Abstract
Algae–bacteria symbiosis can promote the growth of microalgae and improve the efficiency of wastewater treatment. Attached culture is an efficient culture technique for microalgae, with benefits of high yield, low water consumption and easy harvesting. However, the promoting effects of bacteria on microalgae in attached culture are still unclear. In this study, different forms of a nitrogen-fixing bacteria, Azotobacter beijerinckii (including bacteria supernatant, live bacteria, and broken bacteria), were co-cultured with Chlorella pyrenoidosa in an attached culture system using wastewater as the culture medium. The results showed that the broken A. beijerinckii form had the best growth promotion effect on C. pyrenoidosa. Compared with the pure algae culture, the biomass of C. pyrenoidosa increased by 71.8% and the protein increased by 28.2%. The live bacteria form had the best effect on improving the efficiency of wastewater treatment by C. pyrenoidosa, with the COD, PO43− and NH4+–N removal rates increased by 20.8%, 18.5% and 8.9%, respectively, in comparison with the pure algae culture. The attached co-culture mode promoted the growth of C. pyrenodisa better than the suspended co-culture mode. This research offers a new way for improving microalgae biomass and wastewater treatment by attached algae–bacteria symbiont.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae, as a large group of organisms with photosynthetic autotrophic ability, are diverse in species and component. The world energy crisis in the 1970s led humans to recognize microalgae as an ideal biomass energy source due to its renewable and sustainable characteristics, and promote numerous researches on microalgae in various fields [1]. However, microalgae cultivation suffers from high energy consumption, high cost, and low biomass, which seriously hindered the industrialization development of microalgae [2, 3].
Recently, the construction of algae–bacteria system has attracted much attention, to enhance microalgal biomass and wastewater treatment efficiency. In some cases, microalgae are completely dependent on bacteria for growth and division [4, 5]. Cho et al. established an artificial bacterial community and characterized its growth pattern with microalgae, and found that the algae–bacteria symbiotic system contributed to both algal biomass and lipid productivity [6]. Tadashi et al. observed that the biomass of the following three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris, and Chlamydomonas thingy, increased by 1.5, 1.8–2.8 and 2.1 times, respectively, compared to the pure algae culture [7]. Xue et al. also reported an increase in microalgal biomass and biodiesel quality in the algae–bacteria co-culture system [8]. Oswald et al. showed that the symbiotic relationship between microalgae and bacteria could not only keep microalgae from toxic compounds in wastewater, but also contributed to removing harmful pollutants [9]. Obviously, the microalgae–bacteria co-culture system can eliminate high concentration of nutrients, maintain ecological balance, as well as produce a large number of biological resources for recycling.
Suspended culture is a kind of culture in which microalgal cells grow in suspension state in liquid without dependence on the surface of supporting material. It is the most common method for microalgae culture. However, there are some problems in suspended culture, including poor light transmittance, slow CO2 dissolution rate, low biomass yield and high dehydration cost [10,11,12]. Attached culture is a culture method in which cells are attached to a certain solid surface, which can solve the above problems of suspended culture [13]. Liu et al. achieved biomass productivity of 50–80 g/m2 d by cultivating microalgae in an attached mode, which is seven times higher than that in a normal running pond [14]. Ozkan et al. inoculated microalgae Botryococcus braunii onto a concrete surface and showed that the microalgae biomass concentration was significantly increased to 96.4 g/L [15]. Moreover, microalgae biomass of biofilms could be harvested easily from the concrete surface by gentle mechanical scraping with a squeegee, which reduce the energy requirement for dewatering process by 99.7%. Water requirement per unit of microalgal biomass production was decreased by 45% compared with that in an open pond [15].
Nitrogen is indispensable for the growth of microalgae and is the main source for protein synthesis. When co-culturing nitrogen-fixing bacteria with microalgae, the nitrogen-fixing bacteria can assimilate nitrogen in the air for use by microalgae. One study found that the co-culture of Chlorella vulgaris with Azotobacter Mesorhizobium sp. in nitrogen-deficient conditions increased the biomass and lipid of Chlorella vulgaris by 66.3% and 47.7%, respectively [16]. Juan-Pablo et al. made immobilized colloidal balls composed of a nitrogen-fixing bacteria and Chlorella vulgaris, and found that Chlorella vulgaris could grow well in nitrogen-free medium [17]. Xu et al. co-cultured Azotobacter chroococcum with Chlamydomonas reinhardtii, and found that the biomass, lipid accumulation and cellular activity of Chlamydomonas reinhardtii were all increased [18]. The current studies on the co-culture of nitrogen-fixing bacteria and microalgae are mainly focused on the impact of live nitrogen-fixing bacteria on microalgae in suspended culture. However, there is no report on the impact of different forms of nitrogen-fixing bacteria on microalgae in attached mode.
In this study, a nitrogen-fixing bacteria A. beijerinckii was used to investigate the impact of its different forms (bacteria supernatant, live bacteria, and broken bacteria) on the promotion of C. pyrenoidosa growth as well as the removal of pollutants in wastewater. The purpose was to investigate the promotion impact of different forms of A. beijerinckii addition on C. pyrenoidosa in attached cultivation, and to provide reference for its application in microalgae scale culture and wastewater treatment.
Materials and methods
Microalgae species and culture conditions
The microalgae used in this study was Chlorella pyrenoidosa (FACHB-9), obtained from Institute of Aquatic Biology, Chinese Academy of Sciences. The microalgae were cultured in BG11 medium at (25 ± 1) °C, with a light cycle of 12 h:12 h and a light intensity of 9600 lx.
Bacteria strain and culture conditions
The nitrogen-fixing bacteria strain was Azotobacter beijerinckii (CGMCC 1.9044), obtained from the National Microbiology Resource Center, China. Luria–Bertani medium was used for bacteria cultivation, after sterilized at 121 °C for 20 min. The bacteria were cultured in a shaker (THZ-320, China) at 30 °C and 200 r/min.
Culture system construction
As shown in Fig. 1A, the attached culture device was constructed by placing sponge in a rectangular plastic box as absorbent and supporting medium. The surface of the plastic box was sealed with plastic wrap to keep the inner environment stable. Quantitative culture medium was poured into the rectangular box to make the sponge fully soaked. The culture medium was simulated brewery wastewater with the following ingredients: C6H12O6 1.0 g/L, Yeast 1.0 g/L, KH2PO3 1.0 g/L, CaCl2 0.5 g/L, MgSO4·7H2O 0.01 g/L, NaHCO3 1.0 g/L, NH4Cl 1.0 g/L, MnSO4·H2O 0.01 g/L, and FeSO4 0.02 g/L. The experiments were carried out indoors at an ambient temperature of (22 ± 1) °C. Natural light was used as the light source and carbon dioxide in the air was used as the inorganic carbon source for microalgae growth.
As shown in Fig. 1B, conical flack sealed with parafilm was used as the suspended culture device. The culture medium was placed into the conical flask and shaken regularly. The culture conditions were the same as the attached culture.
Establishment of algae–bacteria co-culture system
C. pyrenoidosa (algae) and A. beijerinckii (bacteria) were separately cultured to logarithmic growth phase. The inoculum density of C. pyrenoidosa and A. beijerinckii were 0.35 g/L and 1 × 108 cfu/mL, respectively. The supernatant of A. beijerinckii was obtained by centrifugation. Broken A. beijerinckii was obtained by adding lysis buffer to bacteria, and then breaking the bacteria with ultrasound. C. pyrenoidosa and different forms of A. beijerinckii was mixed to form the algae–bacteria mixture in accordance with the ratio of 10:1 (v:v). The algae–bacteria mixture was filtered onto acetate fiber filter membrane with pore size of 0.45 μm. Then, the filter membrane was attached to the surface of the sponge to complete the inoculation of the attached culture system. As for the inoculation of the suspended culture system, the algae culture was centrifuged to remove the supernatant. Then, the algae precipitation and different forms of bacteria with the ratio of 10:1 were mixed and poured into conical flasks to complete the inoculation. Each system was set up in four groups, respectively: (1) pure algae, (2) algae + bacteria supernatant, (3) algae + live bacteria, and (4) algae + broken bacteria. These were incubated in a chamber for 16 days, with the pure algae culture as the control.
Determination of microalgae growth parameters
Biomass determination
Dry weight method was used to determine the biomass of C. pyrenoidosa [14]. Attached culture: algae attached to the membrane was rinsed off with distilled water and resuspended in liquid, and then re-filtered onto a pre-weighed 3 μm diameter membrane (W0) and dried at 105 °C to a constant weight (Wn). Suspended culture: algae biomass was determined using the same method as the attached culture. Algae liquid was filtered onto a pre-weighed 3 μm filter membrane and dried at 105 ℃ to a constant weight. The diameter of the A. beijerinckii cell is approximately 2 μm and the diameter of C. pyrenoidosa cell is greater than 3 μm. The re-filtration onto the 3 μm membrane was designed to remove salts and bacteria (A. beijerinckii) sticking to the microalgal cell surface. The medium volume used in the attached culture was the same as the suspended culture system. In order to compare with the suspended culture system, the biomass unit in attached culture system was converted from g/m2 to g/L. The calculation method of biomass dry weight is as follows:
The calculation method of C. pyrenoidosa specific growth rate (μ) is as follows:
where W refers to the dry weight (g/L) of cells at the initial (m) time or final (n).
Chlorophyll extraction
Methanol extraction method was used to measure chlorophyll [19]. The microalgae solution was centrifuged at 10,000 rpm for 5 min in a high-speed refrigerated centrifuge. After removing the supernatant, the microalgae mud was resuspended in the centrifuge tube by adding methanol, and then the microalgae mud was placed in a water bath for 24 h at 45 °C in the dark. The solution to be tested after the water bath was centrifuged in a high-speed refrigerated centrifuge at 10,000 rpm for 5 min, and then the supernatant was taken to measure the absorbance (OD value) at 470 nm, 652.4 nm, and 665.2 nm, respectively.
Biochemical component analysis
The Bradford method was used to determine protein content [20]. 50 mg algal powder was weighed, 5 ml NaOH (0.5 mol/mL) solution was added and extracted in 100 °C water bath for 10 min, cooled to room temperature, the supernatant was then transferred by centrifugation, and the above operation was repeated 2–3 times until complete extraction, supernatant was combined, and standard curve was made with bovine serum protein.
Phenol–sulfuric acid method was used to determine carbohydrate [21]. 50 mg algal powder was weighed, 5 mL H2SO4 (0.5 mol/L) was added, and the supernatant was placed in a 100 °C constant temperature water bath for 4 h. The supernatant was transferred by centrifugation. Phenol and concentrated sulfuric acid were quickly added and shaken, and the absorbance value was measured at 490 nm after cooling.
Chloroform–methanol method was used to extract lipid [22]. The microalgae powder was ground, and a certain amount of chloroform–methanol (1:2, V:V) was added in the grinding process to extract lipid. After being shook overnight, the solution was added with a certain amount of trichloromethane and distilled water, stratified by centrifugation, and then nitrogen was blown into remove the organic phase, and the lipid layer was measured by weighing.
Determination of superoxide dismutase activity
20 mL of algae culture was centrifuged for 10 min at 4 °C, 10,000 rpm, and then the supernatant was discarded. 1 mL 0.02 M PBS buffer was added to the algae mud, and ultrasonic treated for 30 min under the condition of ice water bath. After the algae cells were fully broken, the algae solution was centrifuged again at 10,000 rpm for 10 min, and the supernatant was the microalgae crude enzyme liquid. The SOD activity was measured with the SOD kit.
Wastewater nutrients’ analysis
The NH4+–N in wastewater was measured by the Nessler’s reagent spectrophotometric method; PO43− was measured by the ammonium molybdate spectrophotometric method [23]. The potassium dichromate method was applied to determine COD [24].
Cell viability assay
The 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay was adopted for determining the cell viability [25]. With the purpose of forming MTT dye (5 mg/mL), MTT was dissolved in PBS. Then MTT dye was added to microalgal solution and incubated at 37 °C for 4 h. After removal of the MTT dye by centrifugation, 1:1 isopropanol and dimethyl sulfoxide were added. Absorbance was then measured at 570 nm.
Data analysis
Three groups in parallel were set for all experiments, and SPSS 17.0 was used to analyze the differences between the groups by ANOVA method. When P < 0.05, the differences between the groups were significant.
Results
Impact of different forms of A. beijerinckii on the promotion of C. pyrenoidosa growth in attached culture
Biomass
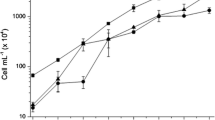
Different forms of A. beijerinckii had different impacts on the biomass of C. pyrenoidosa. Based on Fig. 2, the biomass of C. pyrenoidosa entered a rapid growth phase from the fourth day and stabilized after 14 days. As shown in Fig. 3, at the end of the culture, the biomass of co-culture systems was higher when compared with the pure algae culture system (P < 0.05). Specifically, the biomass of C. pyrenoidosa reached 34.9 g/L with the addition of broken bacteria, and the specific growth rate was 0.3 g/d. The biomass was found to be increased by 71.8% (P < 0.05) in comparison with the pure culture, which was the largest among the four groups. The biomass of bacteria and bacteria supernatant group enhanced by 64.6% and 16.2%, respectively, in relative to pure algae culture group.
Photosynthetic pigments
The change of photosynthesis in microalgae cells is usually reflected by the change in photosynthetic pigments. As can be seen in Fig. 4, The Chlorophyll-a and b contents of the algae + broken bacteria group were 0.8 mg/g and 0.7 mg/g, respectively, which were the highest among the four groups. Compared with the pure culture of C. pyrenoidosa, they increased by 23.1% and 14.1%, respectively. Changes in Chlorophyll content can represent the photosynthetic strength of microalgae, and the ratio of Chl-a/Chl-b can represent the ability of capturing light energy [26]. The ratio of Chl-a/Chl-b in the pure culture of C. pyrenoidosa was 1.01. The ratios of algae + bacteria supernatant group, algae + live bacteria group, and algae + broken bacteria group were 1.15, 1.05 and 1.09, respectively, which showed no significant difference from the C. pyrenoidosa pure culture. Therefore, the addition of different forms of A. beijerinckii did not generate significant changes in Chlorophyll fractions, i.e., the different forms of A. beijerinckii had little effect on the light-response phase, while the effect on photosynthesis of microalgae mainly existed in the dark-response phase, improving the Calvin cycle and the carbon sequestration ability of microalgae [27].
Notably, the proportion of carotenoids increased in the changes of photosynthetic pigments (Fig. 4). Carotenoid synthesis is an innate cellular defense mechanism against oxidative stress, which is caused by adverse environmental conditions. Therefore, changes in stress biomarkers associated with oxidative stress in cells were also examined in this study to further reveal the role of different forms of A. beijerinckii in the attached culture system. Superoxide dismutase (SOD) is an important antioxidant enzyme present in living organisms that regulates oxidative and antioxidant systems, repairs damaged cells and restores free radicals, and plays a crucial role in microalgae [28, 29]. Compared with the pure algae culture, SOD activity increased in co-culture system. In the four groups, the SOD activity of the pure algae group, algae + bacteria supernatant group, algae + bacteria group, algae + broken bacteria group was 10.8 U/mg prot, 11.8 U/mg prot, 20.1 U/mg prot, 23.3 U/mg prot, respectively. It indicated that different forms of A. beijerinckii activated the defensive responses of microalgae cells, reduced oxidative stress capacity, enhanced antioxidant enzyme activity and stimulated the growth of C. pyrenoidosa cells.
Cell viability
To study the impact of different forms of A. beijerinckii on the cell activity of C. pyrenoidosa, the MTT method was used for the assay. As shown in Fig. 5, the OD570 increased with the addition of different forms A. beijerinckii, in which the broken A. beijerinckii group had the highest OD570, followed by algae + live bacteria group, while the effect of the addition of bacteria supernatant on the cell activity of C. pyrenoidosa was not very significant. Therefore, broken bacteria were the best in enhancing the cellular activity of C. pyrenoidosa.
Biochemical components
The components of C. pyrenoidosa were analyzed, and the results are shown in Fig. 6. The analysis of cellular composition indicated that the C. pyrenoidosa components of the pure algae group consisted of 48.3% protein, 24.4% lipids, and 11.3% carbohydrate. Both the addition of live bacteria and broken bacteria promoted the protein accumulation of C. pyrenoidosa. The addition of broken bacteria significantly increased the protein content of C. pyrenoidosa, with an increase of 28.2% in relative to the pure algae culture. The addition of bacteria supernatant did not cause changes in protein content. The lipids’ content of all the co-culture groups decreased when compared with the pure algae culture. But the carbohydrate content in co-culture were all higher than that of pure algal culture. In summary, the protein and carbohydrate contents increased, but the lipid contents decreased in the co-culture system.
Impact of co-culture of C. pyrenoidosa and different forms of A. beijerinckii on wastewater treatment
Using nutrients such as nitrogen and phosphorus in brewery wastewater for microalgal culture can save the cost of microalgae cultivation and improve the economic benefit. Table 1 presents the removal rates of COD, PO43− and NH4+–N in wastewater treated by the attached co-culture. In relative to the pure algae culture system, the co-culture systems all had higher purification efficiency for wastewater. The COD removal rate by the algae pure culture system was 60.5%. The addition of bacteria supernatant and broken bacteria did not improve the removal rate of COD very significantly, which were 61.7% and 63.4% respectively. But the addition of live bacteria made the removal rate of COD reach 73.1%, which was 20.8% higher compared with the pure algae culture system. NH4+–N and PO43− are nutrients necessary for the growth and metabolism of microalgae. As can be seen from Table 1, the addition of different forms of bacteria all improved the removal rates of NH4+–N and PO43− in the wastewater. The removal rates of NH4+–N, PO43− reached 83.0% and 89.1% with the addition of live bacteria, which were the highest among the four groups.
Comparison on the growth of C. pyrenoidosa co-cultured with different forms of A. beijerinckii between attached and suspended culture
Biomass comparison of C. pyrenoidosa in the two cultivation modes were shown in Fig. 7. Compared with the suspended culture mode, the biomass of C. pyrenoidosa in the attached culture mode was higher. In the pure algae group, the biomass of C. pyrenoidosa in attached culture mode was 1.22 times higher than that of suspended culture mode. The addition of broken bacteria caused C. pyrenoidosa to reach a maximum biomass and resulted in a times of 1.32 between the two culture modes. The addition of live bacteria and bacteria supernatant also expanded the times between the two culture modes to 3.77 times and 1.27 times, respectively. It meant that the difference between the two culture modes could be enhanced by the microalgae–bacteria co-culture system.
Discussion
Impact of different forms of A. beijerinckii on the promotion of C. pyrenoidosa growth in attached culture
As presented in this study, different forms of A. beijerinckii significantly stimulated the accumulation of C. pyrenoidosa biomass. During the growth of C. pyrenoidosa, the microalgal cells need to absorb nutrients from the surrounding environment to synthesize organic substances. The addition of different forms of A. beijerinckii may regulate the process of nutrient absorption, thus stimulating C. pyrenoidosa cell growth as well as various physiological activities. Studies have shown that lipopolysaccharides from Aeromonas hydrophila and polysaccharides from Hydrangea can improve the immune function of the host [30]. The biomass improvement of the broken bacteria group was shown to be the highest in this experiment, which meant that the polysaccharides in broken bacteria may have certain effect [31]. Moreover, a certain concentration of indole-3-acetic acid (IAA) could improve the photosynthetic efficiency of microalgae and significantly increase microalgae biomass, and also could generate a certain promotion impact on the accumulation of microalgae pigments [32]. It was reported that the nitrogen-fixing bacteria had the ability to secrete IAA, which could regulate various physiological and biochemical processes in microalgae cells, such as relieving algal cell stress, reducing oxidative damage and improving antioxidant enzyme activity, and, therefore, had a good promotion impact on the growth of microalgae [33]. In this study, broken A. beijerinckii increased the biomass of C. pyrenoidosa better than live A. beijerinckii, which suggested that some active substances from the internal components of the bacteria, such as lipopolysaccharides, polysaccharides and IAA promoted the C. pyrenoidosa growth, while the specific cytoplasmic inclusion were still needed for further study.
As can be seen from Fig. 6, compared with the algae pure culture system, the content of protein and carbohydrate increased, and the content of lipid decreased with the addition of different forms of A. beijerinckii. It has been reported that nitrogen-fixing bacteria can secrete indole-3-acetic acid (IAA), which has a significant effect on the synthesis of protein and other biomolecules in Chlorella vulgaris [34]. Carbon was fixed into algae cells through the Calvin cycle of photosynthesis, and competition between carbohydrates and lipid for carbon has been discovered. It was reported that the lipid content in C. sorokiniana began to increase at the same time as the carbohydrate content decreased [35]. In this study, carbohydrate and lipid also showed opposite trends, suggesting that there was competition between the synthesis pathways of lipid and carbohydrate in cells, and more carbon fixed by photosynthesis flowed to the synthesis pathway of carbohydrate.
Impact of co-culture of C. pyrenoidosa and A. beijerinckii on wastewater treatment
Microalgae provided O2 to facilitate the aerobic bacteria to oxidize and remove organic substances, and could use the CO2 produced from the bacteria respiration, thus made the algae + live bacteria group had the highest COD removal rate [36]. The production of large amounts of C. pyrenoidosa biomass also led to the transfer of large amounts of nutrients from wastewater to C. pyrenoidosa cells. In addition, the cell viability of algae + live bacteria group tested by MTT method was also the higher among the four groups. The higher the cell viability, the higher the ability of microalgae cells to absorb nutrients from the wastewater. As the number of microalgae cells and cell viability increased, the demand for nutrients continued to increase, leading the COD in wastewater to decrease more significantly.
There are two main ways of NH4+–N and PO43− removal from wastewater by microalgae–bacteria system. One way is consumption by the growth of microalgae and bacteria, and another is volatilization of NH4+–N and the precipitation of PO43− as insoluble salts caused by high pH. From the MTT test results (Fig. 5), it can be seen that the addition of A. beijerinckii increased the vigor value of C. pyrenoidosa from 0.12 to 0.18, which significantly improved the vigor of C. pyrenodisa and stimulated its growth rate. The increase of C. pyrenoidosa biomass led to an increase in the demand for nutrients, and the reproduction of A. beijerinckii also absorbed nitrogen and phosphorus in water, thus leading to an improvement of the NH4+–N and PO43−removal rates. Second, when microalgae performed photosynthesis, the pH of the culture medium rose, which led to the volatilization of NH4+–N and precipitation of PO43− [36, 37]. In this study, the pH of algae + live bacteria group increased to 8.5 at day 16, which accelerated NH4+–N volatilization and PO43− precipitation as insoluble salts, thus improving the removal rate of NH4+–N and PO43−.
Comparison of the growth of C. pyrenoidosa co-cultured with different forms of A. beijerinckii between attached and suspended culture modes
Light is one of the most important factors for microalgae growth. Within a certain light intensity range, the growth rate of microalgae increases with the increase of light intensity, while insufficient light will inhibit the pigment absorption and light energy conversion efficiency in algal cells and affect the anabolism of algal cells themselves [38], thus reducing the growth rate of microalgal cells. Light penetration of the attached culture system is better in relative to the suspended culture. Study found that 40% of the algal cells in attached culture system would be effectively irradiated, while only 2.5% were effectively irradiated at the same cell density in suspended culture [36]. CO2 provides a carbon source for algal cells. In suspended culture system, CO2 must overcome the air–liquid interface, so as to transfer to the liquid medium for being used by algal cells. However, in attached culture mode, algal cells are exposed to air and can directly contact with CO2, instead of using the dissolved CO2 in liquid. Thus, the CO2 utilization efficiency is improved due to more efficient mass transfer [37]. Therefore, light transmittance and CO2 transfer efficiency are better in attached culture mode. Besides, the local concentration of probiotics is higher which maybe more conducive to the C. pyrenoidosa growth in attached culture.
Potential of attached algae–bacteria system in microalgae cultivation and wastewater treatment
Compared with suspended culture system, the attached culture system can significantly improve the environmental tolerance, decontamination ability and light utilization efficiency of microalgae. The construction of algae–bacteria symbiotic system can further improve the accumulation of microalgae biomass and the removal of nutrients in wastewater [39]. As shown in this study, the effect of microalgae growth and wastewater purification was obvious. In attached culture, the algae cells are separated from the medium, and the light can directly irradiate on the surface of the cell biofilm without light attenuation. The light utilization rate of unit algae cells is increased, which accelerates the growth of algae cells and also improves the utilization of nutrients such as nitrogen and phosphorus by algae cells [40]. More importantly, after the treatment of wastewater by the attached culture, the algae cells can be collected without centrifugation and simply scraped away, which can save energy consumption, improve the efficiency of algae production and reduce the cost of wastewater treatment.
At present, the wastewater treatment process of algae–bacteria symbiotic system is mainly in the laboratory research stage [41]. The attached algae–bacteria system in this study has certain potential for commercial production due to its high algae growth rate, easy construction, low harvest cost and so on. But the system may also face some challenges in practical application, such as the long-term stability of large-scale symbiotic culture of algae and bacteria, the interference of other organisms (such as zooplankton) in the actual wastewater, the influence of different wastewater components, the optimal design and cost of large-scale bioreactor configuration, and further utilization of algae biomass.
Conclusion
In conclusion, different forms of A. beijerinckii all stimulated the C. pyrenodisa growth and the effect of wastewater treatment. The broken A. beijerinckii had the best promoting effect for the accumulation of C. pyrenodisa biomass and protein, with the biomass increased by 71.8% and protein increased by 28.2%, in comparison with the pure algae group. It indicated that some active substance components inside the A. beijerinckii had the effect of promoting the growth of microalgae cells. The algae + live bacteria group has the best wastewater treatment efficiency, with the removal rates of COD, PO43− and NH4+–N increased by 20.8%,18.5% and 8.9%, respectively, in relative to the pure algae group. The algae–bacteria co-culture system was more conducive to the growth of C. pyrenodisa in the attached culture system than the suspended culture system. The current work offers a new method for improving microalgae biomass and wastewater treatment by algae–bacteria co-cultivation.
Data availability
All date generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
References
Paul C, Pohnert G (2011) Production and role of volatile halogenated compounds from marine algae. ChemInform 42(21):186
Jia H, Yuan Q, Rein A (2016) Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ Sci 2(1):1275089
Liu W, Cui YQ, Cheng PF, Huo SH, Ma XC, Chen QF, Cobb K, Chen P, Ma JJ, Gao XG, Ruan R (2020) Microwave assisted flocculation for harvesting of Chlorella vulgaris. Bioresour Technol 314:123770
Bolch C, Subramanian TA, Green DH (2011) The toxic dinoflagellate gymnodinium catenatum (Dinophyceae) requires marine bacteria for growth. J Phycol 47(5):1009–1022
Grant MA, Kazamia E, Cicuta P, Smith AG (2014) Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures. Isme J 8(7):1418–1427
Cho DH, Ramanan R, Heo J, Lee J, Kim HS (2015) Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour Technol 175:578–585
Tadashi T, Mari K, Tsubasa H, Naoto K, Yasuhiro T, Daisuke I, Kazunari S, Masaaki M, Kazuhiro M (2018) Growth promotion of three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris and Euglena gracilis, by in situ indigenous bacteria in wastewater effluent. Biotechnol Biofuels 11:176
Xue LG, Shang H, Ma P (2018) Analysis of growth and lipid production characteristics of Chlorella vulgaris in artificially constructed consortia with symbiotic bacteria. J Basic Microbiol 58(4):358–367
Oswald WJ, Gotaas HB, Golueke CG, Kellen WR (1957) Algae in waste treatment. Sewage Ind Waste 29(4):437–455
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14(2):557–577
Wang J, Han D, Sommerfeld MR, Lu C, Hu Q (2013) Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J Appl Phycol 25(1):253–260
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energ 88(10):3524–3531
Zhang TY, Hu HY, Wu YH, Zhuang LL, Xu XQ, Wang XX, Dao GH (2016) Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew Sustain Energy Rev 60:1602–1614
Liu TZ, Wang JF, Hu Q, Cheng PF, Bei J, Liu JL, Chen Y, Zhang W, Chen XL, Chen L, Gao LL, Ji CL, Wang H (2013) Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour Technol 127:216–222
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Technol 114:542–548
Wei ZJ, Xiao LI, Wang HN, Yin YH, Jun XL, Sheng GB (2019) Enhanced biomass production and lipid accumulation by co-cultivation of Chlorella vulgaris with Azotobacter Mesorhizobium sp. Chin Biotech 39(7):56–64
Hernandez JP, De-Bashan LE, Rodriguez DJ, Rodriguez Y, Bashan Y (2009) Growth promotion of the freshwater microalga Chlorella vulgaris by the nitrogen-fixing, plant growth-promoting bacterium Bacillus pumilus from arid zone soils. Eur J Soil Biol 45:88–93
Xu LL, Cheng XL, Wang QX (2018) Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front Plant Sci 9:741
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72(1–2):248–254
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Bligh EG, Dyer WJ (1959) A rapid methos of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Eaton AD, Clesceri LS, Greenberg AE, Franson MA (1966) Standard methods for the examination of water and wastewater. Am J Public Health Nations Health 56(3):387–388
Yao N, Liu Z, Chen Y, Zhou Y, Xie B (2015) A novel thermal sensor for the sensitive measurement of chemical oxygen demand. Sensors 15(8):20501–20510
Omar H, Aljudaibiand A, Elgendy A (2018) Abstract: Antimicrobial, antioxidant, anticancer activity and phytochemical analysis of the red algae, Laurencia papillosa. Int J Pharmacol 14(4):572–583
Demmig-Adams B (1996) Chlorophyll and carotenoid composition in leaves of Euonymus kiautschovicus acclimated to different degrees of light stress in the field. Funct Plant Biol 23(5):649–659
Yu Z, Pei HY, Li YZ, Yang ZG, Zhen X, Hou QJ, Nie CL (2020) Inclined algal biofilm photobioreactor (IABPBR) for cost-effective cultivation of lipid-rich microalgae and treatment of seawater-diluted anaerobically digested effluent. Bioresour Technol 315:123761
Liu X, Zhang S, Shan XQ, Christie P (2007) Combined toxicity of cadmium and arsenate to wheat seedlings and plant uptake and antioxidative enzyme responses to cadmium and arsenate co-contamination. Ecotox Environ Safe 68(2):305–313
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26(11):2027–2038
Liu CR, Chen ZP, Zhang ZW (2008) The effects of Aeromonas hydrophila-lipopolysaccharide and Sparasiscrispa-polysaccharide on the immune and digestion function of loach (Misgurnusanguillicaudatus). Mar Sci 32:1–9
Xu YJ, Shan HW, Ma S (2015) Effect of Bacillus sp. and Vibroio alginolyticus on the activities of digestive and immune enzymes disease resistance of Litopenaeus vannamei. Period Ocean Univ China 45:046–053
Zhang B, Chen JC, Su YR, Sun WX, Zhang AL (2021) Utilization of Indole-3-acetic acid–secreting bacteria in algal environment to increase biomass accumulation of ochromonas and Chlorella. Bioenerg Res 15:1–11
Fuentes-Ramirez LE, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J (2018) Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 154:145–150
Zhang B, Chen J, Su Y, Sun W, Zhang A (2021) Utilization of Indole-3-acetic acid–secreting bacteria in algal environment to increase biomass accumulation of Ochromonas and Chlorella. Bioenerg Res 2021:1–11
Li T, Mahmoud G, Feng J (2015) Regulation of starch and lipid accumulation in a microalgal Chorellasorokiniana. Bioresour Technol 180:250–257
Yun H, Wei X, Qiang L, Qian F, Xia A, Zhu X, Sun YH (2016) Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour Technol 222:367–373
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201
Wang JF, Liu JL, Liu TZ (2015) The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae. Biotechnol Biofuels 8:49
Song XY, Luo WH, Mcdonald J (2018) An anaerobic membrane bioreactor-membrane distillation hybrid system for energy recovery and water reuse: removal performance of organic carbon, nutrients, and trace organic contaminants. Sci Total Environ 628–629:358–365
Zhang L, Chen L, Wang J, Chen Y, Gao X, Zhang Z, Liu TZ (2015) Attached cultivation for improving the biomass productivity of Spirulina platensis. Bioresour Technol 181:136–142
Singhl G, Patidar SK (2020) Development and applications of attached growth system for microalgae biomass production. Bioenerg Res 14(3):709–722
Acknowledgements
The current study was partly funded by the Innovation Ability Improvement Project of Small and Medium-sized High-tech Company in Shandong Province (2022TSGC2199); Natural Science Foundation of Shandong Province (ZR2022MC204; ZR2019MB073); Major Science and Technology Innovation Projects in Shandong Province (2019JZZY010723); and National Natural Science Foundation of China (22176104).
Funding
This study was supported by the Innovation Ability Improvement Project of Small and Medium-sized High-tech Company in Shandong Province, 2022TSGC2199, Natural Science Foundation of Shandong Province, ZR2022MC204, ZR2019MB073, Major Science and Technology Innovation Projects in Shandong Province, 2019JZZY010723, and National Natural Science Foundation of China, 22176104.
Author information
Authors and Affiliations
Contributions
HD: conceptualization, data curation, formal analysis, and writing—original draft. WL: supervision, funding acquisition, data curation, and writing—review and editing. HZ: methodology and data curation. ZW: investigation. FF: data curation. LZ: data curation and investigation. HD: data curation and investigation. TX: data curation. XL: investigation. JM: data curation and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
This manuscript does not involve any human participants, human data, human tissue, individual person’s data or animal experiment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, H., Liu, W., Zhang, H. et al. Enhanced biomass production and wastewater treatment in attached co-culture of Chlorella pyrenoidosa with nitrogen-fixing bacteria Azotobacter beijerinckii. Bioprocess Biosyst Eng 46, 707–716 (2023). https://doi.org/10.1007/s00449-023-02855-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02855-8