Abstract
Employing aerobic fermentation, Gram-negative bacteria belonging to the genus Xanthomonas produce the high molecular weight natural heteropolysaccharide known as xanthan. It has various amounts of O-acetyl and pyruvyl residues together with D-glucosyl, D-mannosyl, and D-glucuronyl acid residues in a molar ratio of 2:2:1. The unique structure of xanthan allowed its various applications in a wide range of industries such as the food industry, pharmacology, cosmetics and enhanced oil recovery primarily in petroleum. The cultivation medium used in the manufacture of this biopolymer is critical. Many attempts have been undertaken to generate xanthan gum from agro-based and food industry wastes since producing xanthan gum from synthetic media is expensive. Optimal composition and processing parameters must also be considered to achieve an economically viable manufacturing process. There have been several attempts to adjust the nutrient content and feeding method, temperature, pH, agitation and the use of antifoam in xanthan fermentations. Various modifications in technological approaches have been applied to enhance its physicochemical properties which showed significant improvement in the area studied. This review describes the biosynthesis production of xanthan with an emphasis on the importance of the upstream processes involving medium, processing parameters, and other factors that significantly contributed to the final application of this precious polysaccharide.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbohydrate polymers or polysaccharides can be produced via plants [1], parasites, algae [2] and bacterial fermentation [3]. It has many unique properties, both chemical and physical [4]. Polysaccharides are used by plants as structural materials, energy stores, adhesives and information transmission agents [5]. For microbes, exopolysaccharide (EPS) helps withstand environmental stresses [6] and energy storage [7]. Microbial polysaccharides consist of repeating units of simple sugars with groups of 10 or fewer in which each group of 3–7 monosaccharides is connected [8]. Often these polysaccharides are called slime, or EPS [9]. The chemical compound is secreted through a fermentation cycle involving the conversion of carbon into a medium known as EPS by the different types of micro-bacteria. Compared to synthetic polymers, polysaccharides produced from microbes are biodegradable and environmentally friendly [10]. The combination of carbohydrates found in microbial EPS was reported to be incredibly diverse, although most of the sugars are commonly found in animals and plants [11]. Microbial EPS are primarily ionic, non-ionic and linear molecules that are bound at regular intervals to side chains of varying lengths and complexity [12]. EPS can be classified into two types which are homo-polysaccharides and hetero-polysaccharides [13]. Homo-polysaccharides are formed by monosaccharides such as dextran. Whereas hetero-polysaccharides are complex structures formed by the synthesis in cells that involve many monosaccharides, such as xanthan and gellan.
A number of these microbes have the ability to produce a valuable chemical that finds diverse applications in areas such as food additives, pharmaceuticals, oil recovery and more. Xanthan, pullulan, levan, kefiran and gellan are among the most extensively researched and significant microbial polysaccharides [14,15,16,17,18]. Xanthan, a microbial polysaccharide, holds great commercial significance owing to its manufacturing volume and versatile range of uses [19]. Xanthan has drawn a lot of interest because of its exceptional qualities, including being safe for pharmaceutical and food uses and not poisonous, sensitizing, or irritating to the eye including skin [10]. The European Economic Community has authorized xanthan as a food emulsifier or stabilizer list, E-415, and the United States Food and Drug Administration (FDA) has cleared it for use as a food additive without specifying a quantity [20, 21].
Some of the crucial factors in producing EPS are the availability of carbon and nitrogen sources [22]. The optimal carbon-to-nitrogen ratio is important as carbon increases xanthan production. It is generally known that increased xanthan gum synthesis and accumulation take place in nitrogen-limited environments and higher C/N ratios [23]. The excess amount of nitrogen will trigger the degrading enzymes in which it is unwanted [24]. To obtain a high purity of xanthan products or polysaccharides, further operations such as re-precipitation, deproteinization utilizing chemical or physical approaches, and a variety of membrane techniques are necessary [25]. Traditionally, xanthan is produced using glucose or sucrose as a carbon source, which requires high energy input and contributes to the depletion of fossil fuels. Xanthan can also be produced using agricultural waste as a carbon source, which has the potential to increase sustainability and contribute to the circular economy. Agricultural wastes are rich in cellulose and hemicellulose, which can be hydrolyzed into simple sugars for xanthan production. The use of agricultural waste as a carbon source for xanthan production can reduce the dependency on non-renewable resources, mitigate greenhouse gas emissions and promote sustainable agricultural practices. Xanthan production using agricultural waste can contribute to the circular economy by creating value from waste streams. The use of agricultural waste for xanthan production can increase sustainability and promote the circular economy. The conversion of agricultural waste into xanthan can provide an additional revenue stream for farmers and food processors, as well as reduce the amount of waste sent to landfills. It is a promising approach that can benefit various industries while also contributing to environmental protection and economic development. The wide range of xanthan applications indicates the promise of these substances for industries and the significance of additional research to better understand their manufacturing based on their chemical and physical characteristics.
Xanthomonas campestris
Different polysaccharides with biological activity have been isolated in recent years from a variety of fungi and are categorized according to where they came from. Xanthomonas campestris pv. graminis [26], Xanthomonas campestris pv. mangiferaeindicae [27], Xanthomonas campestris pv. pelagornii [28] and Xanthomonas campestris pv. campestris [29] were among the Xanthomonas species that were successfully discovered. Pv stands for pathovar, which is defined as "a group of strains with the same or comparable traits, distinguished at the infra subspecific level from other strains of the same species or subspecies based on initial pathogenicity to one or more plant hosts" [30]. In different words, Pv. means pathovar, a type of host plant classification that Xanthomonas campestris attacks.
Characteristics of xanthan
Xanthomonas campestris produces xanthan, an EPS, on a chemically specified, semi-synthetic, or entirely complex substrate. It was established in 1963 at the United States Department of Agriculture's (USDA) National Center for Agricultural Utilization Research [31]. It is a secondary metabolite and the by-product of aerobic development, and because of its significant physicochemical characteristics, it is employed as a suspending, emulsifying and thickening agent. It has strong shear stability, pseudo-plastic properties, and can withstand a variety of pH levels and temperatures. Xanthomonas campestris, which is categorised as a heteropolysaccharide, produces xanthan by using carbohydrates as the main sources of pure fermentation. Other Xanthomonas species, including Xanthomonas campestris [32], Xanthomonas pelargonii, Xanthomonas phaseoli and Xanthomonas malvacearum [33], are employed to create xanthan during aerobic fermentation. Figure 1 illustrates the chemical composition of xanthan, which mostly consists of repeated units of a pentasaccharide composed of two glucose units, two mannose units and one glucuronic acid unit with a molar ratio of 2.8:2.0:2.0. The molar mass of xanthan is influenced significantly by the fermentation process and the characteristics of the bacteria used which are significantly higher than 2.0 × 106 g/mol [34].
Chemical structure of xanthan [35]
Xanthan has a unique characteristic whereby a low concentration of its application in solution would show a high degree of viscosity compared to another polysaccharide. Owing to this property make xanthan a perfect thickener and food stabilizer. Xanthan is used to give fruit pulps and beverages a better texture and appearance as its suspension is maintained and a small application of xanthan in sauces would provide the food with high viscosity [35]. Due to its pseudoplastic behavior, xanthan improves the sensory quality of the final product, good pourability and eases the processing [36]. Xanthan powder is dissolved in cold and hot water but is insoluble in most organic solvents.
In the biopharmaceutical industry, xanthan has been adopted into the drug as it is considered safe due to its biodegradability [37, 38], abundant availability, non-toxicity and low cost. It has a remarkable performance as a drug delivery carrier [39]. Apart from its pseudoplasticity, xanthan has found application in enhanced oil recovery (EOR) as well. In this process, it is essential to consider properties such as solubility, viscoelasticity, adsorption [40, 41], thickening ability, salt-resistance and temperature stability of xanthan. The suitability of xanthan for the process needs to be assessed and screened as variations in the oil reservoir can affect the criteria [42].
The production of xanthan can be achieved through various methods, ranging from laboratory scale to complex industrial processes. It all begins with the selection of a suitable substrate to create a medium. Researchers have explored the use of diverse waste materials, including food and agricultural waste, as a carbon source to produce xanthan. Food waste, in particular, offers a high-value substrate and is viewed as a potential source of raw materials for xanthan production [43]. This readily biodegradable waste starchy food can be a good choice for carbon resources [44]. The production of xanthan will have an option in terms of the type of substrate used in the fermentation medium.
Biosynthesis of xanthan
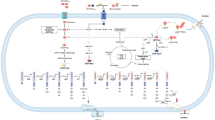
The process of EPS biosynthesis involves multiple steps, such as the synthesis of nucleotide sugar precursors, oligosaccharide repeater units and the direct synthesis of polysaccharides via sequential or progressive glycosyltransferase activity. Once synthesized, the polysaccharides are assembled from the repeater units and exported as the final product [45]. Figure 2 illustrates that the biosynthesis of xanthan, a widely studied EPS, follows a similar trend as Klebsiella pneumonia and other bacterial species, as highlighted in several studies [46].
Biosynthesis pathways of xanthan gum production [46]
Glucose serves as the starting material for the metabolic process involved in xanthan biosynthesis. This process leads to the production of sugar nucleotide precursors, such as UDP-glucose, UDP-glucuronate and GDP-mannose, which are crucial for the construction of the pentasaccharide repeat unit [47]. Xanthan production is linked to the primary carbohydrate metabolism, whereby pentasaccharide repeat units are constructed on undecaprenyl phosphate lipid carriers tethered in the cytoplasmic membrane. Specific glycosyltransferases sequentially transfer sugar moieties from the nucleotide sugar xanthan precursors onto the lipid carriers. Acetyl and pyruvyl residues may also be added as non-carbohydrate embellishments. Mature repeat units are polymerized and exported, following a similar WZY-dependent polysaccharide synthesis pathway as seen in Enterobacteriaceae. The gene cluster responsible for xanthan biosynthesis produces, synthesizes, polymerizes and exports the repeat units.
Researchers have shown a growing interest in the potential of the derivative xanthan in recent studies. Native xanthan has limitations in certain applications due to its poor thermal and mechanical properties, susceptibility to bacterial contamination and inadequate water solubility [42]. Previous scientific studies have explored various chemical modifications of xanthan, including etherification, esterification [48,49,50,51, 51, 52], peptide linkage acetylation, oxidation [53, 54], ionic and covalent crosslinking and mechanical modification [55]. Chemical derivatives resulting from these modifications are generally considered acceptable for use in a variety of applications [56].
Industrial production of xanthan
Xanthan is an industrially produced EPS by fermentation using simple sugars such as sucrose and glucose [57, 58]. The production of xanthan involves the preparation of medium components and inoculum-containing bacteria that will undergo several processes until the production of the final product. Carbon is one of the main ingredients in xanthan production that is obtained through inverted sugar or glucose fermentation. In the production of xanthan, nitrogen sources such as peptone, ammonium, soybeans and yeast extract are also important [59].
In the industrial production of xanthan, it is crucial to sterilize the bioreactors used and closely monitor the fermentation process. Process conditions such as temperature (28–32 ºC) and solution pH (6.5–7.5) must be carefully regulated and batch processing is commonly used. The specific agitation power, typically in the range of 1 kW/m3 or higher, is considered favorable [60]. The supply of oxygen is a critical factor affecting the productivity and concentration of xanthan. An aeration rate greater than 0.3 vvm is recommended [61]. The specific process conditions required may vary depending on the selected strain.
Factors affecting xanthan production
Figure 3 illustrates that medium compositions and processing parameters are the two key factors that impact xanthan production. The medium composition includes two macronutrients, carbon and nitrogen sources, which are involved in xanthan production, while micronutrients such as magnesium, phosphate, potassium and iron can stimulate growth and EPS formation. Processing parameters such as temperature, pH, agitation rate, incubation period and dissolved oxygen are also significant in xanthan synthesis.
Medium composition
The production process of xanthan is greatly affected by the composition of the growth medium [62, 63]. The aim is to develop a medium that can provide the necessary chemical and organic nutrients during the fermentation process, while also standardizing the assembly process to ensure high-quality xanthan production and lower production costs [59]. Xanthan production by Xanthomonas campestris requires macronutrients such as carbon and nitrogen, as well as micronutrients including potassium, phosphate and calcium. Xanthomonas campestris bacteria required a carbon source as a substrate for xanthan production. The employment of various substrates will affect the provision of nutrients for the expansion of the Xanthomonas campestris bacteria. Different substrates affect the structure of the side chain chemical clusters of xanthan but do not change its main structure [64]. The fermentation time of xanthan production would be dependent on the type of substrate used [61]. There are many different types of medium formulations used to produce xanthan. Table 1 shows different types of medium formulations used by previous researchers to produce xanthan.
Carbon sources
Carbon sources play a key role in the fermentation process of xanthan microbes as they act as catalysts for the transfer of sugar to the cells. The glucose concentration was found to have a significant impact on the development of xanthan [44]. In microbial fermentation, the carbon source is a crucial aspect in the formation of cellular structures and also provides an energy source to the microbial [71] and is used in polysaccharide synthesis. The quantity of sugar in the fermentation broth is also significant during secondary metabolites of bacteria, which result in the synthesis of extracellular heteropolysaccharides [4].
Over the years, researchers have focused on finding inexpensive sources of carbon to produce xanthan, leading to the use of various sources such as plant sources and waste products [72]. Xanthomonas species can ferment glucose, sucrose, or lactose to produce xanthan, and it can also be produced from sources such as sugar molasses, cassava starch and crude glycerin, as shown in Table 2. The xanthan polysaccharide is precipitated with isopropyl alcohol or ethanol from the growth medium, then dried and ground into a fine, off-white powder before packaging.
One potential substrate for xanthan production is pineapple waste, which has a high content of biodegradable organic material and suspended particles, as well as significant BOD and low pH levels. The primary nutrients in pineapple juice waste are sucrose, glucose and fructose. This waste can be characterized and recycled to reduce waste or produce higher value-added products such as organic acid, methane, ethanol, SCP and enzymes. Sugar analysis has shown that the main sugars present in liquid waste are sucrose, glucose and fructose [86, 87]. In a study by [88], Lactobacillus delbruckii was used to produce lactic acid using liquid and solid pineapple waste as a substituted carbon source, resulting in a lactic acid production of 54.3 g/L. Pineapple waste can be considered as a valuable alternative carbon source for the production of biodegradable polymers.
The quantity and type of carbon source significantly impact the growth and development of these microbes, which ultimately affects the yield and quality of xanthan. In recent years, researchers have focused on finding cost-effective and sustainable sources of carbon, such as plant sources and waste products. The study of carbon sources in microbial fermentation is crucial for optimizing the production of valuable products and reducing waste generation.
Nitrogen sources
One of the vital nutrients in cell growth and biosynthesis of polysaccharides is nitrogen source. There have been numerous documented facts about nitrogen as a source of energy. One study by [89] revealed the effectiveness of the different types of organic and inorganic nitrogen sources comprise of urea, peptone, potassium nitrate, yeast extract and ammonium sulfate. About 20 g/L of hydrolyzed cassava bagasse was used to produce xanthan using a bacterial culture of Xanthomonas campestris and the inclusion of potassium nitrate as nitrogen in the medium. The xanthan obtained after 72 h of fermentation is about 14 g/L and no significant changes until the fermentation process ends (i.e., 96 h). For xanthan growth, ammonium sulfate was the least effective and affected the use of sugar by the bacterial culture.
Another investigation was carried out on the impact of several organic and inorganic nitrogen sources that were fermented by Xanthomonas campestris E-NRC-3 [90]. The findings demonstrated that the use of complex organic and affordable sources of nitrogen including peptone, yeast extract and maize steep liquor boosted the efficiency of Xanthomonas campestris E-NRC-3 in the manufacture of xanthan. After 5 days of incubation, several inorganic nitrogen sources, including sodium nitrate, ammonium chloride and urea (600 mg/L), showed distinct impacts on the effectiveness of xanthan synthesis by Xanthomonas campestris E-NRC-3. Among the aforementioned nitrogen sources, ammonium chloride has the best efficiency. The addition of 2400 mg ammonium chloride increases the synthesis of xanthan up to 66.3 g/L.
The addition of amino acids, such as glutamate and nitrate, has been shown to increase the yield of xanthan, as reported by [91]. It should be noted that high concentrations of these nitrogen sources can actually inhibit the production of xanthan. This is because rapidly growing cells require high levels of nitrogen, which can also affect the rheological properties of the resulting xanthan when the nitrogen content in the medium is excessive. Media with a high carbon content and a low nitrogen content are more favorable for xanthan polymer accumulation. It can be concluded that nitrogen sources are vital for cell growth and biosynthesis of xanthan. Organic and inorganic nitrogen sources were investigated and it was found that potassium nitrate was effective in producing xanthan using a bacterial culture of Xanthomonas campestris. Ammonium sulfate was the least effective and affected the use of sugar by the bacterial culture. The use of complex organic and affordable sources of nitrogen, including peptone, yeast extract and maize steep liquor, boosted the efficiency of cells in xanthan production. Inorganic nitrogen sources, such as sodium nitrate, ammonium chloride and urea, also had distinct impacts on xanthan synthesis. The addition of amino acids such as glutamate and nitrate stimulated the yield of xanthan, but higher concentrations inhibited production and affected rheological properties. Media with high carbon content and low nitrogen content favor polymer accumulation.
pH condition
Most of the findings regarding the pH variables during the process have reported that the optimised value of pH is within 6–8 for the cultivation of Xanthomonas campestris before the production of xanthan. These pH conditions did not affect the process to produce xanthan. Previous research by [92] stated that the pH values of xanthan fermentation broth increased from 7.0 to 10.0 based on the agitation and temperature used. In the study conducted by [93], production and certain rheological, thermal and emulsifying properties of xanthan have been affected by the effects of alkali stress using a sodium hydroxide solution with a pH value of 11.
In the other finding by [94], they observed that the pH value declined significantly during batch fermentation of xanthan. Since the pH culture has only been controlled without the use of any controlling unit, the accumulation of organic acids in the growth media. As a result, the organic acids can adversely affect the production of xanthan. It's highly recommended to use a pH controller during the process. Xanthan development of Xanthomonas campestris ATCC 1395 under uncontrolled pH conditions at different stirring speeds was evaluated in another analysis by [95]. Uncontrolled pH conditions during processing were observed to result in low output rates of approximately 6.5 g/L at 600 rpm. It can be noted that pH plays a key role in certain EPS development and all these findings indicate that indirect regulation of the pH medium may affect the yield of xanthan.
Temperature
The temperature during the fermentation process plays a crucial role in xanthan production. Maintaining the optimum temperature range is crucial to achieve high-quality xanthan [96]. Studies suggest that the optimal temperature for fermentation is around 28 °C [78, 97]. According to [79], temperatures below 24 °C trigger secondary metabolism, leading to a significant slowdown in xanthan production. Temperatures above 27 °C promote xanthan biosynthesis from the exponential phase to the stationary phase. At 35 °C, the specific growth rate was almost zero, indicating that sustained cell growth is not possible at this temperature.
Other experiments showed that the optimal temperature condition during the process was 30 °C [23]. In comparison, lowering the temperature from 37 to 30 °C can result in higher xanthan production yields. The temperature became an important element in the fermentation of Xanthomonas campestris. It also is related to the activation of enzymes involved in the xanthan polymerization process [98]. The published research by [99] also supports the idea that decreasing the temperature will increase the production of xanthan when carbon is used as an energy source. To conclude, the best optimal temperature for xanthan production is between 28 °C to 30 °C. Maintaining an optimum process temperature is important for producing high xanthan.
Dissolved oxygen
The availability of oxygen is crucial for the bacteria to function effectively during the aerobic fermentation process [100]. To fulfill the oxygen requirement in the fermentation process, it is important to ensure adequate oxygen transfer. A new bioreactor design may be required to address the limitation in oxygen transfer. The design strategy could be based on the use of a porous fibrous matrix to facilitate the free movement of culture medium and air, thereby enhancing the interaction between cells, support of the fibrous matrix and nutrients. This overall approach is intended to improve oxygen transport and may increase the reaction rate while simultaneously reducing the rapid growth of mutants [101].
Several studies have been conducted to study the effect of oxygen concentration on the synthesis of xanthan. According to [102], the decrease in aeration rates induced an increase in glucose and mannose concentrations during xanthan production. Increasing airflow contributes to the high viscosity of xanthan and biomass, as well as xanthan with strong thermal degradation properties. A study report by [103] states that the production of polysaccharides creates a high-viscosity medium, which ultimately results in an oxygen transfer constraint affecting the production of xanthan. The viscosity of polysaccharides is also correlated with pyruvate content and molecular weight. In a study by [104], the impact of varying oxygen rates (0.75 to 1.5) on the pyruvilation degree was investigated and no significant differences were observed. Increasing agitation and supplying sufficient oxygen to the broth resulted in increased molecular weight of xanthan, indicating that the availability of oxygen can affect the physicochemical properties of the polysaccharide [105].
Different strategies for improving the transfer of oxygen in bioreactors have been implemented. Some previous researchers have applied an approach to disperse a liquid phase from organic compounds such as vegetable oils [106, 107] and hydrocarbons [108], later known as the organic phase, which is immiscible to the system. This approach allows more oxygen gas to be carried through the modified medium in the presence of organic phases [109]. This phase increases the rate of oxygen solubility in water because the organic phase has a strong affinity and was successfully implemented in the past [110]. Kuttuva et al. [111] have used this technique in the fermentation of xanthan and have found that the rate of oxygen flow to the broth increases. The limited transfer of oxygen in traditional bioreactor designs has led to a need for a new design that allows for closer interaction between cells, support and nutrients to improve oxygen transport and increase the reaction rate. Increasing airflow can contribute to higher viscosity and thermal degradation properties, while high viscosity medium can result in an oxygen transfer constraint. Different strategies for improving oxygen transfer in bioreactors have been implemented, including the use of organic compounds, which have been shown to increase the rate of oxygen flow to the broth in xanthan fermentation.
Incubation time
The incubation period also provides insight into the overall process of xanthan production. Each of the microorganisms would react to several factors during the process. The output of biopolymer for a period of 96 h seems to be insignificant in one research conducted by [67]. They discovered that there is no discernible change in the number of sugar components in all examined mediums because microorganism cells employ carbon as a source of energy, proliferation and xanthan production. Theoretically, even though the culture time was decreased to 24 h, it was possible to see that there was no negative influence on the intended product yield.
Some researchers have documented an increase in acetate radical concentration throughout fermentation [112]. This effect on the degree of pyruvilation is not sufficiently clear; according to several studies, the radical content of pyruvate increases over time [113, 114] but other authors have reported that when the growth of the cell reaches the stagnant phase, this concentration achieves a stable value [104].
Agitation rate
Many studies conducted on xanthan production highlighted the importance of the agitation rate during the fermentation process. Instead of independent factors such as the source of carbon source, nitrogen source and phosphorus, the agitation rate in submerged fermentation was also assessed for its effects on biomass and xanthan production. Xanthomonas campestris is an aerobic microorganism and the agitation rate is very important in the production of xanthan gum [115]. Zakeri et al. [116] reported that the maximal yield of xanthan was derived using an agitation rate of 500 rpm. In another study conducted by [117], the optimum conditions for maximal xanthan production were using an agitation rate of 394.8 rpm. Reconfirmation testing was performed for xanthan synthesis and the experimental value at ideal conditions was around 6.72 g/L, which was similar to the predicted values of 6.51 g/L. An agitation rate of 387.4 rpm was employed to increase the biomass output. Other studies that reported similar results showed that 500 rpm was the best for xanthan production [94]. Moshaf et al. [117] reported that an agitation rate of 394.8 g/L was the best for xanthan production. From this, we can conclude that a high agitation rate is favorable for the maximal production of xanthan. The viscosity of the culture medium has an impact on the aeration rate, nutrient distribution and uniformity. As the viscosity of the medium increases, the aeration rate decreases, affecting the nutrient distribution and uniformity [61]. Xanthan synthesis can lead to extracellular deposition, reducing the oxygen mass transfer rate [20]. The agitation rate is crucial during the fermentation process to maximize xanthan production. A high agitation rate of up to 500 rpm can enhance oxygen mass transfer, maintain nutrient distribution and uniformity, and prevent a decrease in the aeration rate caused by the rise in viscosity.
Stress condition
When fermentation is performed, the bacteria in the solution will be exposed to some type of stress. This stress can occur physically, chemically, or biologically. Bacteria will respond to various stresses such as pH, temperature, nutrient limitation and osmotic stress for survival. During the process of cell growth, the concentration of solute in the cell will maintain the flow of water for metabolism. To continue to survive, bacteria will produce biopolymers products that can protect cells from physical conditions, help improve the cell's ability to bind, form symbiotic relationships and create biofilms [118].
One study conducted by [16] on the production of kefiran stated that polysaccharide production was affected when NaCl was added at different concentrations during the fermentation process. Sheng et al. [119] studied the effects of NaCl and CaCl2 on the photosynthetic bacteria Rhodopseudomonas acidophila, which produced water-soluble EPS via anaerobic mechanisms. The results showed that high NaCl concentrations resulted in higher EPS, whereas CaCl2 concentration had little or no effect on EPS generation.
Xanthan production by Xanthomonas campestris B82 is influenced by acetic acid [120]. During the exponential and stationary phases of growth, acetic acid doses ranging from 1.56 to 6.25 mM were added to the medium. Quantitative investigations demonstrated a significant increase in xanthan production and viscosity following two cycles of acetic acid addition at 24 and 26 h of fermentation. EPS production in Pseudomonas putida was found to be influenced by acidic and alkaline environments, as demonstrated by [121], while [122] showed similar findings for Xanthomonas campestris during alkali stress conditions. This behavior can be utilized to enhance xanthan gum production.
An alkaline condition study by [93] showed that the production of xanthan was increased by up to 133.80% using sucrose as the main carbon. Even if the mixture of sucrose and crude glycerol has been applied in the medium, it will somehow reach a higher percentage of xanthan production (164.86%). Little information is available about the impact on yield and, mainly, on its technological properties. Bacteria are exposed to physical, chemical and biological stress during the fermentation process which can affect cell growth and xanthan production. To protect themselves, cells produce biopolymers like polysaccharides that improve their ability to bind, form symbiotic relationships and create biofilms. NaCl can increase EPS production in some bacteria, while acetic acid can increase xanthan gum production. Alkaline conditions have also been found to increase xanthan production, particularly when using sucrose as the main carbon source. More research is needed to fully understand the impact of osmotic stress on cell growth and xanthan production.
Applications of xanthan
Xanthan was a promising EPS among the others in a way that it is improving some of the properties or characteristics of added materials. Xanthan gum provides the texture, rheological qualities, taste release, appearance and moisturizing properties that are currently required by food products [123]. In the food industries, a wide range of products such as tea, fruit pulp and powdered drinks, chocolate late, cookies, jellies, dairy goods, margarine, yogurt, baking products, packaged foods sauces and gravel all contain xanthan as an emulsifier and thickener. In the packaging industry, it was reported that xanthan has the potential to improve banana preservatives in methylcellulose/xanthan novel composite film [124]. Banana shelf life has qualitatively improved with XH composite film for food preservation.
In addition to its use as a gelling agent, stabilizing agent, suspending agent and viscosity-enhancing agent, xanthan has also been extensively investigated as a possible polymeric material in various floating drug delivery technologies [125]. Xanthan has been utilized as a gelling agent, binder and disintegrant in pharmaceutical formulations for various drugs, such as rosiglitazone maleate [126] and acyclovir [127]. One of the primary applications of xanthan in pharmaceuticals is drug release. Controlled release of drugs is crucial for enhancing the effectiveness of drug delivery to the targeted location while minimizing adverse effects. Due to its gelling properties, xanthan can reduce the rate of drug release and can also trap the substance inside the gel matrix [128]. Carboxymethylated xanthan has a lower viscosity than xanthan and enhances the rate of drug delivery [129].
In recent years, xanthan has become a promising ingredient in the cosmeceutical industry. According to [130], xanthan has been used in toothpaste, creams, lotions, shower gel, shampoo, sunscreens and skincare. In cosmetics, xanthan is commonly used as a thickener, binding agent, emulsifier, stabilizer, suspending agent and controlled release agent. For example, in toothpaste, xanthan gum provides the shape and consistency of the toothpaste texture to make it easier to pump or squeeze. In lotion or creams, it helps in adjusting the viscosity to make the cream and lotion perform their shear-thinning properties. While in shampoos, xanthan acts as a stabilizer to suspend insoluble particles such as pigments and active ingredients [131].
Xanthan has potential applications in construction engineering to improve soil properties such as aggregate strength, erosion resistance and stability. Research has shown that xanthan can effectively reinforce high-graded soil, with the reinforcing effect being dependent on soil moisture conditions. Xanthan fibers interact with charged clay surfaces to form xanthan matrices, which behave like hard plastic between uncharged particles. This environmentally friendly product is being considered as an alternative to traditional methods for enhancing soil strength [132].
Xanthan is also widely used in the energy industry for oil extraction and output lubrication in the drilling of mud. Xanthan is an excellent suspension agent for the extraction of drilling rock cuttings; it is also compatible with barytes used to resolve strong reservoir pressures [133]. Xanthan solutions have also been introduced to boost the recovery of oil from depleted rivers by injecting solutions containing viscosifiers [134]. To enhance oil recovery and stabilize oil production, Liang et al. [131] have developed a biopolymer of xanthan that can be used under conditions of high temperature (80 °C) and high salt concentrations of 170,000 ppm. They use the xanthan flooding process that could efficiently change the wettability of oil in the reservoir and can also help improve the performance of oil recovery. In recent years, de Souza [135] has studied the thermoviscosifying behavior of produced water xanthan (PWX) and its potential to adapt to harsh conditions inside reservoirs. The PWX has been shown to increase oil recovery during the late injection process (tertiary recovery) by 9% compared to commercial xanthan gum, making it an environmentally friendly ex-situ microbial-EOR metabolite. Figure 4 summarizes the various industrial applications of xanthan.
The use of heavy metal-based wastewater treatment catalysts can result in secondary pollution, making it essential to find more environmentally friendly alternatives [136,137,138]. Xanthan, with its numerous hydroxyl groups, can serve as a site for the graft copolymerization of synthetic monomers [139]. Understanding the mechanism of graft copolymerization is crucial in developing new materials with desirable properties. Graft copolymerization induces physical, chemical, thermal and morphological changes that can enhance the adsorption of hazardous heavy metals and artificial dyes from wastewater and other industrial effluents. This approach presents a promising strategy for developing effective and sustainable wastewater treatment methods.
Due to xanthan’s unique rheological properties, it is used extensively in the food, pharmaceutical and petroleum industries. The ability of xanthan to form stable suspensions, gels and emulsions makes it a versatile ingredient in many applications. Despite its widespread use, the production of xanthan remains complex and expensive due to the difficulty in cultivating the producing microorganisms and the limited knowledge of the biosynthesis pathway. To meet the growing demand for xanthan, it is important to understand the biosynthesis process and the characteristics of xanthan. The biosynthesis pathway of xanthan has been extensively studied, but there is still much to be learned about the genetic regulation of xanthan production and the metabolic pathway involved. Advances in biotechnology and genetic engineering may provide new strategies to improve xanthan production and tailor xanthan characteristics to meet specific industrial needs. The characterization of xanthan is also crucial for its successful application. Factors such as molecular weight, degree of acetylation and conformational properties can affect the rheological properties of xanthan. Understanding these properties can aid in the selection of the appropriate xanthan for specific applications and can lead to the development of new applications. A better understanding of the biosynthesis pathway and the characterization of xanthan will provide opportunities for the development of new strategies for xanthan production and the tailoring of xanthan characteristics to meet specific industrial needs.
Future outlook
The review on biosynthesis, production and xanthan applications provides an overview of the current knowledge of xanthan gum, its production and potential applications. Based on the findings of this review, several future directions can be suggested to further advance the field. More research is needed to explore the potential of novel microbial strains for xanthan production. The isolation and characterization of new strains can lead to the development of more efficient and cost-effective xanthan production processes. Furthermore, genetic engineering of microbial strains to increase the production of xanthan or modify its properties can also be explored. The development of new fermentation techniques and bioreactors can improve xanthan production. The optimization of fermentation parameters such as temperature, pH and nutrient supplementation can further enhance xanthan production. The use of advanced monitoring and control systems can also improve the efficiency and consistency of the fermentation process.
The development of new applications for xanthan gum can further expand its commercial potential. Xanthan gum is primarily used as a thickening and stabilizing agent in the food and pharmaceutical industries. Research can be conducted to explore its potential applications in other areas such as cosmetics, agriculture and environmental remediation. In addition to that, sustainability and environmental impact should be considered in the future development of xanthan production and applications. Efforts can be made to reduce the use of fossil fuels and develop more sustainable and environmentally friendly production processes [141]. The potential environmental impact of xanthan gum use should be evaluated and addressed. In general, the review on biosynthesis, production and xanthan applications has highlighted the potential of xanthan gum as a versatile and valuable biopolymer. To further advance the field, future research should focus on exploring new microbial strains, optimizing fermentation techniques, developing new applications and addressing sustainability and environmental concerns.
Conclusion
Xanthan is an EPS produced through submerged fermentation by Xanthomonas sp. strains. The production process includes upstream processes, such as cell banking and fermentation and downstream processing, including cell separation, recovery and product purification. Several important factors influence the final yield of xanthan, including medium components, bacterial strains, pH, temperature, dissolved oxygen, agitation, incubation time, agitation rate and stress conditions. Xanthan's unique properties make it suitable for a variety of applications, ranging from food to the construction industry. In the food industry, it is widely used as an emulsifier, thickener, gelling agent, fat substitute and food packaging material. There is potential for new applications of xanthan and further research is necessary to uncover them. Scaling up xanthan production with high yield and purity is essential to maximize its potential for broader applications on a larger scale.
Data availability
Data will be made available on request.
References
Cheah KW, Yusup S, Chuah LF, Bokhari A (2016) Physio-chemical studies of locally sourced non-edible oil: prospective feedstock for renewable diesel production in Malaysia. Proc Eng 148:451–458. https://doi.org/10.1016/j.proeng.2016.06.460
Khanra A, Vasistha S, Rai MP, Cheah WY, Khoo KS, Chew KW, Chuah LF, Show PL (2022) Green bioprocessing and applications of microalgae-derived biopolymers as a renewable feedstock: Circular bioeconomy approach. Environ. Technol Innov 28:102872. https://doi.org/10.1016/j.eti.2022.102872
Chuah LF, Chew KW, Bokhari A, Mubashir M, Show PL (2022) Biodegradation of crude oil in seawater by using a consortium of symbiotic bacteria. Environ Res 113721. https://doi.org/10.1016/j.envres.2022.113721
Mahmoud YAG, El-Naggar ME, Abdel-Megeed A, El-Newehy MH (2021) Recent advancements in microbial polysaccharides: synthesis and applications. Polymers 13(23):4136
Tosif MM, Najda A, Bains A, Kaushik R, Dhull SB, Chawla P, Walasek-Janusz M (2021) A comprehensive review on plant-derived mucilage: characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 13(7):1066
Dailin DJ, Selvamani S, Michelle K, Mohd Jusoh YM, Chuah LF*, Bokhari A, El Enshasy HA, Mubashir M, Show PL (2022) Production of high-value added exopolysaccharide by biotherapeutic potential Lactobacillus reuteri strain. Biochem Eng J 188: 108691. https://doi.org/10.1016/j.bej.2022.108691.
Liu H, Lian B (2019) Quantitative evaluation of different fractions of extracellular polymeric substances derived from Paenibacillus mucilaginosus against the toxicity of gold ions. Colloids Surf, B 175:195–201
Kabir SF, Rahman A, Yeasmin F, Sultana S, Masud RA, Kanak NA, Haque P (2022) Occurrence, distribution, and structure of natural polysaccharides. In Radiation-Processed Polysaccharides (pp. 1–27). Academic Press
Zeidan AA, Poulsen VK, Janzen T, Buldo P, Derkx PMF, Oregaard G, Neves AR (2017) Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol Rev 41:168-S200
Suryawanshi N, Naik S, Jujjawarapu SE (2022) Exopolysaccharides and their applications in food processing industries. Food Sci Appl Biotechnol 5(1):22–44
Tiwari ON, Sasmal S, Kataria AK, Devi I (2020) Application of microbial extracellular carbohydrate polymeric substances in food and allied industries. 3 Biotech. 10(5):1–17
Gangalla R, Gattu S, Palaniappan S, Ahamed M, Macha B, Thampu RK, Fais A, Cincotti A, Gatto G, Dama M Kumar A (2021) Structural characterisation and assessment of the novel Bacillus amyloliquefaciens RK3 exopolysaccharide on the improvement of cognitive function in alzheimer’s disease mice. Polymers 13(17):2842
Qamar SA, RiasatA JM, Fatima R, Bilal M, Iqbal HM, Mu BZ (2022) Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J Basic Microbiol 62(11):1319–1336
Nordin NZ, Rashidi AR, Dailin DJ, Abd Malek R, Wan Azelee NI, Abd Manas NH, Selvamani S, Abg Zaidel DN, Abd Alsaheb RA, Sukmawati D, El Enshasy H (2020) Xanthan biopolymer in pharmaceutical and cosmeceutical applications: critical review. Biosci Res 17:205–220
Dailin DJ, Low LZMI, Malek RA, Izyan N, El Enshasy H (2019) Pullulan, a biopolymer with potential applications in pharmaceutical and cosmeceutical: a review. Biosci Res 16(30):2604–2616
Dailin DJ, Elsayed EA, Malek RA, Hanapi SZ, Selvamani S, Ramli S, Sukmawati D, Sayyed RZ, El Enshasy HA (2020) Efficient kefiran production by Lactobacillus kefiranofaciens ATCC 43761 in submerged cultivation: Influence of osmotic stress and nonionic surfactants, and potential bioactivities. Arab J Chem 13(12):8513–8523
Ragab TI, Malek RA, Elsehemy IA, Farag MM, Salama BM, Abd EL-Baseer MA, Gamal-Eldeen AM, El Enshasy HA, Esawy MA (2019) Scaling up of levan yield in Bacillus subtilis M and cytotoxicity study on levan and its derivatives. J biosci. Bioeng. 127(6):655-662
Fonseca LR, Santos TP, Czaikoski A, Cunha RL (2022) Microfluidics-based production of chitosan-gellan nanocomplexes encapsulating caffeine. Food Res Int 151:110885
Berninger T, Dietz N, Gonzalez Lopez O (2021) Water-soluble polymers in agriculture: xanthan gum as eco-friendly alternative to synthetics. Microbial Biotechnol 14(5):1881–1896
Garcı́a-Ochoa F, Castro EG, Santos VE (2000) Oxygen transfer and uptake rates during xanthan gum production. Enzyme Microbial Technol. 27(9):680-690
García-Ochoa F, Santos VE, Casas JA, Gómez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18(7):549–579
Miri M, Bergayou H, Belmouden A, Moukrim A, Baazizi H, Boum’handi N (2021) Medium optimization for exopolysaccharides production by Bacillus Zhangzhouensis BZ 16 strain isolated from Khnifiss Lagoon. In E3S Web of Conferences (Vol. 234, p. 00099). EDP Sciences
Ozdal M, Kurbanoglu EB (2019) Use of chicken feather peptone and sugar beet molasses as low cost substrates for xanthan production by Xanthomonas campestris MO-03. Fermentation 5(1):9
Kumar A, Rao KM, Han SS (2018) Application of xanthan gum as polysaccharide in tissue engineering: a review. Carbohydr Polym 180:128–144
Roca C, Alves VD, Freitas F, Reis MAM (2015) Exopolysaccharides enriched in rare sugars: bacterial sources, production, and applications. Front Microbiol 6:288
Mooter MV, Steenackers M, Maertens C, Gossele F, Vos PD, Swings J, Kersters K, Ley JD (1987) Differentiation between Xanthomonas campestris pv. graminis (ISPP List 1980), pv. phleipratensis (ISPP List 1980) emend., pv. poae Egli and Schmidt 1982 and pv. arrhenatheri Egli and Schmidt 1982, by numerical analysis of phenotypic features and protein G. J Phytopathology. 118(2):135-156
Rottava I, Batesini G, Silva MF, Lerin L, Oliveira D, Padilha FF, Toniazzo G, Mossi A, Cansian RL, Luccio MD, Treichel H (2009) Xanthan gum production and rheological behavior using different strains of Xanthomonas sp. Carbohydr Polym 77(1):65–71
Niknezhad SV, Asadollahi MA, Zamani A, Biria D (2016) Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int J Biol Macromol 82:751–756
da Silva JA, Cardoso LG, de Jesus Assis D, Gomes GV, Oliveira MB, de Souza CO, Druzian JI (2018) Xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl. Biochem. Biotechnol. 186(3):750–763
Dye DW, Bradbury J, Goto M, Hayward AC, Lelliott RA, Schroth MN (1980) International standards for naming pathovars of phytopathogenic bacteria and a list of pathovar names and pathotype strains. Rev Plant Pathol 59(4):153–168
Margaritis A (1978) Biotechology review: mixing, mass transfer, and scale-up of polysaccharide fermentations. Biotechnol Bioeng 20(7):977–993
Farhadi GB, Khosravi-Darani K, Nejad BN (2012) Enhancement of xanthan production on date extract using response surface methodology. Asian J Chem 24(9):3887–3890
Leela JK, Sharma G (2000) Studies on xanthan production from Xanthomonas campestris. Bioprocess Eng 23(6):687–689
Miranda AL, Costa SS, de Jesus AD, Andrade BB, de Souza CO, Oliveira MB, Guimarães AG, Druzian JI (2018) Investigation of cellular fatty acid composition of Xanthomonas spp. as chemical markers of productivity and quality of xanthan gum. Carbohydr Polym 192:291–298
Miranda AL, Costa SS, Assis DD, de Jesus CS, Guimarães AG, Druzian JI (2020) Influence of strain and fermentation time on the production, composition, and properties of xanthan gum. J Appl Polym Sci 137(15):48557
Alhalmi A, Alzubaidi N, Altowairi M, Almoiliqy M, Sharma B (2018) Xanthan gum; its biopharmaceutical applications: an overview. World J Pharm Pharm Sci 7(1):1536–1548
Dailin DJ, Rithwan F, Hisham AM, Rasid ZIA, Azelee NIW, Sapawe N, Chuah LF, Yusof AHM, Enshasy HE (2022) A Review on current status of plastic waste biodegradation using microbial approach. Biosci Res 202219(3):1599–1606
Dailin DJ, Nordin NZ, Tan LT, Ramli S, Chuah LF, Sapawe N, Mohd Jusoh YM, Abang Zaidel DN, Sukmawati D, El-Enshasy H (2022) State of the art Bioremediation of textile dye in wastewater: a review. Biosci Res 19(2):914–924
Prajapati VD, Jani GK, Moradiya NG, Randeria NP (2013) Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym 92(2):1685–1699
Aziz T, Farid A, Haq F, Kiran M, Ullah N, Faisal S, Ali A, Ullah Khan F, You S, Bokhari A, Mubashir M, Chuah LF, Show PL (2023) Role of silica-based porous cellulose nanocrystals in improving water absorption and mechanical properties. Environ Res. 222. 115253. https://doi.org/10.1016/j.envres.2023.115253.
Gul S, Ahmad Z, Asma M, Ahmad M, Rehan K, Munir M, Bazmi AA, Ali HM, Mazroua Y, Salem MA, Akhtar MS, Khan MS, Chuah LF, Asif S (2022) Effective adsorption of cadmium and lead using SO3H-functionalized Zr-MOFs in aqueous medium. Chemosphere 307: 135633. https://doi.org/10.1016/j.chemosphere.2022.135633
Pu W, Shen C, Wei B, Yang Y, Li Y (2018) A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J Ind Eng Chem 61:1–11
Chatterji BP, Amonkar MV, Jose A, Almeida D, Acharya K, Nasir N, De O, Agera R, Darne R (2015) Utility of xanthan gum produced from agro food waste. Proceedings of the India International Science Festival-Young Scientists’ Meet, Department of Science and Technology, Government of India 4–8
Demirci AS, Palabiyik I, Apaydın D, Mirik M, Gumus T (2019) Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: process optimization and fermentation kinetics. LWT 101:40–47
Becker A (2015) Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front Microbiol 6:687
Alves A, Miguel SP, Araujo AR, de Jesús Valle MJ, Sánchez Navarro A, Correia IJ, Ribeiro MP, Coutinho P (2020) Xanthan gum-konjac glucomannan blend hydrogel for wound healing. Polymers 12:99
Becker A, Vorhölter F-J (2009) Xanthan biosynthesis by Xanthomonas bacteria: an overview of the current biochemical and genomic data. In: Rehm BHA (ed) Microbial production of biopolymers and polymer precursors: applications and perspectives. Caister Academic Press, Norfolk, United Kingdom, pp 1–11
Chuah LF, Bokhari A, Yusup S, Saminathan S (2017) Optimisation on pretreatment of kapok seed (Ceiba pentandra) oil via esterification reaction in an ultrasonic cavitation reactor. Biomass Conversion and Biorefinery 7(1):91–99. https://doi.org/10.1007/s13399-016-0207-9
Chuah LF, Bokhari A, Yusup S, Klemeš JJ, Abdullah B, Akbar MM (2016) Optimisation and kinetic studies of acid esterification of high free fatty acid rubber seed oil. Arab J Sci Eng 41(7):2515–2526
Yusup S, Bokhari A, Chuah LF, Ahmad J (2015) Pre-blended methyl esters production from crude palm and rubber seed oil via hydrodynamic cavitation reactor. Chemical Engineering Transactions 43: 522
Bokhari A, Yusup S, Chuah LF, Kamil RNM (2016) Relative efficiency of esterified rubber seed oil in a hydrodynamic cavitation reactor and purification via distillation column. Chemical Engineering Transactions 52:775–780. https://doi.org/10.3303/CET1652130
Chuah LF, Klemes JJ, Bokhari A, Asif S (2021) A review of biodiesel production from renewable resources: chemical reactions. Chem Eng Trans 88:943–948. https://doi.org/10.3303/CET2188157
Chuah LF, Klemeš JJ, Bokhari A, Asif S, Cheng YW, Chong CC, Show PL (2022) A review of intensification technologies for biodiesel production. In: Gutierrez-Antonio, C., Gomez Castro, F.I. (eds). Biofuels and Biorefining. Volume 2: Intensification Processes and Biorefineries. Elsevier Inc. 87–116. https://doi.org/10.1016/B978-0-12-824117-2.00009-0
Haq F, Farid A, Ullah N, Kiran M, Khan RU, Aziz T, Mehmood S, Haroon M, Mubashir M, Bokhari A, Chuah LF, Show PL (2022) A study on the uptake of methylene blue by biodegradable and eco-friendly carboxylated starch grafted polyvinyl pyrrolidone. Environmental Research: 114241. https://doi.org/10.1016/j.envres.2022.114241
Chuah LF, Bokhari A, Asif S, Klemeš JJ, Dailin DJ, Enshasy HE, Yusof AHM (2022) A review of performance and emission characteristic of engine diesel fuelled by biodiesel. Chemical Engineering Transactions, 94, 1099–1104.https://doi.org/10.3303/CET2294183.
Fantou C, Roy AN, Dé E, Comesse S, Grisel M, Renou F (2017) Chemical modification of xanthan in the ordered and disordered states: An open route for tuning the physico-chemical properties. Carbohydr Polym 178:115–122
Patel J, Maji B, Moorthy NH, Maiti S (2020) Xanthan gum derivatives: review of synthesis, properties and diverse applications. RSC Adv 10(45):27103–27136
Jin C, Huang Z, Bao J (2020) High-titer glutamic acid production from lignocellulose using an engineered corynebacterium glutamicum with simultaneous co-utilization of xylose and glucose. ACS Sustain Chem Eng 8(16):6315–6322
Jin C, Hou W, Yao R, Zhou P, Zhang H, Bao J (2019) Adaptive evolution of Gluconobacter oxydans accelerates the conversion rate of non-glucose sugars derived from lignocellulose biomass. Biores Technol 289:121623
Lopes BD, Lessa VL, Silva BM, La Cerda LG (2015) Xanthan gum: properties, production conditions, quality and economic perspective. J Food Nutr Res 54(3):185–194
Murad HA, Abo-Elkhair AG, Azzaz HH (2019) Production of xanthan gum from nontraditional substrates with perspective of the unique properties and wide industrial applications. JSMC Microbiol 1(6)
Palaniraj A, Jayaraman V (2011) Production, recovery and applications of xanthan gum by Xanthomonas campestris. J Food Eng 106(1):1–12
El Enshasy H, Then C, Othman NZ, Al Homosany H, Sabry M, Sarmidi MR, Aziz RA (2011) Enhanced xanthan production process in shake flasks and pilot scale bioreactors using industrial semidefined medium. Afr J Biotechnol 10(6):1029–1038
Elsayed EA, Othman NZ, El Enshasy HA (2016) Bioprocess optimization of xanthan production by Xanthomonas campestris using semi-defined medium in batch and fed-batch culture. Pharm Lett 8(19):288–296
Esmaeili S, Noorolahi Z (2017) Application of starch processing enzymes in food technology-a review. Carpathian J Food Sci Technol 9(3):114–127
Moravej R, Alavi SM, Azin M, Salmanian AH (2020) Production and physicochemical characterization of xanthan gum by native lactose consuming isolates of Xanthomonas citri subsp. Citri Ukrainian Biochem J 92(1):92–102
Gondim TS, Pereira RG, Fiaux SB (2019) Xanthan gum production by Xanthomonas axonopodis pv. mangiferaeindicae from glycerin of biodiesel in different media and addition of glucose. Acta Sci. Biol. Sci. 41(1): 43661
Rončević Z, Grahovac J, Dodić S, Vučurović D, Dodić J (2019) Utilisation of winery wastewater for xanthan production in stirred tank bioreactor: bioprocess modelling and optimization. Food Bioprod Process 117:113–125
Jazini M, Ameri A, Sohrabi D, Karimi K (2018) Efficient xanthan gum production from phosphoric acid-pretreated cedar wood and elm wood. Adv res microb metab technol 1:51–64
Soleymanpour Z, Nikzad M, Talebnia F, Niknezhad V (2018) Xanthan gum production from acid hydrolyzed broomcorn stem as a sole carbon source by Xanthomonas campestris. 3 Biotech. 8(7): 1–2
Mohsin A, Zhang K, Hu J, Tariq M, Zaman WQ, Khan IM, Zhuang Y, Guo M (2018) Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: a kinetic model approach’. Carbohydr Polym 181:793–800
Aqeel M, Ran J, Hu W, Irshad MK, Dong L, Akram MA, Eldesoky GE, Aljuwayid AM, Chuah LF, Deng J (2023) Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 318. 137924. https://doi.org/10.1016/j.chemosphere.2023.137924
Obidah JS, Owuama CI (2017) Xanthan yield and conversion efficiency of pre-treated rice husk, sweet potato and cassava flours from Xanthomonas campestris fermentation. IJETT 49(8):477–481
de Sousa Costa LA, Inomata Campos M, Izabel Druzian J, de Oliveira AM, de Oliveira Junior EN (2014) Biosynthesis of xanthan gum from fermenting shrimp shell: yield and apparent viscosity. Int. J. Polym. Sci.
Moreno J, Lopez MJ, Vargas-Garcia C, Vazquez R (1998) Use of agricultural wastes for xanthan production by Xanthomonas campestris. J Ind Microbiol Biotechnol 21:242–246
Molina O, Fitzsimons R, Perotti N (1993) Effect of corn steep liquor on xanthan production by Xanthomonas campestris. Biotechnol. Lett. 15(5)
Lopez MJ, Ramos-Cormenzana A (1996) Xanthan production from olive-mill wastewaters. Int Biodeterior Biodegradation 38(3–4):263–270
Druzian JI, Pagliarini AP (2007) Xanthan gum production by fermentation from residue of apple juice. Food Sci Technol 27(1):26–31
Vidhyalakshmi R, Vallinachiyar C, Radhika R (2012) Production of xanthan from agro-industrial waste. J Adv Sci Res 3(2):56–59
Jazini MH, Fereydouni E, Karimi K (2017) Microbial xanthan gum production from alkali-pretreated rice straw. RSC Adv 7(6):3507–3514
Ngowatana N, Rudisirisak K (2016) Xanthan gum from sugar cane residue. Int J Geomate 11:2851–2856
Zhang Z (2015) Xanthan production with the hydrolysate of wheat straw. 6th International Conference on Manufacturing Science and Engineering. Guangzhou, China, 947–952.
Bajić B, Rončević Z, Puškaš V, Miljić U, Dodić S, Grahovac J, Dodić J (2015) White wine production effluents are used for biotechnological production of xanthan. J Process Energy Agric 19(1):52–55
Niknezhad SV, Asadollahi MA, Zamani A, Biria D, Doostmohammadi M (2015) Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci Biotechnol 24(2):453–460
Bhatia SK, Kumar N, Bhatia RK (2015) Stepwise bioprocess for exopolysaccharide production using potato starch as carbon source. 3 Biotech 5(5): 735–739
Katherine RF, Muthukumaran C, Sharmila G, Kumar NM, Tamilarasan K, Jaiganesh R (2017) Xanthan gum production using jackfruit-seed-powder-based medium: optimization and characterization. 3 Biotech, 7(4): 248
Abdullah A, Mat H (2008) Characterisation of solid and liquid pineapple waste. Reaktor 12(1):48–52
Jusoh N, Othman N, Idris A, Nasruddin A (2014) Characterization of liquid pineapple waste as carbon source for production of succinic acid. J Teknol 69(4):11–13
Abdullah A (2017) Solid and liquid pineapple waste utilization for lactic acid fermentation. Reaktor 11(1):50–52
Woiciechowski AL, Soccol CR, Rocha SN, Pandey A (2004) Xanthan gum production from cassava bagasse hydrolysate with Xanthomonas campestris using alternative sources of nitrogen. Appl Biochem Biotechnol 118(1–3):305–312
Abd El-Salam MH, Fadel MA, Murad HA (1994) Bioconversion of sugarcane molasses into xanthan gum. J Biotechnol 33(1):103–106
Souw P, Demain AL (1979) Nutritional studies on xanthan production by Xanthomonas campestris NRRL B1459. Appl Environ Microbiol 37:1186–1192
Psomas SK, Liakopoulou-Kyriakides M, Kyriakidis DA (2007) Optimization study of xanthan gum production using response surface methodology. Biochem Eng J 35(3):273–280
Trindade RA, Munhoz AP, Burkert CAV (2018) Impact of a carbon source and stress conditions on some properties of xanthan gum produced by Xanthomonas campestris pv. Mangiferaeindicae Biocatal Agric Biotechnol 15:167–172
Gilani SL, Najafpour GD, Heydarzadeh HD, Zare H (2011) Kinetic models for xanthan gum production using Xanthomonas campestris from molasses. Chem Ind Chem Eng Q 17(2):179–187
Papagianni M, Psomas SK, Batsilas L, Paras SV, Kyriakidis DA, Liakopoulou-Kyriakides M (2001) Xanthan production by Xanthomonas campestris in batch cultures. Process Biochem 37:73–80
Sherley KI, Priyadharshini RD (2015) Review on production of xanthan gum in batch and continuous reactors. Int J Chemtech Res 8(2):711–717
Shu CH, Yang ST (1990) Effects of temperature on cell growth and xanthan production in batch cultures of Xanthomonas campestris. Biotechnol Bioeng 35(5):454–468
Salah RB, Chaari K, Besbes S, Ktari N, Blecker C, Deroanne C, Attia H (2010) Optimisation of xanthan gum production by palm date (Phoenix dactylifera L.) juice by-products using response surface methodology. Food Chem. 121(2) : 627–633
Esgalhado ME, Roseiro JC, Collaço MA (1995) Interactive effects of pH and temperature on cell growth and polymer production by Xanthomonas campestris. Process Biochem 30(7):667–671
Hii YS, Law AT, Chuah LF, Mohamed Shazili NA, Abdul Rashid MK, Yong JC (2009) Inhibitive chemical cue of Pseudomonas pseudoalcaligene on biodegradation of anthracene in seawater medium. J Sustain Sci Manag 4(1):1–9
Rosalam S, England R (2006) Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb Technol 39(2):197–207
de Jesus AD, Brandão LV, de Sousa Costa LA, Figueiredo TV, Sousa LS, Padilha FF, Druzian JI (2014) A study of the effects of aeration and agitation on the properties and production of xanthan gum from crude glycerin derived from biodiesel using the response surface methodology. Appl Biochem Biotechnol 172(5):2769–2785
Suh IS, Schumpe A, Deckwer WD (1992) Xanthan production in bubble column and air-lift reactors. Biotechnol Bioeng 39(1):85–94
Cadmus MC, Knutson CA, Lagoda AA, Pittsley JE, Burton KA (1978) Synthetic media for production of quality xanthan gum in 20 liter fermenters. Biotechnol Bioeng 20(7):1003–1014
Peters HU, Herbst H, Hesselink PG, Lünsdorf H, Schumpe A, Deckwer WD (1989) The influence of agitation rate on xanthan production by Xanthomonas campestris. Biotechnol Bioeng 34(11):1393–1397
Chuah LF, Abd Aziz AR, Yusup S, Klemeš JJ, Bokhari A (2016) Waste cooking oil biodiesel via hydrodynamic cavitation on a diesel engine performance and greenhouse gas footprint reduction. Chemical Engineering Transactions 50:301–306. https://doi.org/10.3303/CET1650051
Chuah LF, Yusup S, Abd Aziz AR, Bokhari A (2015) Performance of refined and waste cooking oils derived from palm olein on synthesis methyl ester via mechanical stirring. Aust J Basic Appl Sci 9(37):445–448
Mohd Shamsuddin NA, Yusup S, Ibrahim WA, Bokhari1 A, Chuah LF (2015) Oil extraction from Calophyllum inophyllum L. via soxhlet extraction optimization using response surface methodology (RSM). A paper presented at the 10th Asian Control Conference (10th ASCC). 31 May -3 June, 2015 at Sutera Harbour Resort, Kota Kinabalu, Sabah, Malaysia. IEEE Xplore. Doi.https://doi.org/10.1109/ASCC.2015.7244791
Mohd Sauid S, Krishnan J, Huey Ling T, Veluri MV (2013) Enhancement of oxygen mass transfer and gas holdup using palm oil in stirred tank bioreactors with xanthan solutions as simulated viscous fermentation broths. Biomed Res Int. Article ID 409675.
Rols JL, Goma G (1991) Enhanced oxygen transfer rates in fermentation using soybean oil-in-water dispersions. Biotechnol Lett 13(1):7–12
Kuttuva SG, Restrepo AS, Ju LK (2004) Evaluation of different organic phases for water-in-oil xanthan fermentation. Appl Microbiol Biotechnol 64(3):340–345
Tait MI, Sutherland IW, Clarke-Sturman AJ (1986) Effect of growth conditions on the production, composition and viscosity of Xanthomonas campestris exopolysaccharide. J Gen Microbiol 132(6):1483–1492
Flores F, Torres LG, Galindo E (1994) Effect of the dissolved oxygen tension during cultivation of X. campestris on the production and quality of xanthan gum. J Biotechnol 34(2): 165–173
Holzwarth G, Ogletree J (1979) Pyruvate-free xanthan. Carbohydr Res 76(1):277–280
Bhat IM, Wani SM, Mir SA, Masoodi FA (2022) Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol 102328.
Zakeri A, Pazouki M, Vosoughi M (2017) Use of response surface methodology analysis for xanthan biopolymer production by Xanthomonas compestris: focus on agitation rate, carbon source, and temperature. Iran J Chem Chem Eng 36(1):173–183
Moshaf S, Hamidi EZ, Azizi MH (2014) Statistical optimization of xanthan gum production and influence of airflow rates in lab-scale fermentor. Appl Food Biotechnol 1(1):17–24
Nichols CM, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol 7(4):253–271
Sheng GP, Yu HQ, Yue Z (2006) Factors influencing the production of extracellular polymeric substances by Rhodopseudomonas acidophila. Int Biodeterior Biodegradation 58(2):89–93
Shehni SA, Soudi MR, Hosseinkhani S, Behzadipour N (2011) Improvement of xanthan gum production in batch culture using stepwise acetic acid stress. Afr J Biotechnol 10(83):19425–19428
Lee Y, Seo H, Yeom J, Park W (2011) Molecular characterization of the extracellular matrix in a Pseudomonas putida dsbA mutant: implications for acidic stress defense and plant growth promotion. Res Microbiol 162(3):302–310
Luvielmo MD, Borges CD, Toyama DD, Vendruscolo CT, Scamparini AR (2016) Structure of xanthan gum and cell ultrastructure at different times of alkali stress. Braz J Microbiol 47(1):102–109
Hadde EK, Mossel B, Chen J, Prakash S (2021) The safety and efficacy of xanthan gum-based thickeners and their effect in modifying bolus rheology in the therapeutic medical management of dysphagia. Food Hydrocolloids for Health 1:100038
Zheng M, Chen J, Tan KB, Chen M, Zhu Y (2022) Development of hydroxypropyl methylcellulose film with xanthan gum and its application as an excellent food packaging bio-material in enhancing the shelf life of banana. Food Chem. 131794
Jagdale SC, Patil SA, Kuchekar BS (2012) Design, development and evaluation of floating tablets of tapentadol hydrochloride using chitosan. Asian J Pharm Clin Res 5(4):163–168
Kavitha K, Puneeth KP, Mani TT (2010) Development and evaluation of rosiglitazone maleate floating tablets using natural gums. Int J Pharmtech Res 2(3):1662–1669
Kar R, Mohapatra S, Bhanja S, Das D, Barik B (2010) Formulation and in vitro characterization of xanthan gum-based sustained release matrix tables of isosorbide-5-mononitrate. Iran J Pharm Sci 9(1):13
Benny IS, Gunasekar V, Ponnusami V (2014) Review on application of xanthan gum in drug delivery. Int J Pharmtech Res 6(4):1322–1326
Ahuja M, Kumar A, Singh K (2012) Synthesis, characterization and in vitro release behavior of carboxymethyl xanthan. Int J Biol Macromol 51(5):1086–1090
Savary G, Grisel M, Picard C (2015) Cosmetics and personal care products. natural polymers: industry techniques and applications. Springer, Switzerland, pp 219–261
Liang K, Han P, Chen Q, Su X, Feng Y (2019) Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer 4 : 10620-10628
Chang I, Im J, Prasidhi AK, Cho GC (2015) Effects of xanthan gum biopolymer on soil strengthening. Constr Build Mater 74:65–72
William JK, Ponmani S, Samuel R, Nagarajan R, Sangwai JS (2014) Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J Petrol Sci Eng 117:15–27
Sutherland I (2002) A sticky business. Microbial polysaccharides: current products and future trends. Microbiol Today 29:70–71
de Souza ER, Rodrigues PD, Sampaio IC, Bacic E, Crugeira PJ, Vasconcelos AC, dos Santos Silva M, dos Santos JN, Quintella CM, Pinheiro AL, de Almeida PF (2022): Xanthan gum produced by Xanthomonas campestris using produced water and crude glycerin as an environmentally friendlier agent to enhance oil recovery. Fuel. 310(PB): 122421
Han N, Wang S, Rana AK, Asif S, Klemeš JJ, Bokhari A, Long J, Thakur VK, Zhao X (2022) Rational design of boron nitride with different dimensionalities for sustainable applications. Renew Sustain Energy Rev 170:112910
Han N, Wang S, Yao Z, Zhang W, Zhang X, Zeng L, Chen R (2020) Superior three-dimensional perovskite catalyst for catalytic oxidation. EcoMat 2(3):e12044
Jiang M, Zhang M, Wang L, Fei Y, Wang S, Núñez-Delgado A, Bokhari A, Race M, Khataee A, Klemeš JJ, Xing L (2022) Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem Eng J 431:134104
Maji B, Maiti S (2021) Chemical modification of xanthan gum through graft copolymerization: Tailored properties and potential applications in drug delivery and wastewater treatment. Carbohydr Polym 251:117095
Malik S, Fatima F, Imran A, Chuah LF, Klemes JJ, Khaliq IH, Asif S, Aslam M, Jamil F, Durrani AK, Akbar MM, Shahbaz M, Usman M, Atabani AE, Naqvi SR, Yusup S, Bokhari A (2019) Improved project control for sustainable development of construction sector to reduce environment risks. J Clean Prod 240:118214. https://doi.org/10.1016/j.jclepro.2019.118214
Acknowledgements
The authors would like also to thank the Ministry of Higher Education Malaysia through Fundamental Research Grant Scheme (FRGS/1/2020/TK0/UTM/02/16), Universiti Malaysia Terengganu and Research Management Center at UTM, Malaysia. Heartfelt appreciation to Dr. Loy Kak Choon, Chew Kuan Lian, Teh Bee Bee, Timmy Chuah Tim Mie and Ong Shying Weei for their support.
Author information
Authors and Affiliations
Contributions
Data collection and analysis were performed by [ARR and NIWA]. The first draft of the manuscript was written by [ARR]. The manuscript was reviewed and edited by [DNAZ]. The manuscript was reviewed and edited by [HE-E, LFC and AB]. Funding, supervision and project management were done by [DJD]. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest. The authors have no relevant financial or non-financial interests to disclose in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashidi, A.R., Azelee, N.I.W., Zaidel, D.N.A. et al. Unleashing the potential of xanthan: a comprehensive exploration of biosynthesis, production, and diverse applications. Bioprocess Biosyst Eng 46, 771–787 (2023). https://doi.org/10.1007/s00449-023-02870-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02870-9