Abstract
This study evaluated the flow properties of viscosity and flow rate for water and two common pediatric liquids. The flow properties of the test liquids are of interest to create a cup simulation model and “smart” prototype training cup. Two objective methods of determining flow properties were utilized: a rheometer to assess viscosity and a modified version of the International Dysphagia Diet Standardization Initiative (IDDSI) to assess flow rate. Rheometer results concluded that the pediatric supplements were less than 50 cP at all shear rates evaluated and exhibited shear-thinning properties, placing both liquids into the “thin” category. The IDDSI, which was performed according to standardized protocol and also with experimental modifications of varying syringe volumes, determined that all three test liquids had greater than 1 mL/s flow rate across all syringe types/sizes. The experimental modification of the IDDSI with 60 mL syringe volume was found to be the most consistent and applicable with discrete values obtained across all liquids tested. A flow rate factor equation can be determined with the use of a 60 mL syringe, with our laboratory setup, to create the cup simulation model. This computer-generated cup simulation model also aims to integrate engineering with clinical practice to develop a “smart” prototype training cup equipped with software to control flow rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective feeding skills in typical children develop across a protracted timeframe, allowing for adequate nutrition and hydration through self-feeding. For the task of open-cup drinking, children are required to develop advanced sensorimotor skills in order to manage variable quantities and consistencies of liquids. Clinical reports suggest that introduction to an open-cup occurs within the first year, with refinement of skills through toddlerhood [1,2,3]. National and international policies for pediatricians and other health professionals encourage specific timelines for typically developing children to wean from bottles. The design of these guidelines occurred from a nutritional perspective rather than an examination of the skills required in successfully achieving open-cup drinking. For example, the World Health Organization (WHO) recommends a gradual wean until 2 years of age, the American Academy of Pediatrics recommends that this occurs by 15 months of age [4] and in the United Kingdom bottle drinking is “actively discouraged” after the first year [5]. Developing children have widely varied experiences while transitioning to open-cup drinking, which further complicates the establishment of a firm developmental timeline. A recent survey of families in the UK found that despite the 12-month UK recommendation to be transitioned to an open-cup, the majority of infants continue to use a combination of bottles and cups at 1 year [6]. Similarly, an earlier study in the US determined that open-cup drinking exposure varies widely and is highly influenced by parental practices [7].
This lengthy developmental timeline has been capitalized on by companies internationally who market “no-spill” training cups as a seemingly convenient way to prevent frequent liquid spills during the transition period. However, these “no-spill” cups require a wide variability of suction pressures across brands to obtain and/or maintain the flow of liquid, which trains a different set of physiologic features than utilized in a mature open-cup drinking experience [8]. Because “no-spill” spouted cups—those that require suction, prolong both non-nutritive patterns and exaggerated intraoral pressures during nutritive sucking, the American Dental Association has cautioned against their use [9]. As a result, these “no-spill” cups act similarly to bottles which have known negative sequelae for prolonged use including dental caries, changes in occlusions patterns, temporomandibular joint dysfunction, otitis media, myofunctional disorders, iron deficiencies, and obesity [8].
Concerns have arisen that these commercially prevalent cups will alter the developmental timeframe and underlying physiologic mechanisms for mature drinking patterns to emerge [6, 8]. Further, with the influx of no-spill cups on the market and because we no longer know if standard practice is effective, the critical time periods in which infants and toddlers should be encouraged to practice new oral skills is now unknown. Similarly, children who utilize “no-spill” cups for extended periods have fewer opportunities to master open-cup drinking, and therefore may present with slower development of the skill [3, 8]. A subset of typical feeders, who require a structured practice environment, may struggle to integrate sensorimotor patterns learned from highly variable “no-spill” training cup to open-cup designs. Regardless, the majority of typically developing children still transition to open-cup drinking in an apparent seamless fashion despite the use of “no-spill” training cups.
Children with atypical feeding development are generally not appropriate candidates for “no-spill” or open-cup designs. For example, children with decreased muscle tone or altered sensory processing may not be able to break the initial vacuum seal of a “no-spill” sippy cup or maintain suction pressures resulting in a lack of liquid flow from the cup [8]. In contrast, children with developmental delays and oropharyngeal dysphagia are at risk for aspiration with the high flow rate of true open-cup designs [10]. Speech-language pathologists and other feeding professionals often modify cups and liquids to bridge the gap between no-flow and high flow challenges.
In addition to modifications of cup designs, clinicians regularly modify liquid flow rate during assessment and treatment of dysphagia by altering the “thickness” of a fluid, in hopes of decreasing the rate at which the bolus moves through the oropharyngeal cavity. As a result, dysphagia research has focused on the measurement of viscosity. As a flow factor, viscosity can be defined as the tendency of a liquid to resist flow [11]. Viscosity is a function of two factors: shear stress, defined as the applied force per unit area that creates or produces flow (Fig. 1), and shear rate, defined as the change in velocity at which the particles are displaced. Viscosity is determined instrumentally with a rheometer, a device that calculates the rheological properties of a liquid at various defined shear rates in order to determine behavior of the liquid in response to stress [12]. Common pediatric supplements do not typically have accessible rheologic information or a standard viscosity available in the literature. In fact, most commercially manufactured pediatric supplements in the United States report a general classification of viscosity utilizing the National Dysphagia Diet (NDD) standards [13] on the packaging, such as “thin” or “nectar-thick,” in lieu of precise ranges of rheological measurements [14, 15]. This practice of broadly classifying viscosity using the NDD standards is typically clinically sufficient; however, our cup model requires more precise information.
Flow rate, unlike viscosity, does not have a device equivalent to a rheometer to form an instrumental classification. However, a global framework called the International Dysphagia Diet Standardization Initiative (IDDSI) was recently released as an objective form of liquid flow classification using a continuous rating scale from 0 to 4 [16, 17]. While the NDD classifications of liquids are based on viscosity values at a set shear rate, the IDDSI scale is based on the flow rate of a liquid through a 10 mL luer slip tip syringe in a 10-s period, measured in mL/s ([15]; Fig. 2).
The National Dysphagia Diet Task Force defines viscosity in standardized viscosity measurements in centipoise (cP) with strict class boundaries at a shear rate (s−1) of 50 s−1 [13, 18]. The International Dysphagia Diet Standardization Initiative utilizes flow rate as an indicator for liquid level; the scale of 0–4 is determined by the amount of liquid remaining in a 10 mL syringe following a free flow period of 10 seconds [19]
In a bid for safe, autonomous, and standardized feeding practices, our laboratory is developing a flow-regulated training cup for use with liquids of varying viscosities as a transitional aid to open-cup drinking. Our prototype utilizes a pinch valve system in order to program flow rate (U.S. Patent No. 8432249) and a lid that mimics the lip of an open-cup. Initial prototype cup trials have been performed with water, a known high-flow, low-viscosity liquid, which allowed for stable baseline measurements. Yet numerous liquids are likely to be consumed by transitioning feeders in real-world applications of the training cup. In an effort to determine the response of our prototype cup to a variety of liquids dispensed, the flow factors of viscosity and volumetric flow rate (flow rate) are of interest for programming. Specifically, because flow rates of pediatric nutritional supplements have not been reported in the literature, the primary aim of this study is to establish a reliable method of liquid classification within the context of our cup model, through objective flow factor measurements of common pediatric supplements. We hypothesize that flow rate is more relevant than viscosity for liquid classification due to (a) ease of accessibility and (b) reliability in both clinical and research design applications. This study will examine the flow properties in select liquids to determine a valid methodology of liquid classification specifically for the use of development of a training cup prototype through the integration of engineering and clinical methodologies.
Methods
Sample Liquid Selection
PediaSure© and PediaSure with Fiber© (Abbott Laboratories, Columbus OH) chocolate flavors were selected as test liquids given the popularity of these supplements with both typical and atypical transition feeders. Viscosity, measured in centipoise (cP), for both supplements has been reported as “1–50 cP at room temperature and chilled” [17], which corresponds with the National Dysphagia Diet Task Force [13] class boundaries for thin liquids (Table 1). Water was also selected as a test liquid, because of its known viscosity, in order to determine flow rate as compared to pediatric supplements in a controlled laboratory setting. Prior to testing, PediaSure©, PediaSure with Fiber©, and distilled drinking water (Kroger Co., Cincinnati OH) were procured and stored at ambient room temperature within an insulated cooler (Coleman Co., Wichita KS). All test liquids remained sealed until immediately prior to testing, and any opened bottles were discarded at the close of each day to ensure that there were no adverse effects on flow properties as a result of exposure to the elements.
Rheometer Procedures
TA Instruments Discovery HR-1 Rheometer (TA Instruments, New Castle, DE) equipped with Trios software and a Peltier plate was used to determine apparent viscosity for PediaSure© and PediaSure with Fiber©. Water was not tested with this form of measurement, as water has a known viscosity. A flow sweep protocol was used to evaluate apparent viscosity as a function of shear rate. All experiments were conducted at ambient temperature (25 °C), and instrument calibration was confirmed prior to all trials. Experimental operations were performed jointly by two researchers, following two training sessions with a qualified chemical engineer. Both PediaSure© and PediaSure with Fiber© trials were repeated six times. Researcher shook manufacturer bottle manually 15× prior to opening the bottle, tapped the top of the bottle to release air bubbles, then extracted 500 µL liquid sample using a pipette. The 500 µL sample was loaded onto the Peltier plate. A parallel plate geometry 20 mm in diameter was lowered to contact the sample with a constant gap of 0.95 mm. Shear rates were varied in a logarithmic sweep from 0.1 to 100 s−1 and viscosity results were recorded at 5 points per logarithmic decade. Data were transferred from Trios software to Excel to allow for offline data analysis.
Flow Rate Procedures
Both the standardized IDDSI protocol and a modification of the protocol were conducted in a laboratory setting. The IDDSI protocol was conducted in a laboratory setting with recommended syringes (one concentric luer slip tip; BD, Franklin Lakes NJ and one eccentric luer slip tip; Henke Sass Wolf, Tuttlingen, Germany). In addition, a modification of the protocol consisting of non-approved syringes and varied volumes was conducted; however, the IDDSI flow-test procedure remained the same (Table 1). Each syringe of varied brands and characteristics was examined with distilled drinking water, PediaSure©, and PediaSure with Fiber©. Three 10 mL syringes and one 12 mL syringe (filled to 10 mL) were employed in this study; initially the recommended luer tip syringes were difficult to obtain and more readily available options were purchased. Once we had access to syringes that met the protocol (Table 1) we determined that there was value in testing all of the purchased syringes to examine if minute changes in syringe brand/type affect the results of the IDDSI in a laboratory setting. In order to examine flow behavior over an extended period of time, standard IDDSI trials were modified to be completed with increased syringe capacities (20, 35, and 60 mL). Prior to the initial trial, all syringes were calibrated using a graduated cylinder, and determined to be accurate representations of specified mL markers. The trials were randomized using www.random.org for a total of 105 randomized trials, with each liquid/syringe combination repeated 5× for reliability.
Our research team wanted to examine more precise details regarding flow-rate features than the IDDSI protocol provided; therefore, we created a testing apparatus (Fig. 3) that allowed for a software system to collect flow rate data from a free-flowing syringe. The basic design of the testing apparatus involved securing the test syringe in a syringe clamp, which allowed the syringe to be positioned above the platform scale (Omega Engineering Inc., model LSC7000-5) in a manner easily maintained and repeated across trials. The platform scale collected voltage data at a rate of 100 points per second, and transmitted voltage proportional to the scale load to the panel meter (Omega Engineering Inc., model DP25B-S-A). The panel meter amplified the voltage signal and transmitted the signal to a data acquisition module (DAQ) (Measurement Computing, model USB-1408SF). The DAQ module transformed the signal to a data stream of changes in voltage as a function of time (simultaneous collection of voltage changes during liquid flow and timestamps), and the data were stored and graphed in real time with the InstaCal/TracerDAQ™ data logger software on a secure laptop (Dell Latitude, E6510). The data stored in the software system allowed for conversion to mL/s in the data analysis stage.
The testing apparatus secured the test syringe (A) in a syringe clamp (B) at a fixed height of 10 inches, directly above a load cell-based platform scale (C). The platform scale (C) transmitted a voltage current to the panel meter (D). The panel meter (D) amplified the voltage signal and transmitted the signal to a data acquisition module (DAQ) (E). The DAQ module (E) transformed the signal to a data stream of changes in voltage as a function of time to a secure laptop (F). Also included standard brass weights (G) of 500, 200, and 50 g used for scale calibration, and 500 mL plastic beaker (H) used to collect liquid on the scale during the procedure
At the start of each trial, the appropriate syringe was chosen according to the randomization chart and the plunger was removed. The test syringe was mounted on the test clamp directly above the scale. A vertical level was used to ensure the test syringe was perpendicular to the scale, and a horizontal level was used to ensure the scale was centered. For PediaSure© and PediaSure with Fiber© trials, the sealed bottles were hand-shaken 15× and the top of the bottle was tapped prior to drawing liquids with the measurement syringe. One investigator placed a gloved finger on the open end of the test syringe to prevent outflow of liquid, and a measurement syringe was used to fill the test syringe with the appropriate liquid and mL based on the randomization table. A second investigator verified the liquid volume in the syringe to ensure measurement accuracy and used a digital high-temperature infrared thermometer (Craftsman, model 50499) to determine the liquid temperature.
Following setup completion, the connections between the scale, panel meter, DAQ, and laptop were verified. The InstaCal/TracerDAQ™ software programs were activated on the secure laptop, and collection of the data stream from the software to the computer was initiated. The scale was calibrated with 500, 200, and 50 g standard brass weights respectfully, for 5 s each. An empty 500 mL plastic beaker (Kartell, ISO 7056) was placed on the scale and the scale was zeroed. An audible 10-count was completed to obtain a stable baseline for the computer software before liquid flow began. An investigator then removed the finger from the mounted test syringe tip and allowed the liquid to flow freely into the plastic beaker on the scale below. The computer software was programmed to collect data automatically for 2 minutes. If the syringe drained completely (including drips from the syringe tip) prior to the 2-minute mark, the computer software was manually discontinued 5 s after cessation of drips. Any residual liquid in the test syringe was documented following each trial. The data collected from the software were saved separately following each trial, and analyzed offline. All syringe and measurement tools were washed and dried thoroughly prior to beginning the next trial.
Data Analysis
The data collected via the rheometer were analyzed in the following manner. Data points were formulated by a logarithmic sweep at shear rates 0.1–100 s−1, collected at 5 points per decade via Trios software. A linear regression analysis was performed on a logarithmic–logarithmic scale to determine best fit for the data. The Statistics Add-On in Google Sheets was used to generate two logarithmic–logarithmic linear regression graphs, one for both liquid types: PediaSure© and PediaSure with Fiber©. A regression fit, confidence band, prediction band, and spline were determined for both liquids. The data were utilized to generate a box plot, filtered by the two liquid types. All strain rates were present within each liquid’s boxplot filter, and a mean was generated to determine the average liquid viscosity across all strain rates as well as at specified strain rates.
The IDDSI output data from each experimental test were imported from the software system into a 2-column format spreadsheet application (column A=time, Column B=voltage). A spreadsheet template was created in Excel to a convert the imported data into a graphical representation. The graphical representation was directly linked to the 2-column format; therefore, the investigator was able to determine the precise timestamp and voltage at section of the graph by hovering the mouse over the desired section (x-axis=voltage, y-axis=time). Four investigators (blinded within and across trials) visually analyzed each graph. Each investigator began by selecting key baseline data points in all graphs, including point at which standard brass weights were added and removed from the scale, the point of beaker placement on the scale, and the 10-count baseline period prior to liquid flow (Fig. 4a). Selection of these baseline data points allowed for the spreadsheet template to perform the conversion from voltage as a function of time, to mL/s. Then, each investigator selected the point in all graphs at which the liquid began flowing out of the test syringe, and ceased flowing out of the test syringe (Fig. 4a). Selection of these two flow rate points allowed for the spreadsheet template to calculate average flow rate in mL/s using a slope calculation. For data sets that were exceptionally noisy, the investigators scaled the x/y-axis on the graphical representation manually for increased visualization of the start/end flow points (Fig. 4b). Inter-rater reliability was calculated and outliers were individually examined.
a, b Example screen shots of key components for data analysis. a The data imported from the software system were converted into a graphical representation (x-axis = voltage, y-axis = time) via Excel template. Investigators visually identified the following key points from the generated graphs: 500 g weight calibration added to scale (A), 500 g weight calibration removed from scale (B), similar identification was repeated for 200 and 50 g (similarly shaped rectangular spikes), point of beaker placement on the scale (C), 10-count zeroing of the scale (D), liquid flow start point (E), and liquid flow end point (F). b Enlarged view of Fig. 4, with a focus on the 10-count zeroing of the scale (D), liquid flow start point (E), and liquid flow end point (F). The method of scaling the x/y-axis manually was utilized for noisy data sets, in order to gain increased visualization of liquid flow start/end points
Results
Viscosity
Across a range of shear rates from 0.1 to 100 s−1, the supplements demonstrated the following viscosities: PediaSure© 7–38 cP with mean viscosity value of 18 cP ± 7, and PediaSure with Fiber© 11–31 cP with mean viscosity value of 19 cP ± 5 (Fig. 5a). The high standard deviation values are a result of the supplements exhibiting decreased viscosity with increased shear rate; both liquids demonstrate similar behaviors, with PediaSure with Fiber© slightly more viscous than PediaSure© at all data collection points. At lower shear rates, both liquids exhibited increased variation in viscosity across trials, yet values became more predictable with increased shear rate. NDD standards for viscosity are set at a shear rate of 50 s−1; however, the rheometer used in this study did not evaluate at a shear rate 50 s−1. The evaluated shear rates that most closely approximated 50 s−1 (41.90 and 66.41 s−1, for our rheometer) were used to determine a predictive viscosity value for PediaSure© and PediaSure with Fiber© (Fig. 5b). Predictive measures concluded that both liquids are less than 50 cP, at a shear rate of 50 s−1.
a, b Range of viscosity by liquid type and shear rate. a Box plot of PediaSure© and PediaSure with Fiber© range of viscosity, from shear rates 0.1 to 100 s−1. Both liquids exhibit a range of viscosities; however, all values are less than 50 cP. b As noted in (a), all viscosity values for PediaSure© and PediaSure with Fiber© are less than 50 cP at all shear rates tested (0.1–100 s−1). Viscosity values for both liquids were not collected at a shear rate of 50 s−1 (the shear rate for the NDD standards); however, values were collected at a shear rate of 41.90 s−1 (PediaSure© 11 cP ± 0.00, PediaSure with Fiber© 14 cP ± 0.00) and 66.41 s−1 (PediaSure© 10 cP± 0.00, PediaSure with Fiber© 13 cP ± 0.00). These precise values were used to create a predictive band (seen above as a gray shaded area) of the liquids’ viscosity values at 50 s−1. The predictive area falls within 1–50 cP, indicating that liquids would be “thin” by NDD standards at this shear rate
A linear regression analysis was performed on a logarithmic–logarithmic scale of each liquid to determine the best fit for the data sets, given the continually decreased viscosity with increased shear rate. The model was able to account for 90% of the variance in PediaSure© viscosity (Fig. 6a), and 84% of the variance in PediaSure with Fiber© viscosity (Fig. 6b).
a, b Logarithmic regression models for PediaSure and PediaSure with fiber. Scatterplots of PediaSure© and PediaSure with Fiber© viscosity values fit with a logarithmic–logarithmic regression model. The Statistics Add-On was applied to determine the model, which consisted of a sample size of 66 test points with zero test points excluded. The 66 test points were achieved by collecting viscosity data at 11 shear rates, in six separate trials. a PediaSure©: model accounted for 90% of the variance in viscosity with r2 = 0.90, residual standard deviation = 0.13, sample standard deviation = 0.42. b PediaSure with Fiber©: model accounted for 84% of the variance in viscosity with r2 = 0.84, residual standard deviation = 0.11, sample standard deviation = 0.27
Flow Rate
All tested liquids were within the range of ambient room temperature for all trials performed, found to be within 22–24 °C immediately prior to trial by the laser thermometer. While the computer software was programmed to collect data automatically for 2-minutes following the initiation of liquid flow, no tested syringe required that length of time to fully drain. Therefore, all trials performed were manually discontinued 5-seconds following cessation of drips. No measureable liquid remained in any test syringe following the procedure.
Of the 105 trials, 103 were found to be within 0.5 mL/s flow rate between all four investigators. Two outliers from 20 mL syringe trials were noted by all the raters compared to the other 20 mL trials. Upon a review of the software formatting, the data for the two trials were inadvertently collected at 60 points per second instead of 100 points per second; this error was remediated manually by adjusting to the correct ratio. Following this remediation, all four investigators presented flow rates that were within at least 0.5 mL/s range. The four calculated flow rates for each of the 105 trials were used to create an “average flow rate” per trial; the low and high values from each trial were excluded, and the middle two values were averaged.
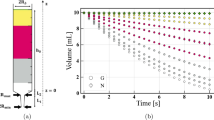
A summary of mean flow rate values for the tested liquids, across all syringe sizes, was compiled (Fig. 7a). Results were further broken down by specific syringe type in order to more closely examine the flow properties in each experimental setting (Fig. 7b–f). The 10 mL syringes were first examined as a group, i.e., all four syringe brands were included in determining the flow rate of the test liquids (Fig. 7b). Due to a notable range of flow rate values within and across brands, the data were further broken down to assess variability in flow rate across 10 mL syringe brand (Fig. 7c). The IDDSI standard syringe used in our procedures (BD, Franklin Lakes NJ) was assessed independently to determine congruence with the IDDSI liquid levels, and resulted in the following flow rates in mL/s: water 1.5 ± 0.1, PediaSure© 1.3 ± 0.2, and PediaSure with Fiber© 1.2 ± 0.1. The remaining syringe volumes (20, 35, 60 mL) were examined individually. The larger syringes exhibited more consistent determinations of flow rate, as syringe volume increased (Fig. 7d–f).
a–f Visual representation of varying flow rates across syringes and liquid types. a Box plot of flow rate values across syringe sizes, divided by liquid type. The liquids presented with the following range of flow rates in mL/s: water 2.1 ± 0.5, PediaSure© 1.7 ± 0.3, and PediaSure with Fiber© 1.4 ± 0.3. b, c Box plots of 10 mL syringe volume flow rate, divided by test liquid (b) and syringe brand (c). b Combination 10 mL syringes (in mL/s): water 2.2 ± 0.6, PediaSure© 1.7 ± 0.4, and PediaSure with Fiber© 1.4 ± 0.4. c 10 mL syringe brands (in mL/s), listed from syringe with the highest variability to the least variability: Walgreens oral syringe 2.5 ± 0.5, Ideal Instruments 1.5 ± 0.4, Norm-Ject 1.8 ± 0.4, and BD 1.3 ± 0.2. d–f Box plot of higher volume syringe (20, 35, 60 mL) flow rate, divided by test liquid. d 20 mL syringe (in mL/s): water 2.0 ± 0.1, PediaSure© 1.5 ± 0.1, PediaSure with Fiber© 1.3 ± 0.1. e 35 mL syringe (in mL/s): water 2.3 ± 0.1, PediaSure© 1.7 ± 0.1, PediaSure with Fiber© 1.4 ± 0.1. f 60 mL syringe (in mL/s): water 2.0 ± 0.1, PediaSure© 1.6 ± 0.1, PediaSure with Fiber© 1.3 ± 0.0
A linear regression analysis performed to calculate flow rate, utilizing the data sets collected with each volume syringe. The Statistics Add-On was applied to determine a linear regression model and calculate the standard deviation of flow rate, for each liquid tested. Each model consisted of thirty-five trials (n=35), with zero trials excluded. Graphical scatterplot representations highlight similar patterns of variability across 10 mL syringe types in all test liquids, with increased consistency of flow rate as syringe volume increases (Fig. 8a–c).
a–c Scatterplots of liquid flow rate values. a Water: r2 = 0.0, residual standard deviation = 0.48, sample standard deviation = 0.47. b PediaSure: r2 = 0.02, residual standard deviation = 0.34, sample standard deviation = 0.34. c PediaSure with Fiber©: r2 = 0.01, residual standard deviation = 0.32, sample standard deviation = 0.318
Discussion
Our lab’s goal is to create a cup simulation model utilizing a flow rate factor equation that will require experimentally determined flow factors for specific liquids in order to predict flow behavior. This computer-generated cup simulation model aims to integrate engineering with clinical practice to develop a “smart” prototype training cup with embedded software to control flow rate. In order to predict flow patterns, we chose to examine two flow properties: viscosity and flow rate. The flow properties of viscosity and flow rate are typically considered to be inversely proportional—that is, if you increase the viscosity of a liquid, the flow rate will decrease.
Liquid standards are based on viscosity measurements with designated boundaries that determine a class of liquids; for example, a liquid with a viscosity 1–50 cP falls within the thinnest category, closest to water [18] (Fig. 2). However, it is difficult for clinicians to test the viscosity of a liquid outside of the research setting. Clinicians have improvised with other objective and subjective forms of measurement such as pouring from a cup or spoon, stirring, visual inspection, oral tasting, the line spread test, and gravity flow tests [16]. None of these methods of measurement are fully standardized—or able to classify liquids reliably based on rheological data. Recently, the International Dysphagia Diet Standardization Initiative (IDDSI) was introduced as a form of liquid standardization that bases liquid levels on flow rate instead of viscosity [16], excluding the need for viscosity measurements when describing or comparing liquids based on thickness. We speculated that minute differences in viscosity and/or flow rate across liquids would make a difference in the model’s ability to control for flow rate with the pinch valve design.
Relevant Viscosity Findings
Our finding support the manufacturers claim that both PediaSure© and PediaSure with Fiber© demonstrated ranges of viscosity between 1 and 50 cP. Our results showed that PediaSure© had a range from 7 to 38 cP and PediaSure with Fiber©, 11–31 cP. PediaSure with Fiber© was slightly more viscous than regular PediaSure© at all shear rate intervals, except the final shear rate 100 s−1 (Fig. 5a). Regardless, viscosity findings were not found to be relevant for the cup simulation model because we cannot reliably program a cup simulation model based on a broad range of viscosity values–even if the values are all considered in one category (i.e., thin liquids).
Finding a range of viscosity values for the pediatric supplements was not surprising, given how liquids behave under applied stress. Some liquids, such as water, can be classified as Newtonian fluids with a linear relationship between shear rate and shear stress [11]. Other liquids exhibit non-Newtonian characteristics with a non-linear relationship between shear rate and shear stress, wherein viscosity varies dependent upon shear rate. Our findings (Fig. 6a, b) confirmed that PediaSure© and PediaSure with Fiber© are “shear-thinning” non-Newtonian liquids. The term “shear-thinning” is used to describe non-Newtonian liquids that decrease in viscosity as shear rate increases. The range at which these liquids exhibit shear thinning is negligible because both liquids fall well within the boundaries for a “thin” liquid (1–50 cP), despite any thinning behaviors.
It is interesting to consider the implications of a viscosity range on a transitioning feeder or populations with oropharyngeal dysphagia. Given our understanding of the inverse relationship between viscosity and flow rate, a liquid with a 7 cP viscosity would seemingly flow more quickly than one with a 38 cP viscosity. However, at this time we do not have the research to determine how specific changes in viscosity will affect changes in flow rate. But, we speculate that children who are transitioning feeders or populations with oropharyngeal dysphagia are more sensitive to changes in flow rate as compared to children who have mastered open-cup drinking.
Relevant Flow Rate Findings
The observed flow rates of the three liquids tested were consistent across trials in that water always had the fastest flow rate, followed by PediaSure© and then lastly PediaSure with Fiber©. The differences in the flow rate of the liquids were not easily perceived with the smaller syringe sizes (10 mL) using our laboratory setup (Fig. 3). This finding is likely a limitation of the signal error from the experimentation hardware and the associated software program which calculates slope at regular intervals. Averaging routines used for slope determination can exhibit large fluctuations with each additional data points when smaller data sets are utilized.
In contrast, the 60 mL syringes were able to provide almost discrete flow rate values for each liquid tested (in mL/s): water 2.0 ± 0.1, PediaSure© 1.6 ± 0.1, and PediaSure with Fiber© 1.3 ± 0.0. The flow rate trials are plotted in a scatterplot fit with a linear regression model (Fig. 8a–c). The flow rate values across trials are most consistent at 60 mL syringe volume for all three tested liquids. Because the 60 mL syringe provides distinct values for flow rate between the tested liquids, this method is the most applicable means of developing a flow rate coefficient for our computer simulation model that will control for minute differences in flow rate.
An incidental finding of this research was the wide variation in flow rate within 10 mL syringes (i.e., 10 mL syringe used to measure flow rate of PediaSure© five times, and resulting in noticeably different flow rates across trials). The two syringe brands that were not IDDSI approved [19]—the oral syringe (Walgreens Distribution, Perrysburg OH) and the 12 mL syringe filled to the 10 mL marker (Neogen, Lexington KY)—demonstrated the most variability (Fig. 7b). The 10 mL syringe specifically suggested in the IDDSI protocol (BD, Franklin Lakes NJ) was the most consistent 10 mL syringe across trials and also had the lowest overall flow rate as compared to the other 10 mL syringes tested (Fig. 7c).
At this time, there is no research that describes the effect of small changes in flow rate (i.e., 1 versus 2 mL/s) clinically with transitioning feeders or persons with oropharyngeal dysphagia. Yet, for patients who might need more calibrated control of flow rate with liquid intake, we hypothesize that small differences in flow rate may be the difference between control of the bolus and penetration/aspiration. For example, a patient with a known delay initiating a pharyngeal swallow may benefit from a controlled flow rate in which both the volume and rate of flow are monitored. The ability to acutely regulate flow rate with a controlled training cup would allow for future physiologic research into the effects of liquid flow rates with populations of interest, such as transitioning feeders, feeding disorders, or persons with dysphagia.
Limitations
By testing three methodologies (rheometer, IDDSI flow test, modified IDDSI flow test with varied size syringes) with only three liquids, we closely examined each method and determined the method that is most effective for our purpose. We strayed from the IDDSI protocol by modifying syringe sizes, which is a limitation as far as standardization. However, the modifications were necessary the development of a flow rate-controlled prototype training cup which requires carefully detailed research as part of iterative r design purposes.
Additionally, the temperature of tested liquids was kept at a constant room temperature for testing; however, in real-world application liquids may be warmed or refrigerated. Changing the temperature of liquids is known to alter viscosity and can be postulated to change flow rate as well. Lastly, the study tested a small number of common pediatric liquids (water and two nutritional supplements). Water was selected as a consistent baseline for testing, because it is easily accessible and enables easy clean-up in experimental conditions. Nutritional supplements were chosen due to their popularity in both typical populations as well as children with feeding/swallowing disorders as a means of increasing caloric consumption. As development of the training cup progresses, a wider collection of pediatric liquids will be tested for the flow rate factor to better represent current pediatric drink markets (i.e., milk, juice, nutritional beverages)
Conclusion and Future Directions
Overall, the results of this study revealed that known viscosity is not a necessary factor in continued prototype cup development. Instead, calculations solely of flow rate will be necessary for each liquid used in the prototype design, in order to precisely program the cup for a variety of liquids. In a research setting, we were able to modify the IDDSI flow test to extend the length of liquid flow, and determine a mean flow rate for tested liquids. Along with the PediaSure© and PediaSure with Fiber©, we will need to test other frequently used liquids/supplements, such as milk and juices, in order to collect additional flow factor data for further development of the cup. In future testing, the 60 mL syringe flow rate testing is all that is needed to contribute to the model; the need for rheological testing or varied volume syringes is no longer necessary.
Future research is also needed to determine the point at which variation in flow results in significant or noticeable changes for a typical “transitioning feeder” versus a feeding disordered or dysphagic population—especially given wide breadth of transitioning feeders that may benefit from a flow rate-controlled training cup. Primary care providers frequently report parental concerns regarding feeding disorders, operationally defined a wide range of eating activities that may or may not be accompanied by a difficulty with swallowing foods and liquids [20]. Incidence and prevalence of feeding disorders varies due to the wide breadth of diagnoses that fit the definition; however, feeding disorders are generally estimated to affect 25–35% of otherwise typically developing children and nearly 80% of children with developmental delays [21]. Our laboratory is attempting to meet the needs of transitioning feeders as well as dysphagic populations by producing a training cup that can control the rate of liquid flow. This device would aid future research into the effects of changing flow rate on liquid consumption.
References
Alexander RA. Oral-motor treatment for infants and young children with cerebral palsy. Semin Speech Lang. 1987;8:87–100. https://doi.org/10.1055/s-2008-1064279.
Arvedson J, Lefton-Greif MA. Anatomy, physiology, and development of feeding. Semin Speech Lang. 1996;17(4):261–7.
Morris S, Klein M. Pre-feeding skills: a comprehensive resource for mealtime development. 2nd ed. Austin: Pro-Ed; 2000. p. 59–95.
Avery A, Baxter A. ‘Change to cup’: an audit to determine parental awareness and practices in changing from bottle to cup. J Hum Nutr Diet. 2001;14:217–23. https://doi.org/10.1046/j.1365-277X.2001.00288.x2009-3583.
Maguire JL, Birken CS, Jacobson S, Peer M, Taylor C, Khambalia A, Mekky M, Thorpe KE, Parkin P. Office-based interventions to reduce bottle use among toddlers: TARGet Kids! Pragmatic, randomized trial. Pediatrics. 2010;126:e343–50. https://doi.org/10.1542/peds.2009-3583.
Milton J, King C. Cup introduction, drink type and vitamin supplementation in preterm babies at 11–25 months. J Hum Nutr Diet. 2012;25:148–54. https://doi.org/10.1111/j.1365-277x.2012.01227.x.
Carruth B, Skinner J. Feeding behaviors and other motor development in healthy children (2–24 months). J Am Coll Nutr. 2002;21(2):88–96.
Scarborough DR, Bailey-Van Kuren M, Frey KE, Knatz ED, Lape AR, Wambaugh NM. Study of pressure and flow rate properties of training cups to characterize parameters in a developmental context. J Pediatr Rehabil Med. 2010;3:187–96. https://doi.org/10.3233/PRM-2010-0128.
American Dental Association Division of Communications. From baby bottle to cup. JADA. 2004;135:387.
Van den Hoek-Engel L, Harding C, van Gerven M, Cockerill H. Pediatric feeding and swallowing rehabilitation: an overview. J Pediatr Rehabil Med. 2017;10(2):95–105. https://doi.org/10.3233/PRM-170435.
Coster S, Schwarz E. Rheology and the swallow-safe bolus. Dysphagia. 1987;1:113–8. https://doi.org/10.1007/BF02412327.
Walters K, Jones WM. Measurement of Viscosity. In: Boyes W, editor. Instrumentation reference book. 4th ed. Burlington: Butterworth Heinemann; 2010. p. 69–75.
National Dysphagia Diet Task Force (NDD). National dysphagia diet: standardization for optimal care. Chicago, IL: American Dietetic Association; 2002.
Mills RH. Rheology overview: control of liquid viscosities in dysphagia management. Nutr Clin Pract. 1999;14:52–6. https://doi.org/10.1177/0884533699014005S11.
Steele CM, van Lieshout P, Goff H. The rheology of liquids: a comparison of clinicians’ subjective impressions and objective measurement. Dysphagia. 2003;18:182–95. https://doi.org/10.1007/s00455-002-0104-1.
Cichero JAY, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, Duivestein J, Kayashita J, Lecko C, Murray J, Pillay M, Riquelme L, Stanschus S. Development of international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: the IDDSI framework. Dysphagia. 2017;32:293–314. https://doi.org/10.1007/s00455-016-9758-y.
Abbott Laboratories Inc. Improving lives through science-based nutrition: Abbott nutrition pocket guide. The Betty Mills (2010). Accessed Electronically 27 Jan 2017.
Cichero JAY, Steele C, Duivestein J, Clave P, et al. The need for international terminology and Definitions for texture modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Reports. 2013;1:280–91. https://doi.org/10.1007/s40141-0130024-z.
International Dysphagia Diet Standardization Initiative (IDDSI). Detailed descriptors, testing methods and evidence: Drink levels 0-4; 2015.
Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14:118–27. https://doi.org/10.1002/ddrr.17.
Burklow KA, Phelps AN, Schultz JR, Rudoph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27:143–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The original version of this article was revised: Modifications have been made to the Figure 2 and its caption and also to the 3rd subheading under the section Methods.
Rights and permissions
About this article
Cite this article
Creech, T.N., Bailey-Van Kuren, M., Sparks, J. et al. A Comparison of Measurements of a Pediatric Supplement. Dysphagia 34, 257–268 (2019). https://doi.org/10.1007/s00455-018-9931-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9931-6