Abstract
As an important five-carbon platform chemical to synthesize polyesters and polyamides, glutaric acid is widely used in numerous biochemical fields such as consumer goods, textile, and footwear industries. However, the application of glutaric acid is limited by the low yield of its bio-production. In this study, a metabolically engineered Escherichia coli LQ-1 based on 5-aminovalerate (AMV) pathway was used for glutaric acid fed-batch fermentation. Given the significance of nitrogen source in the bio-production of glutaric acid by AMV pathway, a novel nitrogen source feeding strategy feedbacked by real-time physiological parameters was proposed after evaluating the effects of nitrogen source feeding (such as ammonia and ammonium sulfate) on glutaric acid bio-production. Under the proposed nitrogen source feeding strategy, a significantly improved glutaric acid production of 53.7 g L–1 was achieved in a 30 L fed-batch fermentation by the metabolically engineered E. coli LQ-1, which was an improvement of 52.1% over pre-optimization. Additionally, a higher conversion rate of 0.64 mol mol–1 (glutaric acid/glucose) was obtained compared with the previously reported bio-production of glutaric acid with E. coli. These results indicated that the nitrogen source feeding strategy proposed in this study will be useful for achieving the efficient and sustainable bio-based production of glutaric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glutaric acid is an important dicarboxylic acid which can be polymerized with different diamines to produce novel polyamides [1]. With the introduction of the odd-numbered carbon units into the polyamides, they have new properties such as high elasticity and water absorption performance due to the different networks of hydrogen bonds between the amide functions [2, 3]. Glutaric acid is also built into organometallics for antimicrobial agents, transistors, and capacitors [4]. Moreover, glutarate is a synthetic precursor of 1, 5-pentanediol, widely used in polyesters and polyurethanes [5].
At present, high-purity glutaric acid can be prepared by multi-step crystallization of mixed acids from the by-product of adipic acid production. The crystallization process is complicated with a much lower yield, which consequently hampers the application of glutaric acid [6]. There are some reports on glutaric acid production by chemical routes which are performed under harsh reaction conditions [7, 8]. Due to the cost and the environmental impact of these chemical methods, it is desirable to establish an effective microbial production of glutaric acid.
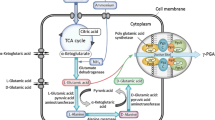
In recent years, several pathways have been demonstrated for microbial production of glutaric acid via 2-oxogularate reduction [9, 10], carbon chain extension from 2-oxogularate to α-ketoadipate and decarboxylation [11], reverse adipate degradation pathway [12], and L-lysine catabolism [13]. L-lysine can be degraded to glutaric acid via 5-aminovalerate (AMV) pathway as shown in Scheme 1A. Firstly, L-lysine is sequential catalyzed to AMV by L-lysine 2-monooxygenase and delta-aminovaleramidase. Then, AMV is further converted to glutaric acid by 5-aminovalerate transaminase and glutarate semialdehyde dehydrogenase. AMV pathway has been considered to be most efficient for microbial production of glutaric acid which offers the highest theoretical yield [4]. L-lysine-overproducing strains such as Corynebacterium glutamicum and Escherichia coli have been extensively investigated for glutaric acid production based on AMV pathway [14,15,16,17,18]. The reported highest titer reached 105.3 g L–1 with a yield over 0.7 mol mol–1 (glutaric acid/glucose) by an engineered C. glutamicum with 11 manipulated genes to increase precursor and a glutaric acid exporter encoded by ynfM overexpressed [16]. Though E. coli is another L-lysine-overproducing strain, reconstructing AMV pathway in an E. coli resulted in only 0.82 g L−1 of glutaric acid [17]. In order to improve the productivity of glutaric acid with E. coli, carbon flux toward glutaric acid biosynthesis was maximized and native transporters were applied to overcome the extracellular accumulation of the intermediates. With these efforts, 54.5 g L−1 of glutaric acid was achieved and the yield was 0.54 mol mol−1 (glutaric acid/glucose) [18].
Nevertheless, studies on microbial production of glutaric acid mainly focus on metabolic engineering of microorganisms, but lack of research on glutaric acid fermentation optimization. During the fermentation process of glutaric acid based on AMV pathway as shown in Scheme 1A, nitrogen source is used to synthesize the precursor L-lysine for further deamination to form the target product. Therefore, nitrogen source supply strategies are expected to affect the biosynthesis of L-lysine and production of glutaric acid as well. In this study, the effect and mechanism of nitrogen source supply strategies on glutaric acid production were systematically investigated using a metabolically engineered E. coli LQ-1.
Materials and methods
Bioreactor system
A 10 L bioreactor (Guoqiang Bioengineering Equipment Co., Ltd, Shanghai, China, FUS-10L) was for seed cultivation and a 30 L bioreactor (Guoqiang Bioengineering Equipment Co., Ltd, Shanghai, China, FUS-30L) for main fermentation was equipped with sensors to monitor and control online measurable parameters [19]. Temperature and pH were monitored by Pt100 temperature sensor (Omega, USA) and pH electrode (Mettler Toledo, InPro 3253), respectively. Dissolved oxygen (DO) was measured using low-drift polarographic electrode (Mettler Toledo, InPro 6800). Off gases (O2 and CO2) during the fermentation were measured by multi-channel off-gas analyzer (Hateman Co., Ltd, Chongqing, China, GQ200-4). All the parameters were collected and calculated using real-time viewing software named Biostar 2.0 (National Center of Bio-Engineering & Technology, Shanghai, China). The real-time OUR, CER and RQ were calculated automatically by Biostar 2.0 using the equations proposed by Wang et al. [20].
Microorganism and media
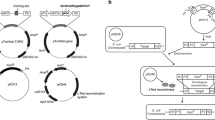
A metabolically engineered E. coli LQ-1 based on AMV pathway developed by our lab was used for glutaric acid fed-batch fermentation. The methods and materials used to get E. coli LQ-1 were carried out according to previous literature reported [17]. Firstly, feedback resistant mutants of aspartate kinaseIII and dihydrodipicolinate synthase were introduced to E. coli BW25113. Then, L-lysine 2-monooxygenase and delta-aminovaleramidase (encoded by davBA), glutaric semialdehyde dehydrogenase and 5-aminovalerate aminotransferase (encoded by davDT) were overexpressed by introducing the plasmids to the strain. In addition, coding genes of L-lysine permease (LysP) and 5-aminovalerate transporter (GabP) were overexpressed by introducing pSTV-LysP-GabP to obtain E. coli LQ-1. Shake flask culture medium and seed tank medium for seed quantity expansion step by step were the same. The culture medium contained 4 g L–1 of KH2PO4, 0.5 g L–1 of MgSO4, 4.5 g L–1 of (NH4)2SO4, 5 g L–1 of yeast extract, 8 g L–1 of tryptone, 20 g L–1 of glucose, 50 μg mL–1 of ampicillin, 50 μg mL–1 of chloramphenicol. The initial medium of fed-batch fermentation was composed of 1.6 g L–1 of MgSO4, 0.03 g L–1 of FeSO4, 0.032 g L–1 of MnSO4, 10 g L–1 (NH4)2SO4, 5 g L–1 of KH2PO4, 30 g L–1 of glucose, 25 g L–1 of corn steeped liquor, 0.7 g L–1 of threonine, 2.2 g L–1 of betaine, 0.006 g L–1 of vitamin B1, 50 μg mL–1 of ampicillin, 50 μg mL–1 of chloramphenicol.
Fed-batch fermentation
Shake flask cultivation was performed in a shaking incubator at 37 °C and 170 rpm. Single fresh colonies were inoculated into a 500 mL shake flask containing 50 mL of culture medium and grown for 7 h. Then, 50 mL of the culture broth was transferred into a 10 L bioreactor with 5 L of seed medium and cultivated for 16–18 h. Aeration ratio, temperature, and head space pressure were controlled at 0.4 vvm, 37 °C and 0.05–0.08 MPa, respectively. The pH was maintained at 6.7 by feeding 25% (w/v) ammonia aqueous solution or 30% (w/v) sodium hydroxide aqueous solution. The stirring speed was 400 rpm during the cultivation. When OD600 of the culture broth reached above 20, 1.5 L of the culture broth was inoculated into a 30 L bioreactor with 15 L of the initial volume. The pH, temperature, pressure and aeration ratio for fed-batch fermentation were the same as seed cultivation. At the beginning of the fermentation, the stirring speed was set to 400 rpm, and as DO decreased, the stirring speed was improved to keep it between 20 and 40%. 70% (w/v) glucose aqueous solution was fed to keep the residual sugar concentration of the fermentation broth not less than 0.5% (w/v). Several nitrogen source feeding strategies were performed to investigate the effects on glutaric acid fermentation as following: if only 25% (w/v) ammonia aqueous solution was fed to provide nitrogen, the feeding rate was automatically controlled by feedback of the pH at ~ 6.7. If 30% (w/v) sodium hydroxide aqueous solution and 25% (w/v) ammonia aqueous solution were fed, sodium hydroxide aqueous solution was fed to maintain the pH at ~ 6.7 until NH4+ concentration in the fermentation broth decreased to 0.14 mol L–1. Then, ammonia aqueous solution feeding started. If NH4+ concentration was above 0.14 mol L–1, feeding of ammonia aqueous solution was stopped and sodium hydroxide aqueous solution was fed to maintain pH. If feeding 50% (w/v) ammonium sulfate aqueous solution to supply nitrogen and sodium hydroxide aqueous solution was fed to maintain pH, when NH4+ concentration was below 0.14 mol L–1, ammonium sulfate aqueous solution started to feed, and the feeding rates were based on different OUR and CER values.
Analytical methods
Bacterial growth was monitored by measuring the absorbance at 600 nm (OD600) using an ultraviolet spectrophotometer UV1900 (SHIMADZU, Japan). Glucose and L-lysine concentration in the fermentation broth was determined by colorimetry. A certain amount of fermentation broth was diluted and reacted with 3,5-Dinitrosalicylic acid in boiling water bath. Then, the absorbance of the reacted broth at 520 nm was measured to determine glucose concentration. A certain amount of fermentation broth was diluted and reacted with ninhydrin in boiling water bath to measure absorbance at 475 nm to obtain L-lysine concentration. The concentration of glutaric acid was determined by Gas chromatography. A certain amount of fermentation broth was taken and centrifuged for 5 min at 5000 rpm. Then, GC2010pro (SHIMADZU, Japan) was used for the determination. The inlet temperature was set to 290℃ and nitrogen gas flow rate was 1.2 mL min–1. Sample injection amount was 0.5 μL and split ratio was 20:1. Temperature of FID detector was 320℃. The column temperature was set as following: the initial temperature was 180 °C and maintained for 2 min, then increased to 300 °C and maintained for 7 min. Column type: Wondacap-5 30 m × 0.25 mm × 1 µm. NH4+ concentration in fermentation broth was determined by Kjeldahl method [21].
Results and discussion
Nitrogen source supply strategies for glutaric acid production
Microbial production of L-lysine requires a large amount of nitrogen sources, such as ammonium sulfate and ammonia; while glutaric acid production via L-lysine catabolism needs stepwise deamination and transamination reactions. Therefore, nitrogen source supply strategies will interfere with the synthesis of L-lysine and thus glutaric acid. According to AMV pathway (Scheme 1A), L-lysine to 5-aminovaleramide requires oxygen and generates carbon dioxide, which are related to microbial physiological parameters such as oxygen uptake rate (OUR), carbon dioxide evolution rate (CER) and respiratory quotient (RQ) during glutaric acid fermentation process. Therefore, the effect of nitrogen source feeding strategies on glutaric acid synthesis can be monitored through real-time physiological parameters.
A metabolically engineered E. coli LQ-1 with AMV pathway was used for glutaric acid fed-batch fermentation in this study. Given the significance of nitrogen source in the bio-production of glutaric acid, a novel nitrogen source feeding strategy feedback by real-time physiological parameters was proposed by evaluating the effects of nitrogen source feeding (such as ammonia and ammonium sulfate) on glutaric acid bio-production as shown in Scheme 1B. Under the proposed nitrogen source feeding strategy, the glutaric acid bio-production was explored detailly in a 30 L fermentor at fed-batch fermentation.
Effects of ammonia feeding on glutaric acid production
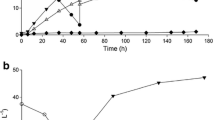
Nitrogen is an essential requirement for growth of microorganisms, and different microorganisms have different preferences for nitrogen sources [22, 23]. Ammonia is an important nitrogen source for industrial fermentation of L-lysine. In this study, 25% (w/v) ammonia aqueous solution was fed to provide nitrogen source for bacterial growth, L-lysine synthesis and neutralize the glutaric acid produced during fermentation to maintain pH at ~ 6.7 as described. Another nitrogen source supply strategy of feeding 25% ammonia aqueous solution to provide nitrogen source and feeding 30% (w/v) sodium hydroxide aqueous solution to keep pH of the fermentation broth was also investigated. Concentration of NH4+ in the fermentation broth, biomass (OD600), L-lysine and glutaric acid (GA) were used to compare effects of feeding strategies on glutaric acid fed-batch fermentation. Only feeding ammonia caused excessive accumulation of NH4+ in the broth, even more than 0.39 mol L–1 (Fig. 1A), and the maximum OD600 was only about 60 (Fig. 1B). When ammonia and sodium hydroxide were fed during the fermentation, NH4+ concentration could be controlled to about 0.05 – 0.10 mol L–1 after 40 h (Fig. 1A) and the maximum OD600 was over 100 (Fig. 1B). It seemed that higher NH4+ concentration inhibited growth of microorganisms during the fermentation. The synthesis rate of L-lysine (Fig. 1C) and GA (Fig. 1D) were lower if only feeding ammonia than feeding ammonia and sodium hydroxide. When ammonia and sodium hydroxide were fed during the fermentation, 45.9 g L–1 of glutaric acid was produced, 30% higher than only feeding ammonia (35.1 g L–1) which might benefit from the fact that the growth of microorganisms was not inhibited.
Microbial physiological parameters such as OUR, CER, RQ are closely related to bacterial growth and carbon flux orientation, which were used for real-time regulation of the fermentation [24, 25]. As shown in Fig. 2A, when only ammonia was fed, OUR and CER reached 140 mmol L–1 h –1 at 12–15 h and then dropped sharply and maintained 60–80 mmol L–1 h –1 until to the end of the fermentation. OUR and CER of feeding ammonia and sodium hydroxide were relatively higher and remained between 120 and 140 mmol L–1 h–1 after 30 h (Fig. 2B). Biosynthesis of glutaric acid requires oxygen as a substrate and generates carbon dioxide, thus, microbial physiological parameters were related to microbial growth and glutaric acid production. From the above ammonia feeding strategy, it could be seen that low OUR and CER during the fermentation meant low synthesis rate of glutaric acid. Because 1.0 mol of oxygen was needed and 1.0 mol of carbon dioxide was generated when 1 mol of L-lysine was converted to 1.0 mol of glutaric acid, RQ almost equal to 1 revealed carbon metabolic flux was towards to glutaric acid without other migration (Fig. 2).
Effects of ammonium sulfate feeding on glutaric acid production
Although feeding ammonia and sodium hydroxide created relatively higher OUR and CER to obtain 30% higher glutaric acid productivity, both of them increased pH of the fermentation broth, so it was difficult to automatically control the pH to ~ 6.7 during the fermentation. If pH was controlled by manual, glutaric acid productivity was severely reduced [16]. Theoretically, feeding ammonium sulfate and sodium hydroxide could accomplish pH–stat fermentation. In order to investigate the correlation of DO, nitrogen source feeding strategies, microbial physiological parameters, glutaric acid productivity and eliminate the effect of pH fluctuation on fermentation, two strategies were conducted by feeding ammonium sulfate and sodium hydroxide. Strategy (1): the stirring speed increased gradually to ~ 600 rpm between 15 to 25 h to create relative higher OUR and CER and when NH4+ concentration was below 0.14 mol L–1 after 25 h, 50% (w/v) ammonium sulfate aqueous solution was fed and the feeding rate was adjusted by real-time OUR and CER feedback to keep them at 60–80 mmol L–1 h–1 from 40 h to the end of the fermentation; Strategy (2): the stirring speed increased gradually to ~ 580 rpm between 15 – 25 h to create relative lower OUR and CER and when NH4+ concentration was below 0.14 mol L–1, ammonium sulfate aqueous solution was fed and the feeding rate was adjusted by real-time OUR and CER feedback to keep them at 80 – 100 mmol L–1 h–1 from 40 h to the end of the fermentation. As seen in Fig. 3A and B, higher agitation speed led to higher OUR and CER (160 – 180 mmol L–1 h–1 versus 140 – 160 mmol L–1 h–1). A little higher titer of glutaric acid was achieved between 15 – 25 h in the fermentation process when OUR and CER were controlled at 160 – 180 mmol L–1 h–1 than that at 140 – 160 mmol L–1 h–1 (Fig. 4A and B), this because higher stirring speed created higher DO, since synthesis of glutaric acid based on AMV pathway needed oxygen. After 25 h, strategy (2) resulted in higher OUR and CER (Fig. 3B) and higher glutaric acid productivity (Fig. 4A and B), in contrast, the productivity of glutaric acid was 12.5% lower if nitrogen feeding rate was adjusted by feedback of OUR and CER at 60 – 80 mmol L–1 h–1. Microbial growth was not limited based on two nitrogen feeding strategies and OD600 of both strategies reached ~ 105 (Fig. 4A and B). As Fig. 4A and B shown, after 40 h to the end of the fermentation, the NH4+ concentration in the fermentation broth of strategy (1) and strategy (2) was 0.03 mol L–1 and ~ 0.07 mol L–1 respectively, which meant the decrease in glutaric acid productivity was due to insufficient nitrogen source supply. As shown in Figs. 3 and 4, it was also clear that the different glutaric productivity after 30 h between two feeding strategies was not because of oxygen supply difference, since the DO of strategy (1) was slightly higher than strategy (2) after 30 h.
An efficient nitrogen source feeding strategy feedback by physiological parameters
Higher NH4+ concentration inhibited bacterial growth, which hindered biosynthesis of glutaric acid, while insufficient nitrogen supply (NH4+ concentration was ~ 0.03 mol L–1) could also reduce the productivity of glutaric acid seriously, although microbial growth was not inhibited. It was also clear that better oxygen supply at the early stage of fermentation (15–25 h) increased glutaric acid productivity. Based on these understandings, an efficient nitrogen source feeding strategy named strategy (3) was designed. Specifically, the stirring speed increased gradually to ~ 600 rpm between 15 and 25 h to create relative higher OUR and CER to ~ 180 mmol L–1 h–1, then 50% sulfate aqueous solution was fed and the feeding rate was set according to the feedback of OUR and CER to maintained them at 160 – 180 mmol L–1 h–1 until to the end of fermentation. As shown in Fig. 5, OUR and CER rose slowly until ~ 7 h, which presented stationary phases of OUR and CER at the beginning of fermentation based on strategy (3). The main reason may be that the seed liquor used for strategy (3) was placed in the refrigerator at 4 °C overnight before inoculation, thus delaying the growth of Escherichia coli LQ-1. The agitation speed increased sharply to 600 rpm at 20–30 h of fermentation, which was superimposed effect of the oxygen demand for microbial growth and glutaric acid synthesis. After 30 h, the growth of microorganisms entered a stable period, and the oxygen supply was mainly used to meet the synthesis of glutaric acid. During this period, although the agitation speed decreased from 600 to 450 rpm, the DO was still over 20%. From 40 h to the end of the fermentation, NH4+ concentration in the fermentation broth was ~ 0.10 mol L–1 (Fig. 6) and OUR and CER remained at 160–180 mmol L–1 h–1, indicating that the synthesis rate of glutaric acid was not limited by NH4+ concentration and microbial growth (Fig. 6). With strategy (3), a metabolically engineered E. coli LQ-1 achieved 53.7 g L–1 of glutaric acid (Fig. 6), 52.1% higher than only feeding of ammonia (Fig. 1D) and the yield from glucose to glutaric acid was 0.64 mol mol–1, higher than previously reported in the literature on microbial production of glutaric acid with E. coli. The production capabilities (titer of glutaric acid divided by OD600) of strategy (3), strategy (2), strategy (1) were 0.479, 0.421 and 0.386 respectively. The production capability of strategy (3) was 13.9% higher than that of strategy (2) and 24.0% higher than that of strategy (1). Time course of online feed quantities of ammonium sulfate solution with three strategies were shown in Fig. 7, feed quantity of ammonium sulfate solution based on strategy (3) was ~ 2.04 kg, much higher than strategy (2) and strategy (1). This study showed that except endogenous genes designed to generate strong metabolic driving force to maximize carbon flux toward glutaric acid biosynthesis [18], the optimization of nitrogen source supply during the fermentation was also an effective method to improve glutaric acid productivity.
Conclusion
In summary, a novel nitrogen source feeding strategy was proposed based on the evaluating the significance of nitrogen source in the bio-production of glutaric acid by AMV pathway using a metabolically engineered E. coli LQ-1. The efficient and sustainable bio-based production of glutaric acid was achieved with a significantly improved glutaric acid production of 53.7 g L–1 as well as a higher glutaric acid yield of 0.64 mol mol–1 (glutaric acid/glucose) in a 30 L fed-batch fermentation. These outcomes illustrated a positive progress in the bio-based production of glutaric acid by the nitrogen source feeding strategy proposed in this study, which will be an effective method to achieve the efficient and sustainable bio-based production of glutaric acid at industrial-scale.
Data availability
All relevant data are within the manuscript.
References
Navarro E, Puiggali J, Subirana JA (1997) The structure of nylon 12, 5 is characterized by two hydrogen bond directions as are other polyamides derived from glutaric acid. Polymer 38:3429–3432
Navarro E, Franco L, Subirana JA, Puiggali J (1995) Nylon 65 has a unique structure with two directions of hydrogen bonds. Macromolecules 28:8742–8750
Ricart A, Puiggalí J, Franco L, Morales-Gámez L (2012) Study on the brill transition and melt crystallization of nylon 65: a polymer able to adopt a structure with two hydrogen-bonding directions. Eur Polym J 46:2063–2077
Rohles CM, Gläser L, Kohlstedt M, Gießelmann G, Pearson S, Campo AL, Becker J, Wittmann C (2018) A bio-based route to the carbon-5 chemical glutaric acid and to bionylon-6,5 using metabolically engineered Corynebacterium glutamicum. Green Chem 20:4462–4474
Mishra MK, Varughese S, Ramamurty U, Desiraju GR (2013) Odd-even effect in the elastic modulii of alpha, omega-alkanedicarboxylic acids. J Am Chem Soc 135:8121–8124
Li JQ, Gao XQ, Ding SM, Qian XR, Ma YG (2011) Separation and preparation of glutaric acid from mixed dicarboxylic acid. Chem Ind Eng 28:53–56
Chen H, Dai WL, Gao RH, Cao Y, Fan KN (2007) New green catalytic manufacture of glutaric acid from the oxidation of cyclopentane-1,2-diol with aqueous hydrogen peroxide. Appl Catal A 328:226–236
Vafaeezadeh M, Hashemi MM (2016) A non-cyanide route for glutaric acid synthesis from oxidation of cyclopentene in the ionic liquid media. Process Saf Environ 100:203–207
Yu JL, Xia XX, Zhong JJ, Qian ZG (2017) A novel synthetic pathway for gultarate production in recombinant Escherichia coli. Process Biochem 59:167–171
Yu JL, Xia XX, Zhong JJ, Qian ZG (2017) Enhanced production of C5 dicarboxylic acids by aerobic-anaerobic shift in fermentation production of engineered Escherichia coli. Process Biochem 62:53–58
Wang J, Wu Y, Sun X, Yuan QP, Yan YJ (2017) De novo biosynthesis of glutarate via a-keto acid carbon chain extension and decarboxylation pathway in Escherichia coli. ACS Synth Biol 6:1922–1930
Zhao M, Li G, Deng Y (2018) Engineering Escherichia coli for glutarate production as the C5 platform backbone. Appl Environ Microb 84:814–818
Revelles O, Espinosa-Urgel M, Fuhrer T, Sauer U, Ramos JL (2005) Multiple and interconnected pathways for L-lysine catabolism in Pseudomonas putida KT2440. J Bacteriol 187:7500–7510
Rohles CM, Gießelmann G, Kohlstedt M, Wittmann C, Becker J (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15:1–13
Kim HT, Khang TU, Baritugo KA, Hyun SM, Kang KH, Jung SH, Song BK, Park K, Oh MK, Kim GB, Kim HU, Lee SY, Park SJ, Joo JC (2019) Metabolic engineering of Corynebacterium glutamicum for the production of glutaric acid, a C5 dicarboxylic acid platform chemical. Metab Eng 51:99–109
Han T, Kim GB, Lee SY (2020) Glutaric acid production by systems metabolic engineering of an L-lysine–overproducing Corynebacterium glutamicum. Proc Natl Acad Sci U S A 117:30328–30334
Adkins J, Jordan J, Nielsen DR (2013) Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5- aminovalerate and glutarate. Biotechnol Bioeng 110:1726–1734
Li WN, Ma L, Shen XL, Wang J, Feng Q, Liu LX, Zheng GJ, Yan YJ, Sun XX, Yuan QP (2019) Targeting metabolic driving and intermediate influx in L-lysine catabolism for high-level glutarate production. Nat Commun 10:3337
Wang Y, Chu J, Zhuang YP, Wang YH, Xia JY, Zhang SL (2009) Industrial bioprocess control and optimization in the context of systems biotechnology. Biotechnol Adv 27:989–995
Wang ZJ, Wang HY, Li YL, Chu J, Huang MZ, Zhuang YP, Zhang SL (2010) Improved vitamin B12 production by step-wise reduction of oxygen uptake rate under dissolved oxygen limiting level during fermentation process. Bioresour Technol 101:2845–2852
Sáez-Plaza P, Michałowski T, Navas MJ, Asuero AG, Wybraniec S (2013) An overview of the Kjeldahl method of nitrogen determination. Part I. early history, chemistry of the procedure, and titrimetric finish. Crit Rev Anal Chem 43:178–223
Magasanik B, Kaiser CA (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene 290:1–18
Wong KH, Hynes MJ, Davis MA (2008) Recent advances in nitrogen regulation: a comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryot Cell 7:917–925
Chen Y, Wang ZJ, Chu J, Xi BL, Zhuang YP (2015) The glucose RQ-feedback control leading to improved erythromycin production by a recombinant strain Saccharopolyspora erythraea ZL1004 and its scale-up to 372 m3 fermenter. Bioprocess Biosyst Eng 38:105–112
Lu F, Wang ZJ, Zhao W, Chu J, Zhuang YP (2016) A simple novel approach for real-time monitoring of sodium gluconate production by on-line physiological parameters in batch fermentation by Aspergillus niger. Bioresour Technol 202:133–141
Acknowledgements
This work was supported by grants from National Key Research and Development Program of China (2022YFC2106204).
Author information
Authors and Affiliations
Contributions
WB: Investigation, Data curation, Validation, Writing-original draft. CC: Investigation, Visualization, Data curation. TW: Formal analysis, Validation, Writing-review and editing. PY: Validation, Writing-review and editing. NL: Conceptualization, Resources, Writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bi, W., Chen, C., Wang, T. et al. Efficient bio-production of glutaric acid by a metabolically engineered Escherichia coli LQ-1 based on a novel nitrogen source feeding strategy. Bioprocess Biosyst Eng 46, 717–725 (2023). https://doi.org/10.1007/s00449-023-02856-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02856-7