Abstract
UES opening occurs following cricopharyngeus deactivation and submental muscle contraction causing hyolaryngeal elevation and UES distraction. During impedance manometry, the inverse of impedance (admittance) can be used to measure bolus presence and infer UES opening. We hypothesized that the temporal relationship between UES relaxation, opening and hyolaryngeal elevation would change with increasing bolus volume. Simultaneous intramuscular cricopharyngeal (CP) electromyography (EMG), surface submental EMG (SM-EMG), and high-resolution impedance manometry were recorded in eight (aged 27 ± 7 years, 5 M) healthy volunteers, while swallowing 0.9 % saline boluses of 2, 5, 10, and 20 ml. Data were exported and analyzed via Matlab. Statistical analysis comprised repeated measures one-way ANOVA and Pearson correlation. A P value of <0.05 was considered significant. Duration of CP deactivation increased at 20 ml volume (P < 0.001). UES relaxation and opening increased with increasing bolus volume (P < 0.001); however, overall duration of SM activation did not change. As UES opening occurs progressively earlier with increasing volumes, peak SM-EMG activity occurs relatively later (P < 0.001) and shifts from occurring before to following peak UES distention. During healthy swallowing, there is sensory modulation of cricopharyngeal and submental muscle activity. Intrabolus pressures, transmitted from the tongue base and pharynx, play a progressively more important role in sphincter opening with increasing volume. The findings may explain why some healthy elderly and patients with oropharyngeal dysphagia have difficulty swallowing larger while tolerating smaller bolus volumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The upper esophageal sphincter (UES) is a narrowed area in the pharyngo-esophageal segment, consisting of cricoid cartilage anteriorly and muscle posteriorly [1, 2]. The UES functions as a barrier or gatekeeper, preventing air from entering the digestive tract during breathing and swallowed or refluxed contents from returning into the hypopharynx [1]. UES closure occurs through passive elastic forces and contraction by the posterior muscle complex consisting of cricopharyngeus with contributions from inferior pharyngeal constrictor and proximal esophagus [2–4]. In its resting state, the UES remains closed and the CP tonically contracted, with constant brainstem-derived neurogenic input [5]. The UES needs to open during swallowing [6–8], belching [9], and vomiting [10]. UES opening represents a complex interplay of sensorimotor neuromuscular activity, modulated via brainstem and spinal reflexes [5].
UES opening during swallowing facilitates bolus transport through the pharyngo-esophageal segment [7] with bolus clearance occurring through a pharyngeal stripping wave coordinated by a central pattern generator (CPG) in the brainstem [5]. The muscles involved in UES opening are primarily the CP muscle relaxing [2, 5] and suprahyoid, (submental, SM) muscles contracting [6–8, 11]. SM contraction causes sphincter distraction leading to increased UES compliance, through which transmitted bolus forces open the sphincter [7]. UES opening is modulated by bolus factors such as volume and viscosity to facilitate bolus passage [6–8, 12, 13]. The known effects of increased bolus volume are longer UES opening and a greater aperture [7, 13]. Due to its asymmetrical shape, representing an oval or horseshoe, apparent small changes in UES anterior–posterior opening are amplified in cross-sectional area (CSA) [7].
UES opening and deactivation of the CP-EMG are thought to occur simultaneously [5]. However, controversy remains on whether the initiating event for CP deactivation occurs at the level of the CP itself or is caused through activation of stretch receptors during hyolaryngeal elevation [5]. One school of thought holds that tonic CP deactivation and UES opening occur simultaneously due to brainstem-mediated neural inhibition originating in the CPG [3, 5, 14, 15]. An alternate school of thought is that the suprahyoid muscles are primarily involved in UES opening and that traction within the suprahyoid muscle group leads to CP deactivation [1, 7, 16, 17]. The inability of integrative functions to appropriately modulate the individual components, UES relaxation, opening and hyolaryngeal elevation, may have clinical implications for patients with oropharyngeal dysphagia; in particular, for determining the “dysphagia limit” beyond which multiple swallows are needed to clear a bolus effectively through the UES [18, 19].
The ability to simultaneously measure CP deactivation, submental activation, pressure and luminal CSA (opening) through the UES region by using electromyography in combination with high-resolution impedance manometry may enable us to clarify the specific contributions of CP and submental muscles to UES relaxation and opening during swallowing. The aim of this study was to measure the temporal relationships among EMG-measured CP and SM muscle activity and impedance manometry measured UES pressure relaxation and luminal opening. We hypothesized that, in healthy individuals without dysphagia symptoms, these interrelationships would change with increasing volume in concert with normal functioning of sensory-motor neuromodulation of the swallow mechanism.
Methods
Participants
Eight healthy subjects (5 males) between 20- and 43-year old (Ave 27 ± 7 years) were recruited for our study. Subjects completed written informed consent prior to any study-related procedures. The protocol was approved by the Institutional Review Board of the University of Wisconsin–Madison.
Study Procedure
Subjects were instructed not to eat for 4 h or drink for 2 h prior to undergoing the study procedure. All participants completed screening questionnaires and were excluded with any history of swallowing, respiratory or neurological deficits or medication affecting gastrointestinal motility. Electromyography (EMG) and impedance manometric recordings were then obtained as described below during a standardized protocol.
Electromyography
Procedures for EMG placement have been described previously [14, 15, 20–22], and are reviewed here. Prior to CP electrode insertion, the anterior neck was numbed using 1 % lidocaine with epinephrine (1:100,000) via a 30-gage needle. Following this, up to four intramuscular cricopharyngeal (CP) bipolar hook-wire intramuscular electrodes (MicroProbes, Gaithersburg, Maryland) were placed transcutaneously using a 27-gage needle. Placement was confirmed by witnessing the characteristic CP muscle pattern of quiescence during a swallow followed by a burst of post-swallow activity. At most, two CP electrodes were left in situ for recording purposes. Bilateral surface EMG electrodes were placed in the submental region between the mandible and the hyoid bone, each at 1 cm from midline and a surface ground electrode (A10058-SRT; Vermed, Bellows Falls, Vermont) placed on the forehead. The EMG signals were amplified, bandpass-filtered from 100 Hz to 6 kHz (model 15LT; Grass Technologies, Warwick, Rhode Island), and digitized at 20 kHz (LabChart version 6.1.3; ADInstruments, Colorado Springs, Colorado) [21].
Manometry
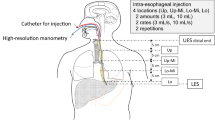
After confirming a successful EMG placement, a 4.2-mm diameter solid-state pressure and impedance manometry catheter incorporating 36 1 cm-spaced pressure sensors and 18 adjoining impedance segments, each of 2 cm length (Given Imaging, Ltd.) was placed via an anesthetized nostril with the recording assembly straddling the velopharynx to proximal esophagus. We confirmed complete capture of the regions of interest, including the velopharynx, tongue base, hypopharynx, and UES, prior to securing the assembly in place. Data were recorded at 50 Hz. Following placement, the subject rested for approximately 5 min to adjust to the catheter prior to undertaking the study protocol.
Swallow Protocol
With the subject sitting upright and in the head neutral position, five bolus swallows of 2, 5, 10, and 20 ml saline solution (0.9 % NaCl) were administered. These were recorded simultaneously by the EMG and pressure-impedance acquisition systems. The boluses were administered at >20-s interval to the mouth via a syringe and subjects asked to swallow on command (i.e., cued volitional swallowing). Volume swallowing was undertaken in sequence from the smallest to the largest volume (i.e., 2 ml followed by 5, 10, and 20 ml).
Data Analysis
Manometric and EMG data were time-linked using a transistor-to-transistor logic signal. Pressure and impedance data for each swallow were exported from the acquisition systems in text file (.txt) format. Pressure, impedance and EMG data were analyzed with a customized Matlab program (MathWorks, Natick, Massachusetts) by a single operator (CC) following export. The EMG signals were rectified, low-pass filtered, then resampled to 50 Hz to match the sampling rate of the HRM signals. The segment of time-series data for UES pressure and the CP muscle voltage were time-aligned for each trial [21].
Impedance data were smoothed using interpolation (Piecewise Cubic Hermite Interpolating Polynomial) to increase the spatial dataset to match the pressure dataset (1 sample per 1 cm). To account for known nonlinearity of the impedance–area relationship [26], the impedance values were converted to the inverse product of impedance (1/impedance), Admittance, expressed in millisiemens (mS). The UES undergoes a 2 cm or more elevation before complete UES relaxation [6], while the manometry catheter elevates approximately 1 cm when swallowing, asynchronous to UES elevation [6, 22]. UES pressure and impedance data were analyzed within an area of interest corresponding to the region from the distal margin of the UES high-pressure zone to the estimated apogee position of the UES during the swallow, and for the time period from 1 s before the onset to 1 s after the offset of CP pause (on CP-EMG). Maximum axial UES pressure during the swallow was measured within the limits of UES area of interest over time. The location of maximum axial pressure was used to track the superior and inferior movement of the UES based on the method of Ghosh et al. [23]. Consecutive pressure and admittance values mapped to the corresponding position of the UES over time were used to derive an optimal profile of pressure and admittance during the swallow that could be correlated with CP-EMG and SM-EMG recordings (Fig. 1).
High-resolution (HR) impedance manometry (a) and simultaneous cricopharyngeal (CP) and submental (SM) electromyography (EMG) (b) for a 10-ml saline swallow. Data exported to Matlab™ and a region of interest defined for the upper esophageal sphincter (UES) (c) used to correlate CP-EMG with UES pressure (d) and SM-EMG with admittance (e) along a line tracking maximum UES pressure/movement (a)
From the EMG data, CP offset (deactivation) and onset (activation), as well as the time and amplitude of the post-swallow CP peak, were measured. CP offset and onset were determined as a reduction below and return above 5 % of maximal CP amplitude per volume [24]. For SM-EMG measurements, the time of onset, peak amplitude, and offset were determined as per Crary et al. [11]. The time from onset to peak was termed the upstroke time, while the time from peak to offset was termed downstroke time. Peak amplitude value was determined. SM onset and offset were determined as a sustained (>100 ms) increase above and return below 5 % of maximal SM amplitude.
Pressure measurements below and above 0 mm Hg for >200 ms were used to define UES relaxation and contraction. Timing relative to CP deactivation was determined for UES relaxation, contraction, and peak post-swallow pressure. Admittance, the inverse product of impedance, expressed in millisiemens (mS, the unit of electric conductance), was used to determine bolus diameter as per Omari et al. [24, 25], and UES opening was thus inferred during bolus presence. Admittance above 1.5 mS was used to define when the lumen was distended by bolus presence [25]. Swallows exhibiting the presence of significant quantities of swallowed air (identified by a sustained drop in admittance) were excluded from further analyses, in practice this related to a subset of 2 ml volume swallows only. The maximum UES admittance allowed estimation of the time of maximal luminal CSA [26, 27]. The time from onset of admittance increase to maximum was termed the admittance upstroke time, while the time from maximal admittance to offset of admittance decrease was called the admittance downstroke time.
Pressure Flow Analysis
Swallows were exported as .txt files and analyzed as previously described [24], using purpose designed software.
In summary, four space–time landmarks were defined on the resulting isocontour plot:
-
1.
The time of onset of complete UES relaxation.
-
2.
The time of offset of complete UES relaxation.
-
3.
The apogee position of the UES high-pressure zone, defined by visualization of the orad movement of the UES high-pressure zone to determine the highest position of the proximal edge of the high-pressure zone during the swallowing event.
-
4.
The distal margin position of the UES high-pressure zone, defined by the lowest position of the distal edge of the high-pressure zone pre- and/or post-swallow.
Guided by definition of these landmarks, the software algorithms can generate values for a range of swallow function variables based on the timings of maximum admittance (maximum distension) and/or maximum pressures. A range of swallow function variables and their derivation have been previously described [28, 29]. Substantial to excellent inter- and intrarater reliability, ranging between 0.77 and 1.00, were achieved among experienced and inexperienced raters for all included variables [29]. In this paper, we present data in relation to UES resting and nadir pressures (mm Hg), duration of UES relaxation (ms), maximal post-swallow relaxation pressure (mm Hg), hypopharyngeal intrabolus pressure (mm Hg), the 0.25 s integrated relaxation pressure (IRP, mm Hg), maximum UES admittance, pharyngeal distention-contraction latency time (ms), and pharyngeal peak pressure (mm Hg).
Statistical Analysis
Statistical analysis was performed on per subject means for each volume. Between volume, differences were determined using repeated measures one-way ANOVA (general linear model with repeated volume measures) in Sigmaplot 13.0 (Systat Software, San Jose, Ca). Further pairwise comparisons (multiple comparison procedures) were undertaken using a Tukey test. A P value of <0.05 was considered statistically significant.
Pearson’s product moment correlation was used to assess correlations between metrics. Data reported relate to differences (RM-ANOVA) across 2, 5, 10, and 20 ml bolus volumes.
Results
Electromyography
The duration of cricopharyngeal EMG (CP-EMG) deactivation increased during swallowing bolus of increasing volume (Fig. 2a; F = 14.44, P < 0.001). Post-swallow peak CP-EMG occurred at a similar time point, and at a similar magnitude among different volumes (F = 2.04, P = 0.14). CP deactivation showed excellent correlation with UES relaxation (r > 0.8, P < 0.001) for all bolus volumes and was particularly closely aligned in the post-swallow period.
Total duration of SM-EMG activation did not significantly increase in duration for different volumes, from 1015 ± 76 ms at 2 ml volume to 1120 ± 55 ms at 20 ml (F = 2.61, P = 0.09). However, the duration of the SM-EMG upstroke time increased (F = 4.90, P = 0.04) with SM-EMG peak occurring progressively later in relation to CP deactivation (Table 1; P < 0.001). SM-EMG peak initially occurred prior to maximal UES opening; however, the SM-EMG peak shifted later to occur following maximal UES opening for volumes over 10 ml. There was also an increase in peak SM-EMG amplitude from 145 ± 23 μV at 2 ml to 195 ± 29 μV at 20 ml (F = 6.22, P = 0.003).
UES Opening
Duration of UES opening increased progressively across volumes (Table 1; F = 46.67, P < 0.001). UES admittance, a correlate for sphincter cross-sectional area [27], peaked progressively earlier (Table 1; P < 0.001) and progressively increased in magnitude with increasing volumes (Fig. 3a; F = 202.7, P < 0.001).
Pressure Flow Measurements
Duration of UES relaxation increased progressively with an increase in bolus volume (Fig. 2b; F = 30.68, P < 0.001). Pharyngeal peak pressure remained constant across volumes from 153 ± 16 mm Hg at 2 ml to 151 ± 18 mm Hg at 20 ml (F = 0.22, P = 0.78). There were no differences in UES resting or nadir pressures and maximal post-swallow pressure occurred at a similar time interval and magnitude at all volumes (data not shown).
Intrabolus pressures (IBP) and the 0.25 s integrated relaxation pressure (IRP) [30] were similar between volumes at the level of the UES. However, at 1 cm above the UES, IBP increased across volumes (Fig. 3b; F = 4.27, P = 0.02) and particularly increased at 20 ml.
Timing variables, such as pharyngeal distention-contraction latency increased with bolus volume (Fig. 3c; F = 23.89, P < 0.001). We interpret this as showing that the timing of maximal pharyngeal distension occurred progressively earlier relative to the timing of maximal pharyngeal contraction.
Discussion
Volume-dependent modulation of upper esophageal sphincter opening is an important mechanism in health that allows swallowing of increasing bolus volumes enabling large boluses to transit the pharyngo-esophageal segment more rapidly [6–8, 12, 13]. Without this compensatory mechanism, flow resistance would increase exponentially placing greater demand on airway protective mechanisms to prevent aspiration. Sensory coding for volume modulates the efferent neural activation of the swallowing muscles leading to the earlier timing of UES opening [12]. Furthermore, the volume and compressibility of the swallowed bolus can influence the extent of UES opening [7, 8, 13]. In the current study, we provide further insights into this mechanism via direct electromyographic recordings of the relevant swallowing muscles. We also show that the mechanisms governing UES relaxation and opening can be elucidated nonradiologically using pressure-impedance recordings. In this study, we have characterized the volume-dependent shifts in the temporal interrelationships of peak submental muscle activity and maximum UES opening during CP deactivation pause.
The swallowing mechanism is notionally most efficient when the peak submental muscle activation and maximum opening of the UES lumen are temporally aligned during a period of complete activity pause of the CP muscle. Our observations showed that the timings of these events during the swallow sequence were volume-dependent. Increasing the volume of bolus swallowed was associated with an earlier UES opening peak and a later submental muscle EMG activity peak. Increasing the volume swallowed also produced a longer pause in CP muscle activity; however, the size of this effect was much smaller when compared to the extent of modulation of peak submental muscle activation and UES opening. The finding of a later submental EMG peak is similar to that of Gokyigit et al. when describing the onset of submental EMG in relation to CP deactivation with increasing volume [34]. In the current study, the timings of peak SM-EMG and UES opening these events ‘crossed over’ as the swallowed volume increased with precise temporal alignment of the different components occurring when 10 ml volumes were swallowed (Fig. 4). This suggests that, in health, the swallowing mechanism is optimally tuned to approximately 10 ml volumes.
Relative timing of swallow related events time correlated to cricopharyngeal deactivation (CP off) for different bolus volumes (mean values). Interestingly, peak submental activation (open diamond) occurs prior to maximal UES opening (open circle) for 2 and 5 ml volume swallows, while peak SM activity occurs following maximal opening at 10 and 20 ml. The inference is that the bolus itself plays an important role in initiating sphincter opening, including CP deactivation, at larger volumes
When subjects swallowed larger 20 ml bolus volumes, maximum UES opening was significantly greater compared to 10 ml and occurred before the peak in submental muscle activity, and this was in turn associated with higher intrabolus distension pressures recorded within the hypopharynx. We interpret these findings as being consistent with the passive distension of the UES during circumstances of contractile force (lingual and then pharyngeal sequentially) being applied to a pharyngeal chamber above, which is full of bolus. In other words, the UES is being pushed open from within by the bolus, and this is occurring before the submental muscles are able to apply maximum extrinsic traction to the UES via their mechanical linkage to the hyoid bone.
The concept of pressurization of a large volume bolus between the advancing pharyngeal stripping wave and the opening UES has been previously described [6–8, 13]. The mechanism of earlier deactivation of the CP muscle, as we have observed in relation to the largest boluses, in not clear; however, we postulate that increased tension, generated by passive distension of a tonically active CP muscle may stimulate deep muscle mechanoreceptors [31], triggering CP deactivation via vagal pathways. Initiation of UES opening at the level of the UES itself, as opposed to occurring within the suprahyoid muscle mechanoreceptors, is a novel concept, suggesting brainstem-based neural deactivation occurs as modulation to enable earlier UES opening during larger bolus volumes. This would be in keeping with findings describing differences in human as compared to animal studies of UES opening, specifically as relates to UES opening at the largest bolus volumes [3, 7, 17].
When subjects swallowed smaller 2 and 5 ml bolus volumes, maximum UES opening was significantly reduced compared to 10 ml and occurred later and after peak submental muscle activity. These findings are consistent with the UES being pushed open by the bolus which is being propelled by the sequential activation of the pharyngeal constrictors only. Hence in the case of smaller volumes, lingual forces play no role in UES opening because the pharyngeal chamber in not full. Having already peaked in activity, the tension in the submental muscles is reducing passively when flow through the UES is occurring. The distension pressures recorded at this time plateau at volumes of 5 and 2 ml, while diameter (maximum admittance) continues to decrease incrementally. This suggests that the level of extrinsic traction applied to the UES, which offers a mechanical advantage for reducing flow resistance during opening, has waned by the time volumes of 2–5 ml transit the UES.
Changes in amplitude and timing of submental peak activity, relative to the contractile components of the swallow provide evidence that hyolaryngeal excursion and the pharyngeal contractile response are functionally decoupled; the former being most important for swallow modulation in relation to volumes [6, 16]. Pharyngeal contractions have previously been shown to be modulated for changed consistency [32], but in keeping with previous observations, occurred in a stereotypical fashion regardless of bolus volume [32]. The pharyngeal stripping wave was closely associated with the bolus tail throughout the swallowing sequence, including during UES closure [24]. Separate neural networks governing suprahyoid and pharyngeal constrictor activation within the brainstem central pattern generator, controlling separate, but related aspects of the swallow response in a flexible, rather than fixed, pattern may explain these observations [33].
The concept of a “dysphagia limit,” the volume above which swallowing occurs in a piecemeal fashion, had been previously described by Ertekin et al. [18, 19]. Their data suggested the dysphagia limit in most subjects is at a volume above 20 ml. Our data show that ‘cross-over’ of SM-EMG peak and maximum UES opening occurs at this volume and therefore is consistent with the notion that 20 ml exceeds the limit of mechanically optimal swallow volume. In heath we believe there is sufficient reserve to allow safe swallowing above this limit. Clearly, any deficiency in function, such as impairment of afferent pathways which modulate the timing of muscle activation, for example, aging effects or overt neurological disease, can lead to rapid decompensation at these limits of normal function.
Interpretation of our data is limited by the lack of simultaneous radiology, which would have enabled us to both directly observe UES opening and measure hyolaryngeal elevation and hyoid movement due to supra- and infrahyoid muscle contraction. To date, we have been unable to record intramuscular EMG during radiology, due to interference from the fluoroscopy unit, rendering the EMG recording uninterpretable. Admittance criteria for bolus presence based on radiological UES opening had previously been described [25] and thus the use of impedance manometry allowed us to substitute admittance-based sphincter opening. We had chosen not to measure hyolaryngeal elevation on manometry, as the interpretation of such data is challenging due to movement not only of the pharyngo-UES in relation to the catheter but also of the catheter itself during swallowing events [6, 22]. We overcame this limitation during interpretation of UES data by constantly tracking the highest pressure within the sphincter zone, as described by Ghosh et al. (Fig. 1c) [23]. CP-EMG is challenging to record, and our study showed that UES relaxation closely correlated with CP deactivation and can substitute CP recording during recording of UES neuromuscular mechanical states [24, 25]. Comparatively, SM-EMG is easy to record via surface electrodes, and we would continue to advocate for the use of SM-EMG in better understanding oropharyngeal dysphagia across bolus volumes and in relation to the efficacy of swallowing maneuvers during studies of UES opening.
In conclusion, our results suggest that at a bolus volume of 20 ml, the bolus itself initiates sphincter opening, leading to CP deactivation and modulation of submental activity to maintaining UES opening at greater aperture for longer. Our study has implications for understanding the dysphagia limit (maximal bolus volume swallowed in a single swallow) in older individuals and patients with neuromuscular oropharyngeal dysphagia. Older individuals and others with overt sensory impairments (e.g., Parkinson’s disease) may be unable to modulate the initiation of UES opening in response to larger volumes. Conversely, individuals with neuromuscular weakness (e.g., motor neurone disease) may have intact modulation of UES opening onset, but may lack the strength to modulate suprahyoid muscle activity.
References
Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterol. 1978;74:514–20.
Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108(4A):27S–37S.
Miller AJ. Deglutition. Physiol Rev. 1982;62:129–84.
Samuel E, Shaker R. Deglutitive pharyngeal and UES pressure phenomena. In: Shaker R, Belafsky PC, Postma GN, Easterling C, editors. Principles of deglutition. New York: Springer; 2013.
Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–44.
Kahrilas PJ, Dodds WJ, Dent J, Logemann J, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterol. 1988;95:52–62.
Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 1989;257:G748–59.
Jacob P, Kahrilas PJ, Lagemann JA, Shah V, Ha T. Upper esophageal sphinicter opening and modulation during swallowing. Gastroenterol. 1989;97:1469–78.
Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol. 1992;25:G621–8.
Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting and swallowing. Am J Physiol Gastrointest Liver Physiol. 2002;283:G529–36.
Crary MA, Carnaby GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. JSLHR. 2006;49:186–93.
Cook IJ, Dodds WJ, Dantas RO, et al. Timing of video fluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4:8–15.
Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, Cook IJ, Lang IM. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol Gastrointest Liver Physiol. 1990;258:G675–81.
Ertekin C, Pehlivan M, Aydogdu I, et al. An electrophysiological investigation of deglutition in man. Muscle Nerv. 1995;18:1177–86.
Ertekin C, Aydogdu I. Electromyography of human cripharyngeal muscle of the upper esophageal sphincter—a review. Muscle Nerv. 2002;26:729–39.
Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume of swallow-induced hyoid movement in normal subjects. AJR. 1988;150:1307–9.
Lang IM, Dantas RO, Cook IJ, Dodds WJ. Videoradiographic, manometric and electromyographic assessment of the canine upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 1991;260:G911–9.
Ertekin C, Aydogdu I, Yűceyar N. Piecemeal deglutition and dysphagia limit in normal subjects and in patients with swallowing disorders. J Neurol Neurosurg Psychiatry. 1996;6:491–6.
Aydogdu I, Kiylioglu N, Tarlaci S, Tanriverdi Z, Alpaydin S, Acarer A, Baysal L, Arpaci E, Yuceyar N, Secil Y, Ozdemirkiran T, Ertekin C. Diagnostic value of “dysphagia limit” for neurogenic dysphagia: 17 years of experience in 1278 adults. Clin Neurophysiol. 2015;126:634–43.
Hammer MJ, Jones CA, Mielens JD, Kim CH, McCulloch TM. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia. 2014;29:564–70.
Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Ann Otol Rhinol Laryngol. 2014;123:174–82.
Jones CA, Cuicci MR, Hammer MJ, McCulloch TM. A mutisensor approach to improve manometric analysis of the upper esophageal sphincter. Laryngoscope. 2015. doi:10.1002/lary.25506.
Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Degluttitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291:G525–31.
Omari TI, Jones CA, Hammer MJ, Cock C, Dinning PG, Wiklendt L, Costa MC, McCulloch TM. Predicting the activation states of the muscles governing upper esophageal sphincter relaxation and opening. Am J Physiol Gastrointest Liver Physiol. 2016. doi:10.1152/ajpgi.00388.2015.
Omari TI, Wiklendt L, Dinning P, Costa M, Rommel N, Cock C. Upper esophageal sphincter mechanical states analysis: a novel methodology to describe UES relaxation and opening. Front Syst Neurosci. 2015;8:241. doi:10.3389/fnsys.2014.00241 eCollection 2014.
Kim HJ, Mittal RK, Patel N, Ledgerwood M, Bhargava V. Esophageal distention during bolus transport: can it be detected by intraluminal impedance recordings? Neurogastroenterol Motil. 2014;26:1122–30.
Cock C, Besanko L, Kritas S, et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterol Motil. 2015. doi:10.1111/nmo.12714.
Omari TI, Dejeager E, Van Beckevoort D, Goeleven A, De Cock P, Hoffmen I, Smet MH, Davidson GP, Tack J, Rommel N. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol. 2011;106:1796–802.
Omari TI, Savilampi J, Kokkinn K, Schar M, Lamvik K, Doeltgen S, Cock C. The reliability of pharyngeal high-resolution manometry with impedance and derivation of measures of swallowing function. Int J Otolaryngol. 2016. doi:10.1155/2016/2718482.
Weijenborg PW, Kessing BF, Smout AJPM, Bredenoord AJ. Normal values for solid-state esophageal high-resolution manometry in a European population; an overview of all current metrics. Neurogastroenterol Motil. 2014;26:654–9.
Nagai T. The occurrence and ultrastructure of a mechanoreceptor in the human cricopharyngeus muscle. Eur Arch Otorhinolaryngol. 1991;248:144–6.
Shaker R, Ren J, Podvrsan B, Dodds WJ, Hogan WJ, Kern M, Hoffmann R, Hintz J. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol Gastrointest Liver Physiol. 1993;264:G427–32.
Jean A, Dellaporta M. Brainstem control of deglutition: swallowing pattern generator. In: Shaker R, Belafsky PC, Postma GN, Easterling C, editors. Principles of deglutition. New York: Springer; 2013.
Gokyigit MC, Pazarci NK, Ercan I, Seker S, Turgut S, Ertekin C. Identification of distinct swallowing patterns for different bolus volumes. Clin Neurophysiol. 2009;120:1750–4.
Acknowledgments
C Cock supported by a grant from the Repat foundation. TM McCulloch and CA Jones supported by NIH Grants DC011130/T32 GM007507. MJ Hammer supported by NIH Grants DC010900/DC014519. TI Omari supported by an NHMRI fellowship. TI Omari has a patent on AIMplot analysis software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author TO owns a patent on AIMplot software. Other authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Cock, C., Jones, C.A., Hammer, M.J. et al. Modulation of Upper Esophageal Sphincter (UES) Relaxation and Opening During Volume Swallowing. Dysphagia 32, 216–224 (2017). https://doi.org/10.1007/s00455-016-9744-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-016-9744-4