence, is due to differences in the measures utilized, varying from 31% for subjective measures to 81% for clinical and instrumental evaluations [1]. Permanent dysphagia emerges in mildly impaired patients scored using Expanded Disability Status Scale [2] (EDSS 2–3) and becomes increasingly common as disability worsens, reaching a prevalence of 65% in the most severely disabled subjects (EDSS 8–9) [3]. Although dysphagia is more often associated with higher levels of disability, it has also been reported in 17% of patients with mild disability (EDSS ≤ 2.5) [4].
The gold standard in the objective assessment and diagnosis of swallowing problems includes the videofluorographic swallowing study and fiber-optic endoscopic examination (FEES) [5]. Both techniques require specific instrumentation and a otorhinolaryngologist for administering the tests and therefore are not used for screening in clinical practice. With FEES, dysphagia is scored using the Dysphagia Outcome Severity Scale (DOSS) [6].
To our knowledge only a few studies have evaluated the presence of dysphagia in MS using FEES.
In particular, Calcagno et al. [7] evaluated swallowing in 143 consecutive patients with primary and secondary progressive MS in an in-patient setting. Evaluations were conducted using the FEES combined with a speech pathologist’s assessment. Specifically, the FEES was administered following a preliminary interview to establish whether the patient had experienced any subjective symptoms related to swallowing. Dysphagia was diagnosed in 49 patients (34.3%) and a significant correlation with illness severity resulted using the DOSS to score the FEES evaluation.
Recently, the Dysphagia in Multiple Sclerosis (DYMUS) questionnaire was validated as a screening tool in MS patients and demonstrated excellent psychometric properties [8, 9]. The questionnaire is easy to administer, reliable, and able to detect characteristics of dysphagia [10]. However, the relationship between DYMUS scores and an objective evaluation, specifically the FEES, is lacking. Only one small study in MS with 26 subjects evaluated dysphagia using the DYMUS questionnaire, while FEES was used to establish the degree of aspiration and penetration, graded using the penetration-aspiration scale (PAS), and reported a positive correlation between DYMUS and PAS [10].
The aim of the current study was to evaluate the correlation between DOSS and DYMUS and to estimate the specificity and sensitivity of both scales in a large random cohort of MS patients.
Material and Methods
Subjects
Study participants were a consecutive sample of patients followed at four Italian MS outpatient clinics (Santa Lucia Foundation Rome, ASL3 Genovese Genoa, Mondino Hospital of Pavia, and University of Catania). Inclusion criteria were as follows: a diagnosis of MS according to the McDonald revised criteria [11], a minimum age of 18 years, currently in a relapse-free phase of the disease and no treatment or training for dysphagia in the last 3 months prior to study entry. Patients with documented dysphagia related to other diseases were excluded from the study.
Instruments
The Dysphagia in Multiple Sclerosis questionnaire (DYMUS) is a 10-item, self-administered screening tool to identify self-reported swallowing problems in adults with MS [8]. The questions assess dysphagia for solids and liquids. Patients are instructed to answer the questions with “yes” or “no” based on their present state, with the exception of the last question, which refers to weight loss over the past 6 months (Affirmation of any of the questions is considered a red flag for dysphagia). Currently data are unavailable that support the DYMUS as an indicator of dysphagia severity.
Fiber-optic endoscopic evaluation of swallowing (FEES) is an instrumental evaluation in which a flexible endoscope is introduced transnasally into the patient’s pharynx providing a view of the laryngeal and pharyngeal structures and their functions [5]. FEES is scored using DOSS levels (scored from 7 to 1). In particular, level 7 is consistent with full oral nutrition with a normal diet. DOSS level 6 implies some functional limitations, such as extra time required for meals, mild oral and pharyngeal delay and retention or trace epiglottal undercoating. At DOSS 6 an individual independently and spontaneously can compensate. DOSS levels 5 to 3 imply modified diet and/or independence and DOSS levels 2 and 1 exclude oral nutrition.
Prior to subject enrollment, participating outpatient clinics agreed on procedures to standardize the administration of the DYMUS and scoring of FEES. After providing written informed consent each subject completed the DYMUS questionnaire. Irrespective of the DYMUS score, each subject underwent FEES. A standardized FEES protocol examined the mouth, teeth, pharyngeal velum, tongue, pharynx, larynx, and voice quality, for both morphological and functional abilities FEES was performed by otolaryngologists who were blinded to subjects’ medical history, disease severity, and the presence of self-reported swallowing symptoms (DYMUS scores).
The study was approved by the Ethics Committees of Centers involved in the study (PI CE: AG4-PROG259-157).
Statistical analyses
To verify the correlation between the DYMUS and DOSS, we calculated the sensitivity and specificity between the different scores of DYMUS (from 0 to 5) and DOSS from 7 to 5. The values from 4 to 1 were combined due to the low number of patients scoring within this range. In order to study the relationship between the DYMUS scores and DOSS levels we computed the Pearson correlation coefficient considering the variables as quantitative. Considering DOSS as a categorical variable, one-way ANOVA were applied to analyze the trend of DYMUS average values within DOSS categories. Subjects were divided into two groups based on the level of independence while consuming meals. Subjects with a DOSS level 7 (no limitations) were considered dysphagia-free. A receiver operating characteristics (ROC) curve was used to assess the sensitivity and specificity to different cutoffs for the DYMUS score. Parametric tests were used to compare age and disease duration (unpaired t tests to compare groups) between subjects with dysphagia and those with no dysphagia, according to DOSS levels. Nonparametric tests were used to assess differences between groups on EDSS (Wilcoxon–Mann–Whitney rank sum test for two groups). Categorical data were compared using Chi-square tests. One-way ANOVA assessed correlations between both DOSS and DYMUS, and demographic and clinical variables including age, gender, age at disease onset, disease duration and EDSS. A p value of < 0.05 was considered statistically significant. The SPSS software version 17 for Windows (SPSS, Chicago IL, 2002) was used for the statistical analyses.
Results
Two hundred nineteen consecutive subjects were enrolled in the study and four were subsequently excluded from the analysis due to incomplete examinations. The final sample included 215 subjects: 82 males (38%) and 133 females (62%), F:M ratio = 1.6:1, with a mean age of 50 years (SD ± 11.9), and a mean disease duration of 15.1 years (SD ± 10.2). The mean EDSS was 5.4 (SD ± 2.4, range 0–9.0). One hundred and three subjects (47.9%) had a relapsing–remitting form of MS, 103 (47.9%) a secondary progressive form, and nine (4.2%) a primary progressive disease course. The mean DOSS score was 6.06 (SD 1.07) and the mean of DYMUS score was 2.81 (SD 2.85). No significant center effect was observed for the distribution of clinical variables.
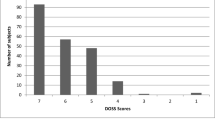
Figure 1 presents the DYMUS distribution and the FEES results scored according to DOSS levels. Table 1 shows the distribution of scores of the DYMUS and the DOSS. One hundred twenty-two subjects (56.7%), according DOSS less than 7, presented with dysphagia. Compared with non-dysphagic subjects, the presence of dysphagia was related to more severe disability (F 7.532, p 0.007), disease duration, and a progressive form of the disease (F 10.101, p 0.002).
In order to evaluate sensitivity and specificity of subjective swallowing symptoms, DYMUS and DOSS scores were compared and are presented in Table 2. Sensitivity for a DYMUS score of 0 was 84% and reached 100% for a DYMUS score of 1, meaning that no subjects showed pathological values on the FEES. The specificity was more than 80% with a DYMUS score greater than 2, meaning that the majority of subjects had a DOSS score of less than 7. The sensitivity of DYMUS 0–1 was also maintained in subjects with lower levels of disability (EDSS < 4). A Pearson correlation coefficient of − 0.621 demonstrated an inverse positive correlation between DYMUS and DOSS scores (significantly different from zero p < 0.001) (See Fig. 2).
Discussion
Early detection of dysphagia in individuals with MS can promote specific and timely intervention leading to the prevention of complications, such as pneumonia and malnutrition [12]. Early detection requires the availability of a screening instrument that can be easily used in a clinical setting by non-swallowing pathology specialists, notably the neurologist. The present study demonstrated a correlation between the DYMUS and FEES, providing evidence that the DYMUS is a highly sensitive, noninvasive means for detecting swallowing problems in subjects with MS.
A recent systematic review of studies on the prevalence of dysphagia in individuals with MS concluded that, based on varied diagnostic methods, swallowing problems are present in more than one-third of subjects [1]. In a large multicenter study that included 1,875 subjects with MS screened with the DYMUS questionnaire, 31.3% of patients self-reported swallowing problems with a score of > 0 [13]. These results are clearly much lower than the 71% found in the present study. This difference may in part be due to the fact that dysphagia is often correlated with disease severity and a progressive course.
In the current sample, 47 of 122 (38.5%) patients with a FEES score < 7 had an EDSS score from 1.0 to 5.5, confirming the relatively high frequency of dysphagia even in subjects with a mild-to-moderate form of MS [4, 14]. Further, among the clinical forms of the disease, the progressive forms of MS (primary progressive and secondary progressive) were more frequently associated with severe dysphagia, while relapsing–remitting MS presented more often associated with mild-to-moderate dysphagia [15].
The DYMUS could overestimate the presence of dysphagia. However, the high variability of the disease within a single subject does not rule out the possibility that the same patient could have a modest, intermittent, or subjective swallowing problem that would not be detected with FEES, especially in those with lower levels of disability [16].
The instrumental gold standards for evaluating swallowing problems in neurological diseases are both video fluoroscopy and FEES [17]. The present study did not include video fluoroscopy due to ethical issues related to administering an invasive procedure in seemingly asymptomatic subjects. FEES allows direct tri-dimensional visualization of the pharynx and larynx, before and after swallowing and may be repeated as often as necessary. The downside to its use in isolation is that it detects the presence of a swallowing difficulty in a specific timeframe lasting only a few minutes, while intermittent or subclinical deficits may be undetected.
The main aim of this study was to evaluate the sensitivity and specificity of DYMUS with respect to the DOSS scoring system, in order to propose the DYMUS questionnaire as the first step in identifying swallowing difficulties. DYMUS questionnaire, a quick and easy tool, is able to screen patients with swallowing problems in clinical practice, especially in MS outpatients. If the patient is identified with a potential dysphagia (DYMUS greater than 2), an instrumental evaluation, as the FEES, is required.
Based on the current findings, we recommend that the DYMUS be administered as part of routine practice especially in MS outpatients and when the score is > 2 the patient be referred for further testing. Subjects with a DYMUS score of 2 should be questioned about symptom frequency and referred for further testing depending on the response. In any case, the DYMUS should be re-administered at follow-up.
However, some questions remain open: in fact, 27 subjects have DOSS 7 and DYMUS greater than 0, so they are or false positive at DYMUS or false negative at DOSS. Several factors such as fatigue, mood disorders, or cognitive impairment could be involved in such discrepancy and explain intermittent swallowing problems. Furthermore currently data are unavailable that support the DYMUS as an indicator of dysphagia severity.
In conclusion, this study represents a large multicenter study of MS people evaluated for dysphagia using both an ad-hoc questionnaire and instrumental examination. DYMUS questionnaire, a subjective, patient-generated outcome can be an easily administered screening tool for use in clinical setting.
References
Guan XL, Wang H, Huang HS, Meng L. Prevalence of dysphagia in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2015;36(5):671–81.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–522.
De Pauw A, Dejaeger E, D’hooghe B, Carton H. Dysphagia in multiple sclerosis. Clin Neurol Neurosurg. 2002;104(4):345–51.
Abraham SS, Yun PT. Laryngopharyngeal dysmotility in multiple sclerosis. Dysphagia. 2002;17(1):69–74.
Keage M, Delatycki M, Corben L, Vogel A. A systematic review of self-reported swallowingassessments in progressive neurological disorders. Dysphagia. 2015;30(1):27–46.
O’Neil KH, Purdy M, Falk J, Gallo L. The dysphagia outcome and severity scale. Dysphagia. 1999;14(3):139–45.
Calcagno P, Ruoppolo G, Grasso MG, De Vincentiis M, Paolucci S. Dysphagia in multiple sclerosis-prevalence and prognostic factors. Acta Neurol Scand. 2002;105(1):40–3.
Bergamaschi R, Crivelli P, Rezzani C, Patti F, Solaro C, Rossi P, Restivo D, Maimone D, Romani A, Bastianello S, Tavazzi E, D'Amico E, Montomoli C, Cosi V. The DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. J Neurol Sci. 2008;269(1–2):49–53.
Bergamaschi R, Rezzani C, Minguzzi S, Amato MP, Patti F, Marrosu MG, Bonavita S, Grasso MG, Ghezzi A, Rottoli M, Gasperini C, Restivo D, Maimone D, Rossi P, Stromillo ML, Montomoli C, Solaro C, DYMUS Group. Validation of the DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. Funct Neurol. 2009;24(3):159–62.
Alfonsi E, Bergamaschi R, Cosentino G, Ponzio M, Montomoli C, Restivo DA, Brighina F, Ravaglia S, Prunetti P, Bertino G, Benazzo M, Fontana D, Moglia A. Electrophysiological patterns of oropharyngeal swallowing in multiple sclerosis. Clin Neurophysiol. 2013;124(8):1638–45.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–330.
Tassorelli C, Bergamaschi R, Buscone S, Bartolo M, Furnari A, Crivelli P, Alfonsi E, Alberici E, Bertino G, Sandrini G, Nappi G. Dysphagia in multiple sclerosis: from pathogenesis to diagnosis. Neurol Sci. 2008 Dec;29(4):360–3.
Solaro C, Rezzani C, Trabucco E, Amato MP, Zipoli V, Portaccio E, Giannini M, Patti F, D'Amico E, Frau J, Lorefice L, Bonavita S, Della Corte M, Grasso MG, Finamore L, Ghezzi A, Annovazzi P, Rottoli M, Gasperini C, Restivo D, Maimone D, Rossi P, Stromillo ML, Bergamaschi R. Prevalence of patient-reported dysphagia in multiple sclerosis patients: an Italian multicenter study (using the DYMUS questionnaire). J Neurol Sci. 2013;331(1–2):94–7.
Prosiegel M, Schelling A, Wagner-Sonntag E. Dysphagia and multiple sclerosis. Int MS J. 2004;11(1):22–31.
Fernandes AMF, de Campos Duprat A, Eckley CA, da Silva L, Ferreira RB, Tilbery CP. Oropharyngeal dysphagia in patients with multiple sclerosis: do the disease classification scales reflect dysphagia severity? Braz J Otorhinolaryngol. 2013;79(4):460–5.
Beckmann Y, Gürgör N, Çakır A, Arıcı Ş, İncesu TK, Seçil Y, Ertekin C. Electrophysiological evaluation of dysphagia in the mild or moderate patients with multiple sclerosis: a concept of subclinical dysphagia. Dysphagia. 2015;30(3):296–303.
Giusti A, Giambuzzi M. Management of dysphagia in patients affected by multiple sclerosis: state of the art. Neurological Sciences. 2008;29(S4):364–366.
Funding
The study was funded by Italian MS Society Research Foundation (Grant No. 2009/R/15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest regards this Original Paper and no primary interest may be influenced by a secondary interest such as financial gain or personal rivalry. All authors made significant contributions to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grasso, M.G., Gamberini, G., Patti, F. et al. The Dysphagia in Multiple Sclerosis Questionnaire Correlates with Fiber-Optic Endoscopic Examination for Detecting Swallowing Deficits in MS. Dysphagia 36, 192–197 (2021). https://doi.org/10.1007/s00455-020-10119-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10119-w