Abstract
Recurrent laryngeal nerve (RLN) injury in neonates, a complication of head and neck surgeries, leads to increased aspiration risk and swallowing dysfunction. The severity of resulting sequelae range from morbidity, such as aspiration pneumonia, to mortality from infection and failure to thrive. The timing of airway protective events including laryngeal vestibule closure (LVC) is implicated in aspiration. We unilaterally transected the RLN in an infant pig model to observe changes in the timing of swallowing kinematics with lesion and aspiration. We recorded swallows using high-speed video-fluoroscopic swallow studies (VFSS) and scored them using the Infant Mammalian Penetration and Aspiration Scale (IMPAS). We hypothesized that changes would occur in swallowing kinematics (1) between RLN lesion and control animals, and (2) among safe swallows (IMPAS 1), penetration swallows (IMPAS 3), and aspiration swallows (IMPAS 7). We observed numerous changes in timing following RLN lesion in safe and unsafe swallows, suggesting pervasive changes in the coordination of oropharyngeal function. The timing of LVC, posterior tongue, and hyoid movements differed between pre- and post-lesion in safe swallows. Posterior tongue kinematics differed for post-lesion swallows with penetration. The timing and duration of LVC and posterior tongue movement differed between aspiration swallows pre- and post-lesion. After lesion, safe swallows and swallows with aspiration differed in timing of LVC, laryngeal vestibule opening, and posterior tongue and hyoid movements. The timing of thyrohyoid muscle activity varied with IMPAS, but not lesion. Further study into the pathophysiology of RLN lesion-induced swallowing dysfunction is important to developing novel therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The timing and duration of several kinematic events are important to the generation of a safe and effective swallow [1, 2]. These events, including tongue, hyoid, and palate movement, as well as the timing and duration of the elevation of the arytenoid cartilages, lead to closure of the larynx during bolus swallowing [3,4,5]. Dysphagia, including either penetration or aspiration of fluid into the airway, occurs at an increased frequency with dysfunction of these kinematics. In particular, this occurs when the hyoid and larynx do not approximate [3], and the closure of the laryngeal vestibule (LVC) fails, which reduces the effectiveness of the protective mechanism that ensures a safe swallow [3]. The timing of hyoid movement is a frequently studied variable in swallowing function [6], as it is thought to be related to opening of the pharynx and movement of the airway out of the path of the bolus, and is thus part of the sequence of events that occur during the swallow to protect the airway. Oral transit kinematic timing plays an important role in preparation and relocation of a food bolus, to ensure a safe swallow. However, the studies of these mechanisms only measure the duration of these events, but not the relative kinematic timing necessary for protection of airway safety during a successful swallow [3, 7]. Dysphagia has been attributed to the duration of the LVC closure and not the timing of closure relative to other kinematic events [3, 7]. Yet, we know for other kinematics, disruptions of timing are associated with unsafe swallows [8].
The studies of LVC and kinematics have been done in adult human subjects; there is no research on LVC timing or kinematics in neonates and children. Infants have a different anatomical configuration of the oropharynx and larynx, as well as obtaining all their nourishment from liquids and not solids [9]. Due to ethical limitations of working with human infants, we do not fully understand the kinematics of infant function. Radiation exposure, necessary for VFSS, has been shown in human studies to increase risk of future development of thyroid cancer and infertility [10], and is ethically unacceptable in infants for research purposes. Chronic EMG evaluation is invasive and subject to patient cooperation, leading to difficulty observing in human infants. Thus, an animal model allows the collection of VFSS and EMG data of higher quality and quantity than that is possible from vulnerable neonates. By studying the maximal and minimal movements in 2D during high frame per second video-fluoroscopic swallow study (VFSS), we can observe important changes in timing of a swallow to understand what contributes to a safe versus unsafe swallow. Infant mammalian feeding in the form of suckling on milk from a nipple is shared among all mammals [11]. Infant pigs are a particularly useful model for human infant feeding [12], having similar anatomy (fleshy tongue and cheeks, undescended larynx), being of similar size, and feeding in a similar posture.
Infant aspiration is a well-documented sequela of head and neck surgeries in neonates. Iatrogenic lesioning of the recurrent laryngeal nerve during head/neck surgeries and closure of persistent ductus arteriosus (PDA) has been described as a cause of childhood dysphagia [13]. One characteristic of both clinical and controlled animal studies is a high level of variability in outcomes of infant patients [14], which may be due to the immature nervous system of infants, and subsequent neural pruning that happens through this period of development. However, variability in the kinematics is poorly understood.
Overall, the mechanisms that produce infant dysphagia are not well understood [15]. Some data suggest that the mechanisms that lead to unsafe swallows are multifactorial [15]. In previous controlled experiments, unilateral RLN lesioning produces changes in swallow safety, specifically an increase in the occurrence of aspiration [14]. Yet, the mechanism that causes dysphagia due to RLN damage is still unclear. The increased information this approach brings grants insights into potential changes in central nervous system reorganization.
The aim of this study was to determine whether changes in the relative timing of kinematic and electromyographic events in several structures throughout the swallow following RLN lesion in infants were associated with swallow safety outcomes. Specifically, we hypothesized that the timing, as well as duration of LVC and other kinematic events would provide insight into how RLN lesions induce such clinical variability among humans and other animal species. We hypothesize that both pre- and post-lesion differences will exist in the timing and duration of LVC, as well as posterior tongue and hyoid movement, between safe and unsafe swallows. We further hypothesize that the relationships between timing of these events and swallow safety will change because of lesion.
Materials and Methods
Experimental Procedures
Six infant pigs, 3–4 days of age (Michael Fanning Farm, How, IN, and Shoup’s Farm, Wooster, OH), were trained to drink milk from a bottle with a sheep nipple (NASCO Farm and Ranch) attached. After approximately 24–48 h of successful training, we carried out a set of validated procedures including radiopaque marker placement, electrode placement, control data collection, RLN lesion [8].
Marker and EMG Surgeries
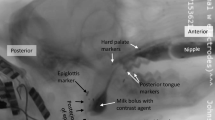
Under inhalant isoflurane anesthesia (2–5%), we implanted radio-opaque makers in the anterior and posterior hard palate, soft palate, and posterior tongue (Fig. 1). A tantalum hemoclip (Weck Ligation Solutions, NC) was attached to the epiglottis. Two to five days later, in an aseptic, intubated surgery under isoflurane anesthesia, we sewed markers to the thyroid cartilage and hyoid bone. During the same surgery, fine wire bipolar electrodes were placed in thyrohyoid, as well as other muscles, not described in this paper. Finally, we identified and marked the right RLN with suture for subsequent lesion. As previously described [16, 17], the bipolar electrode was connected to a microconnector, and exited through a midline incision. Microconnectors were connected to standard 25-pin D-connectors outside the body. The cables were secured with Vetwrap to prevent disconnection and animal injury. Several decades of work examining the physiology of normal swallows has indicated that feeding in these animals is not affected by these procedures [11, 16, 18]. The design of these experiments as self controls also means that the effect of lesion is studied in a ceteris paribus (all things equal) model, thus ensuring that the effect of lesion can be distinguished from any potential effect of surgery per se [19].
Locations of radio-opaque markers. Rostral is to the left and dorsal is up in this image taken from the lateral VFSS film. Marker locations labeled as follows: AP anterior hard palate, PP posterior hard palate, AT anterior tongue, MT middle tongue, PT posterior tongue, HY hyoid, TH thyroid cartilage
Data Collection
Following recovery from the marker placement and EMG surgery procedures, animals were imaged using VFSS while they fed on milk containing barium (E-Z Paque Barium Sulfate, EZ EM Inc., NY). Barium milk was mixed at one cup milk powder:one cup barium:eight cups water. We recorded the animals drinking barium milk in front of biplanar C-arm fluoroscopes (GE9400 C-Arm, 85 kV 4MA) with high-speed digital camera video cameras (XC 1M digital video camera, Xcitex, Cambridge, MA). We recorded 2–5 sessions pre- and post-lesion allowing each animal to serve as its own pre-lesion control. Simultaneous EMG recordings were collected at 10 kHz using a 16-channel digital signal recorder (PowerLab, ADI Instruments), and visualized using digital trace software (LabChart, ADI instruments). Video and EMG signals were synchronized using a nine-volt square wave signal generator which was sent to both the PowerLab and the video camera system.
Recurrent Laryngeal Nerve Lesion Surgery
After sufficient control data were collected, the infant pigs were intubated under isoflurane anesthesia, and lesion of the recurrent laryngeal nerve was performed under full aseptic conditions. On the right side, the recurrent laryngeal nerve was located a few millimeters distal to the point where the RLN enters the larynx at the level of the cricothyroid muscle. The RLN was severed and a small (~2–3 cm) piece removed, and the cut ends were clipped with microhemoclips, tied off with suture, and then displaced with respect to each other. This ensured that regrowth and reconnection of the nerve was not possible during the timescale of these experiments. After recovery from anesthesia, the animals were again recorded with simultaneous biplanar VFSS and EMG (100 frames per second). All experimental procedures were approved by the NEOMED Institutional animal care and use committee (IACUC protocol 13-011 and 16-007).
Assessing Swallow Safety
Swallow safety was scored using the infant mammalian penetration–aspiration scale (IMPAS) [14]. The IMPAS is an ordinal scale where an increased score represents a less safe swallow. IMPAS scores of 1 represent a safe swallow, where no milk enters either the upper of lower airway at any point. IMPAS scores of 7 represent swallows where silent aspiration, or passage of milk below the vocal folds with no attempt to clear, occurs. The same investigator (JO) was blinded to the entire feeding sequences and evaluated each sequence (Table 1).
Obtaining Timing Data
Markers were digitized using computerized auto tracking software (ProAnalyst, Xcitex, MA). Previously published work has indicated inter-user reliability of 95% for auto tracked points and around 80% for manually tracked points [8]. The x and y coordinates of the markers were rotated, scaled to palate length, and translated using anterior and posterior hard palate as reference points.
All timings were calculated relative to the epiglottis flipping, used as a marker of swallow initiation. We extracted a 100-ms window of analysis on either side of swallow initiation, unless swallows were less than 100 ms apart in which case the maximum amount of time before the swallow was used. All swallow windows were normalized to a duration of 1 for comparison purposes. We calculated the following timing variables within each window to test for differences in IMPAS score (1–7) and with treatment (control/lesion):
-
LVC: time that the airway is protected
-
LVO: time that the airway opens
-
LV closure time: LVO–LVC (duration of LV closure)
-
Posterior tongue caudal: timing that posterior tongue reached its caudal-most (minimum x) position (Fig. 2a)
Fig. 2 a and b The posterior tongue and hyoid loops. Anatomical axis (rostro-caudal and dorso-ventral) defined relative to the two hard palate markers (Fig. 1), used as the reference points for the rostro-caudal (x) axis. c This is the key of directions in relation to the posterior palate markers
-
Posterior tongue rostral: time at which the posterior tongue reached its rostral-most (maximum x) position (Fig. 2a)
-
Posterior tongue ventral: time at which the posterior tongue reached its ventral-most (minimum y) position (Fig. 2a)
-
Posterior tongue dorsal: time at which the posterior tongue reached its dorsal-most (maximum y) position (Fig. 2a)
-
Hyoid caudal: timing the hyoid reaches its caudal-most (minimum x) position (Fig. 2b)
-
Hyoid rostral: timing the hyoid reaches its rostral-most (maximum x) position (Fig. 2b)
-
Hyoid dorsal: timing the hyoid reaches its dorsal-most (maximum y) position (Fig. 2b)
-
RTH: timing of right thyrohyoid firing
-
LTH: timing of left thyrohyoid firing.
Statistical Analysis
The swallows were analyzed using complete linear mixed model (ANOVA) with each of the above variables as the dependent or response variable. Individual animal was included as a random effect, and lesion and IMPAS were fixed effects, and a lesion*IMPAS interaction was included in the model. The following specific hypotheses were tested using post hoc independent contrasts, where the interaction term was significant. By identifying these specific tests, with a physiologic justification, we could increase the power of our analyses (Table 2).
-
1.
Control safe swallows (IMPAS 1) differ from lesion safe swallows
-
2.
Control swallows with penetration only (IMPAS 3) differ from lesion swallows with penetration only
-
3.
Control swallows with aspiration only (IMPAS 7) differ from lesion swallows with aspiration only
-
4.
Within controls, safe (IMPAS 1) and aspiration (IMPAS 7) swallows differ
-
5.
Within controls, safe (IMPAS 1) and penetration only (IMPAS 3) swallows differ
-
6.
Within controls, penetration only (IMPAS 3) and aspiration (IMPAS 7) swallows differ
-
7.
Within lesions, safe (IMPAS 1) and aspiration (IMPAS 7) swallows differ
-
8.
Within lesions, safe (IMPAS 1) and penetration only (IMPAS 3) swallows differ
-
9.
Within lesions, penetration only (IMPAS 3) and aspiration (IMPAS 7) swallows differ.
Results
LVC/LVO Timing
There are significant differences in LVC timing, as well as duration of closure, between safe versus unsafe swallows as measured by IMPAS score. The significant interactions between IMPAS and RLN lesioning mean that lesion impacts timing differently in safe and unsafe swallows (Table 2). The timing of opening, LVO, only differs between safe versus unsafe swallows, and lesion does not change this (Fig. 3; Table 3). The specific differences in LVO delay exist between safe swallows (IMPAS 1) and unsafe swallows leading to penetration and aspiration (IMPAS 3, 7).
For timing of LVC, the pattern of significant interactions indicates significant delays in laryngeal vestibular closure in unsafe swallows (IMPAS 3, 7) compared to safe swallows (IMPAS 1) with and without RLN lesion (Fig. 4; Table 4).
In lesioned animals only, changes in duration of laryngeal vestibule closure differ between safe and unsafe swallows. Duration of LVC in swallows with aspiration differs between lesioned and non-lesioned animals (Fig. 5; Table 5).
Tongue Movements
There are significant changes in the time of caudal-most direction for both factors (IMPAS and Treatment), as well as the interaction between them. The results of significant pairwise post hoc tests for the interaction term are presented in Fig. 6; Table 6. In unsafe swallows (IMPAS 3, 7), lesioned animals are delayed in tongue movement to the most caudal position when compared to safe swallows (IMPAS 1). Pre- and post-lesion safe swallows (IMPAS 1) are not significantly different, whereas pre- and post-lesion unsafe swallows (IMPAS 3, 7) are Table 7.
There are significant differences in overall timing of maximum rostral tongue position between lesion and non-lesioned swallows (Fig. 7). However, no effect of IMPAS is found.
There are significant delays in timing of maximal dorsal posterior tongue movement in both lesioned and non-lesioned aspiration (IMPAS 7) swallows compared to non-lesioned safe swallows (IMPAS 1) (Fig. 8; Table 8).
Ventral-most tongue position occurs later in post-lesion than pre-lesion swallows with aspiration. In pre-lesion swallows, there is a significant difference in ventral-most tongue position time between safe swallows and swallows with aspiration (Fig. 9; Table 9).
Hyoid Movement
While the timing of hyoid movement differed with IMPAS score in 3 of 4 hyoid variables, only the timing of reaching the maximum Y position (dorsal) was impacted by treatment, including a lesion* IMPAS interaction. The specific differences were the Safe controls (C1) differing from all others control swallows and the Lesion safe (L1) differing from all others lesion swallows (Fig. 10; Table 10).
There was a significant effect of IMPAS score on hyoid rostral-most position (Fig. 11).
There were no significant differences in the timing of hyoid caudal-most position (Table 11).
Timing of Thyrohyoid Muscle Activity
The only significant difference in timing of thyrohyoid activity was due to IMPAS score on the right (side of lesion) (Fig. 12). Otherwise, this timing was not changed with treatment (Table 12).
Summary of Hypothesis Testing
There was a significant interaction between RLN lesion and swallow safety for six out of the 12 variables examined. Post hoc tests indicate the following:
-
1.
There are differences in timing of safe swallows pre- and post-lesion for three variables (timing of LVC, timing of cranial-most tongue position, timing of cranial-most hyoid position).
-
2.
There are differences in timing of swallows with penetration pre- and post-lesion only for timing of caudal-most posterior tongue position.
-
3.
There are differences in timing of swallows with aspiration pre- and port-lesion in four variables (timing of LVC, duration of LVC, timing of caudal-most posterior tongue position, timing of ventral-most posterior tongue position).
-
4.
Pre-lesion, safe swallows, and swallows with aspiration differed in four variables (timing of LVC, timing of cranial-most posterior tongue position, timing of ventral-most posterior tongue position, timing of cranial-most hyoid position).
-
5.
Pre-lesion, safe swallows, and swallows with penetration differed in two variables (timing of LVC and timing of cranial-most hyoid position).
-
6.
Pre-lesion, swallows with penetration, and swallows with aspiration differed in timing of cranial-most posterior tongue position only.
-
7.
Post-lesion, safe swallows, and swallows with aspiration differed in five variables, with only timing of cranial-most posterior tongue position showing no difference.
-
8.
Post-lesion, safe swallows, and swallows with penetration differed in three variables (timing of LVC, timing of posterior tongue caudal-most position, timing of hyoid cranial-most position).
-
9.
Post-lesion, swallows with penetration, and swallows with aspiration differed only in duration of LVC.
Discussion
LVC/LVO/LV Closure Duration
A safe swallow is characterized by laryngeal closure which includes three distinct events: glottal closure, closure of the laryngeal vestibule (LV), and inversion of the epiglottis [5]. LVC was occurring later in unsafe swallows of both control and lesioned animals. The LVC closing time was highly correlated with hyoid elevation and arytenoid cartilage meeting the base of the epiglottis [5]. This delay caused some change in pharyngeal kinematics that led to increased area for the bolus to penetrate the vestibule and to an increased chance of aspiration. Delaying the closure of the laryngeal vestibule in unsafe swallows led to increases in aspiration risk the longer the LV stays open. Post-RLN lesion, unsafe swallowing occurred at increasing frequency [14]. However, in this study, RLN lesion had no impact on LVC timing, leading us to believe that mechanisms outside of LVC timing cause aspiration due to RLN lesion.
LVO occurred later in unsafe swallows, with a significant lengthening of LV closure time duration in aspiration swallows. This alteration in LVO timing was paradoxical to the idea of aspirations occurring due to lack of airway protection. We are unsure whether a longer duration of LV closure and a delayed opening of the airway were associated with unsafe swallows. Our results from above showed that LVC was the driving force in timing to have a safe swallow. One possibility for increased aspiration when we observed delayed LVO and increased LV closure duration is that the delay in LV closure produced kinematic changes in the oropharynx which in turn produced these adjustments in the swallow.
This adjustment could have produced a longer duration of airway protection, but also a greater risk of failure. As above, RLN lesion did not change the timing of LVO or duration of laryngeal vestibular closure, suggesting that this increase in aspiration could not be a function of the lesion.
Tongue Movement
Caudal tongue movement and dorsal tongue movement was linked to the timing of bolus transit from the oral cavity into the oropharynx, which occurred later in unsafe swallows and in lesioned animals. The changes in the posterior tongue were consistent with the view that bolus transit caudally is delayed in unsafe swallows and after RLN lesion. Previous work shows that RLN lesioning has significant impact on tongue movement and bolus shape [8, 20]. This may increase aspiration risk, in conjunction with LVC timing delay, as the tongue is out of sync with the pharyngeal musculature.
Hyoid Movement
Closure of the LV and LVC timing was correlated with the movement of the hyoid bone and the arytenoid cartilages. Hyoid elevation was delayed in unsafe swallows. This strengthens the claim of LVC timing being delayed due to changes in hyoid kinematics during unsafe swallows. Due to the inadequate closure of the laryngeal vestibule, this would lead to a greater chance of aspiration to occur during a swallow. This delayed hyoid movement, in conjunction with the posterior tongue movement delays, caused the oropharyngeal transit into swallowing to be compromised.
Thyrohyoid Muscle Timing
Previous studies have used thyrohyoid firing as a sign of a swallow [16, 21]. Because cervical rami innervate the thyrohyoid musculature, lesioning of the RLN without any central reorganization should not impact thyrohyoid firing time. Right thyrohyoid firing was significantly delayed in penetration swallows compared to safe swallows. This pathophysiology is not well understood and suggests that further investigation into the role of thyrohyoid in swallow safety is warranted. Although RLN lesioning had no significant impact on thyrohyoid firing time, we have not observed the animals in the long-term to observe if central reorganization may occur due to RLN lesioning.
Mechanism of RLN Lesion Swallowing Dysfunction
Swallowing is a complex physiological process involving the temporal coordination of paired muscles of the tongue, hyoid, pharynx, and larynx. As such there are many possible points along the sequence of movements that may lead to failures of safe bolus control. Lesion of the recurrent laryngeal nerve modifies kinematics of the tongue [8], formation of the bolus [20], as well as the laryngeal effects described here. Furthermore in all cases, these kinematic changes can be related to failure of airway protection in swallows after RLN lesion. The effect of RLN lesion is a subtle, but pervasive, reorganization of swallow kinematics in both the oral and pharyngeal phases that results in pathological aspiration. This most likely reflects a failure of coordination at the level of neural control. Clinically, this suggests that treatment routes based in rehabilitation and movement therapy that target motor pattern learning may be a promising avenue for future research.
Limitations of the Study
Our overall sample size of swallows has very large numbers (n > 100); however, we do have a limited number of animals (N = 6) which may affect subject power, as the swallows within an individual are all linked statistically. We address this issue by including individual as a random factor, which accounts for inter-individual variation, and looks for patterns that exist in addition. Our study evaluated lesions in the right RLN, which is different in human infants undergoing PDA closure that involves potential iatrogenic damage to the left RLN. This decision occurred due to the broader scope of this, which looked at SLN and vagus nerve lesion in addition to RLN. The vagus plexus on the left side gives parasympathetics to the AV node of the heart, leading to a potential increase of harm to animals if lesioned. Due to this limitation, the right side was used.
Future Directions
Continuing research into the neurological effects of sensorimotor integration of swallowing is key in finding new modalities of countering dysphagia and aspiration. Future directions of our research will involve looking at longer term sequelae in swallowing timing, to better see how individual changes in swallowing timing and kinematics occur among individuals to protect their airway. Long-term observation of subjects may highlight key neuroplastic changes that may occur as a result of RLN lesioning, allowing us to better understand the clinical spectrum observed in different patients. Additionally, RLN injury in human infants is associated with preterm birth, and surgical intervention for a patent ductus arteriosus. We are currently examining the interaction between prematurity and RLN damage to understand the separate and joint effects of that insult.
References
Fink BR, Martin RW, Rohrmann CA. Biomechanics of the human epiglottis. Acta Otolaryngol. 1979;87:554–9.
Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol Gastrointest Liver Physiol. 1992;262:G338.
Macrae P, Anderson C, Humbert I. Mechanisms of airway protection during chin-down swallowing. J Speech Lang Hear Res. 2014;57:1251–8.
Calvo I, Sunday KL, Macrae P, Humbert IA. Effects of chin-up posture on the sequence of swallowing events. Head Neck. 2017;39:947–59.
Inamoto Y, Saitoh E, Okada S, Kagaya H, Shibata S, Ota K, et al. The effect of bolus viscosity on laryngeal closure in swallowing: kinematic analysis using 320-row area detector CT. Dysphagia. 2013;28:33–42.
Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia. 2011;26:67–74.
Young JL, Macrae P, Anderson C, Taylor-Kamara I, Humbert IA. The sequence of swallowing events during the chin-down posture. Am J Speech Lang Pathol. 2015;24:659–70.
Gould FDH, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, et al. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol. 2016;120:495–502.
LaMantia A-S, Moody SA, Maynard TM, Karpinski BA, Zohn IE, Mendelowitz D, et al. Hard to swallow: developmental biological insights into pediatric dysphagia. Dev Biol. 2016;409:329–42.
Hersh C, Wentland C, Sally S, de Stadler M, Hardy S, Fracchia MS, et al. Radiation exposure from videofluoroscopic swallow studies in children with a type 1 laryngeal cleft and pharyngeal dysphagia: a retrospective review. Int J Pediatr Otorhinolaryngol. 2016;89:92–6.
German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool. 1992;261:322–30.
German RZ, Crompton AW, Gould FDH, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia. 2017. doi:10.1007/s00455-016-9778-7.
Liu S-C, Chou Y-F, Su W-F. A rapid and accurate technique for the identification of the recurrent laryngeal nerve. Ann Otol Rhinol Laryngol. 2014;123:805–10.
Gould FDH, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, et al. The physiologic impact of unilateral recurrent laryngeal nerve (RLN) lesion on infant oropharyngeal and esophageal performance. Dysphagia. 2015;30:714–22.
Alvarez-Berdugo D, Rofes L, Casamitjana JF, Padrón A, Quer M, Clavé P. Oropharyngeal and laryngeal sensory innervation in the pathophysiology of swallowing disorders and sensory stimulation treatments. Ann N Y Acad Sci. 2016;1380:104–20.
Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol. 2012;112:1512–9.
Holman SD, Waranch DR, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, et al. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. J Neurophysiol. 2013;110:387–96.
Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, Waranch DR, et al. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs: swallowing after palatal anesthesia. Laryngoscope. 2014;124:436–45.
Thompson C, Donley E, Stimpson C, Horne W, Vinyard C. The influence of experimental manipulations on chewing speed during in vivo laboratory research in tufted capuchins (Cebus apella). Am J Phys Anthropol. 2011;145:402–14.
Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia. 2016;32:362–73.
German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102:1017–25.
Acknowledgements
The lab would like to acknowledge the CMU staff and Biomechanical Journal Club. Special thanks to Dr. Vinyard and Dr. Young, as well as everyone who worked in the German laboratory.
Funding
This work was supported by NIH R01 DC9980 NIH R01 HD088561 to R.Z. German.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gross, A., Ohlemacher, J., German, R. et al. LVC Timing in Infant Pig Swallowing and the Effect of Safe Swallowing. Dysphagia 33, 51–62 (2018). https://doi.org/10.1007/s00455-017-9832-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-017-9832-0