Abstract
Replacement of the petroleum-based refineries with the biorefinery is regarded as an essential step towards a “zero” waste (circular) economy. Biobased succinic acid (SA) is listed by the United States Department of Energy among the top ten chemicals with the potential to replace chemicals from petroleum synthesis with renewable sources. Purification of bio-based succinic acid from fermentation by-products such as alcohols, formic acid, acetic acid and lactic is a major drawback of fermentative SA production. This study addresses this issue through a novel chromatographic separation using three distinct anionic resins: Amberlite IRA958 Cl (strong base anion exchange resin), Amberlite HPR 900 OH (strong base anion exchange resin) and Amberlyst A21 (week base anion exchange resin). The influence of process variables such as flow rate (0.18 BV/h, 0.42 BV/h and 0.84 BV/h), eluent concentration (1%, 5% and 10% HCl) and temperature (20, 30 and 40 °C) were investigated. The results indicated SA separation efficiency of 76.1%, 69.3% and 81.2% for Amberlyst A21, Amberlite HPR 900 OH and Amberlite IRA958 Cl, respectively. As the regenerant HCl concentration increased from 1 to 10%, calculated succinic acid separation efficiencies decreased from 80.3 to 70.7%. Notably, as the regenerant strength increased from 1 to 10%, the total amount of organic acids desorbed from the resin sharply increased. At operation temperatures of 20, 30 and 40 °C, SA separation efficacies were 81.2%, 73.9% and 76.4%, respectively. The insights from this study will be of great value in design of chromatographic separation systems for organic acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Succinic acid (or butanedioic acid) has been identified by the US. Department of Energy as a top replacement for petroleum-based platform chemicals [1, 2]. Industrial applications of succinic acid include production of detergents, ion chelators, pharmaceuticals, antibiotics and cosmetics [3, 4]. Furthermore, many essential products such as adipic acid, 1, 4-butanediol, tetrahydrofuran, N-methyl pyrrolidinone, 2-pyrrolidinone, succinate salts and gamma-butyrolactone can be synthesised from SA. Hence, demand for succinic acid is expected to grow rapidly in the next few decades [5]. Succinic acid fermentation from renewable resources like whey, straw hydrolysate and sugarcane molasses is cost-effective and promotes environmental sustainability compared to petroleum-based synthesis [6, 7]. Nevertheless, fermentative production of SA presents by-products such as alcohol, acetate, formate and lactic acid which lessen succinate yield, and as well require efficient downstream purification processes. Although most studies have realised improved succinic acid production through gene sequencing of metabolic pathways, engineered SA-producing strains still generate minor products that require purification. In a study by [8], the concentrations of two by-products, lactic and acetic acids, were reduced from 3.47 to 0.27 and 4.96 to 0.85 g/L, respectively, through genome-based metabolic engineering of Mannheimia succiniciproducens. In another study by [9], fed-batch fermentation succinic acid by engineered strains of Mannheimia succiniciproducens LPK7 resulted in production of 52.4 g/L of SA with a yield and productivity of 1.16 mol/mol of glucose and 1.8 g/L/h, respectively. The formation of by-products was greatly reduced to 0.81 g/L of acetic acid, 0.25 g/L of lactic acid and no formic acid. In this regard, economic viability of biotechnological succinic acid production is highly dependent on the efficiency of associated downstream purification processes. Volatile by-products can successfully be discarded by distillation into their respective fractions, but this still leaves the product unpurified from nonvolatile components [10].
In the fermentation industries, conventional separation of organic acids involves precipitation with calcium hydroxide. As for the case of succinic acid, the formed calcium succinate is filtered and subsequently converted to its acidic form by addition of sulphuric acid. The precipitation technique has the disadvantages of product loss during crystallisation, in addition to the potential environmental threat owing to large quantities of calcium compound (CaSO4) formed [11]. Nanofiltration membranes have been used for purification and separation of SA from other by-products; however, their use is limited in the presence of lactic acid, because of similarity in rejection ratios of the two acids [12]. Reactive extraction of organic acids from fermentation broth using amine-based extractants like tri-n-octylamine (TOA) in liquid–liquid extraction or in the inform of supported liquid membrane have also been investigated in literature, but the method is not selective enough for elimination of lactic acids due to the close range in pKa values (SA pKa1 = 4.2, pKa2 = 5.6; lactic acid pKa = 3.86) [13, 14]. In addition to the afore-mentioned techniques, chromatography-based techniques using ion exchange resin, silica, zeolite and alumina adsorption have been widely explored [15, 16]. In chromatographic techniques, carboxylic acid separation is made possible owing to differences in hydrophobicity and acid strength of the individual acids as they elute through a packed column [17, 18]. Ion exclusion chromatography was investigated by Alén et al. [19] and Hellstén et al.[20] to separate carboxylic acids from salts; however, the overall purity of the generated acids was reported to be low. It is noteworthy that most of the existing literature on chromatographic separation of carboxylic acids has been reported on analytical scale using synthetic mixtures [17, 18, 21]; this is unrealistic in real industrial situations involving separation of SA from fermentation broth. Another benefit of ion exchange coupled with chromatographic elution in our case is the separation of residue salts and sugars from the fermentation broth after bioconversion of whey. As observed in our laboratory investigations and also reported by Blanc et al. [22], both cationic and anionic resins can effectively separate sugars and organic acid; however, only the later can separate organic acids.
In the present study, chromatographic separation was attained through several steps including initial screening of different anionic resins to identify the most suitable candidates for a detailed study on retardation behaviour and understanding of the elution profiles generated. Subsequently, the influence of different process variables such as, flow rate, concentration of the regenerant acid and temperature were investigated. The study demonstrates feasibility of chromatographic purification with anionic resins.
Materials and methods
Strain cultivation and fermentation conditions
Actinobacillus succinogenes 130Z (ATCC 55,618) was obtained from the American type Culture Collection. The inoculum was prepared by anaerobically growing the microorganisms for 20 h in tryptic soy broth (TSB) medium in a shaker (BIOSAN, incubator ES-20/60) at 37 °C and 150 rpm. The TSB medium contained per litre: 17.0 g peptone from casein, 3.0 g yeast extract, 2.5 g glucose, 5.0 g NaCl, 2.5 g K2HPO4. Organic acids were fermented using BIOSTAT® Bplus 5 L benchtop fermenter (Sartorius, Germany) equipped with temperature and pH control systems. Fermentation was performed at 37 °C, pH was automatically maintained at 6.8 by addition of 3 N NaOH solution, whilst agitation was maintained at 200 rpm. Basing on literature studies[23, 24], the prepared fermentation medium contained per litre: 40 g whey lactose, 40 g MgCO3, 5.0 g yeast extract, 1.5 g K2HPO4, 3.0 g KH2PO4, 0.3 g CaCl2, NaCl, 0.3 g MgCl2, and 0.07 g MnCl2. Autoclave sterilisation of the fermentation medium was done at 121 °C for 1 h prior to inoculation with 10% (v/v) of the exponentially growing seed culture. The final composition of fermentation broth used in this study is presented Table 1.
Chromatographic separation
Deionised water for use as eluent was generated from CENTRA-R 60/120 system (ELGA LabWater) available at our laboratory. The salient properties of the anionic resins used in this study are listed in Table 2. The resins were thoroughly washed with deionised water prior to use. Strong base anionic (SBA) resin Amberlite 900 OH and Weak Base Anionic (WBA) resin Amberlyst A21 were purchased in OH− form and converted to Cl− form using 5%HCl. Strong Base Anion (SBA) resin Amberlite IRA958 Cl was shipped in Cl− form.
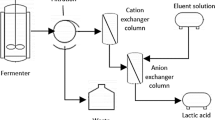
The schematic representation of the experimental setup is illustrated in \* MERGEFORMAT Fig. 1. The experimental setup consisted of a peristaltic fed pump (Masterflex) attached to the inlet of a glass column (Kontes Chromotagrophic Column) equipped with heating jacket. The bed volume (BV) was approximately 500 ml (internal diameter: 4.6 cm, height: 30 cm). The appropriate feed volume for the resins was established by breakthrough curves analyses in preliminary column studies. Each experimental run consisted of two steps. The initial step involved feeding 100 mL of fermentation broth followed by 2 BVs of ultrapure water (eluent) up-flow to the column to washout residual sugars contained in the interstitial resin spacing. In the second step (regeneration), HCl was used as the eluent and organic acids absorbed in the resin were eluted in a chromatographic profile depending on hydrophobicity and acid strength. Samples were collected from the column outlet in fractions of 0.1 BV (50 ml) by an automated fraction collector (ISCO Retriever II) for both feeding and regeneration cycles. Finally, distilled water was used to wash out the HCl contained in the interstitial space, before starting of the succeeding experiments. To investigate the effect of temperature, experiments were carried out at 20, 30 and 40 °C. The effect of flowrate (Q) was studied by varying flow at 0.18, 0.42 and 0.84BV/h; whereas, the effect of regenerant acid concentration was investigated by varying the HCl concentration from 1 to 10%.
Separation efficiency
The efficiency of separation was evaluated based on recovery of succinic acid from its mixer with lactic acid. Other by-products were ignored since they can easily be separated by distillation.
The separation analysis of the chromatography profile is presented in Fig. 2. The holdup volume Vo is the volume of liquid in the whole system including bed porosity, tubing and fitting. The start and end volumes of the lactic acid band are indicated by Vo and VLA,f, respectively. The right-side chromatogram represents succinic acid (SA). Similarly, the start and end volumes of the SA band are indicated by VSA,o and VSA,f, respectively. Integration of the outlet concentration over eluted volume between VSA,o and VSA,f as shown in Eq. (1) corresponds to the total area under desorbed SA chromatogram.
However, the purified succinic acid is the \(Total\,SA_{{area}}\) less volume between VSA,0 and VLA,f, which is the confluence of LA and SA chromatograms.
Recovered succinic acid, illustrated by the shaded area, was obtained from Eq. (3).
Consequently, separation efficiency (\({Y}_{i}\)) was computed from Eq. (4)
Data analysis and experimental reproducibility
Quantitative analysis of organic acids and sugars was done using high-performance liquid chromatography (HPLC) system (Shimadzu LC-20AD, Tokyo, Japan) equipped with a refractive index detector (RID-10A). The HPLC column used was Aminex HPX-87H, 300 mm × 7.8 mm (BIO-RAD), with 0.008 N sulphuric acid mobile phase at a flow rate of 0.6 mL/min and oven temperature of 60 °C. All the samples were filtered through 0.45 µm prior to HPLC analyses. To ensure reproductivity of the results, experiments were performed in duplicate and mean values reported.
Results and discussion
Effect of anionic resin type
To explore the influence of resin type on elution profile of organic acids, three anionic resins in Cl‐ form were studied. Detailed properties of the resins are indicated in Table 2. As a mixture of different components is eluted through a resin column, the individual components slowdown, depending on their affinity for the resin, and thus separation occurs. The mechanisms of separation include ligand exchange, size exclusion, ions exchange and hydrophilic or hydrophobic interactions [25, 26]. The elution profiles for the three resins are shown in Fig. 3. The styrene resins (macroporous) demonstrated a slightly higher absorption capacity than the polyacrylic resin. This is attributable to the hydrophobic nature of polystyrene matrix [27], although the difference in total organic acid absorption of the three resins was not statistically significant (p < 0.05). Another key factor to consider in the operation process is bio-fouling potential. The microporous resins possess large pores, which are less susceptible to bio-fouling [28, 29]. For all the resin types tested, the order of absorption was succinic acid > lactic acid > acetic acid > formic acid. This evidently shows that these acids have different absorption affinities from each another. Another crucial factor to consider in organic acid purification is selectivity of the resins, all the three resins did not adsorb lactose, proteins and other amino acids from the fermentation as indicated in the material balance provided in Table 3. Similar observations have been reported by Li et al. [30] for NERCB 07 and NERCB 09 polystyrene resins. According to Eq. (4), the calculated SA separation efficiency from the elution profiles was 76.1, 69.3 and 81.2% for Amberlyst A21, Amberlite HPR 900 OH and Amberlite IRA958 Cl, respectively. The separation of succinic acid peaks started at 0.9BV. Based on the separation efficiency, Amberlite IRA958 Cl was chosen as a suitable resin for further experimental investigations. Furthermore, the succinic acid chromatogram in the polyacrylic resin demonstrated better band resolution with limited tailing. Whereas, the styrene resins exhibited more prolonged tailing of succinic acid chromatogram, requiring about 2BV to remove the solute components from the column. From economic point of view, the prolonged chromatogram tailings require more eluent, energy and storage volume.
As indicted in Table 4, the organic acid desorption step was free of any residual substrates (lactose, glucose, galactose). This indicates that the anionic exchange resins had no affinity for the residual sugars. In addition, the cleaning step with 2 BVs of ultrapure water (eluent) prior to organic acid desorption with HCl ensured all the solutes retained in interstitial spacing were washed out.
As seen in Table 3, the resin absorbed 0.5–11.2% of residual sugars of lactose, glucose and galactose, whereas traces of sugars were not detected during the desorption cycle (Table 4). The anionic resins work by retaining anionic compounds, slowing their progression through the resin bed. The carboxylic acids will be retained more strongly, causing longer elution time, while salts and non-anionic compounds elute out quickly. Sugars being very weak electrolytes have little tendency to react with ion exchangers and, therefore, form weak complexes. The sugar sorption onto the resin could be attributed to merely partitioning [31]. Since the residual sugars were not detected after washing with 2 BVs of ultrapure water (eluent) prior to organic acid desorption with HCl, it implies that the residual sugars held in the interstitial space microporous resins were washed out with water.
Effect of flow rate
The effect of flow rate on elution profile at constant temperature (20 °C) and regenerant HCl concentration (5%) is shown in Fig. 4. The flow rate affected axial dispersion which in turn affected separation efficiency of succinic acid. Consequently, separation efficiencies were calculated as 81.1%, 81.2% and 55.9% at flow rates of 0.18 BV/h, 0.42 BV/h and 0.84 BV/h, respectively. As flow rate increased, the elution profiles became characterised by shorter and broader peaks. Generally, performance of resin columns in fixed bed operations is dependent on intraparticle diffusion, axial dispersion, adsorption and film diffusion [32]. Therefore, increase in flow rate results in the reduction in resistance of axial dispersion and film diffusion; on the other hand, adsorption and intraparticle diffusion are expected to raise with increase in flow rate. As flow rate increased, the total amount of organic acid sorbed decreased from 6.33 to 5.97 mg/gresin. The slightly higher sorption at low flow rate is due to the adequacy of residence time provided to attain equilibrium. The order of elusion remained as lactic acid–acetic acid–formic acid–succinic acid. This suggests that sorption of lactic acid is poorer compared to succinic acid at these experimental conditions. As mentioned in the previous section, succinic acid is a dicarboxylic acid (pKa1 = 4.2, pKa2 = 5.6) unlike the other acids in the fermentation broth. Dicarboxylic acids dissociate into monocharged and dicharged anions, and each of these species is sorbed by the ion-exchange resin. Since the adsorption cycle was performed at pH > 6, succinic acid was adsorbed in dicharged anion form. This results are very different from those observed by cationic exchange resins in which no particular order was observed in elution profiles [33, 34].
Effect of regenerant acid concentration
Regeneration of the resin was performed in the second cycle of operation to elute the sorbed organic acids as well as prepare the resin column for ensuing tests. For this investigation, concentration of regenerant (HCl) was varied from 1 to 10% and Amberlite IRA958 Cl resin was used at constant flow rate of 0.42 BV/h. Elution profiles are shown in \* MERGEFORMAT Fig. 5. The calculated succinic acid separation efficiencies were 80.3%, 81.2% and 70.7% at regenerant HCl concentration of 1%, 5% and 10%, respectively. Notably, as the regenerant strength increased from 1 to 10%, the total amount of organic acids desorbed from the resin sharply increased (Table 5). This implies that resin regeneration became more efficient at higher regenerant concentration. The mass of total organic acids desorbed from the resin was 4.97, 5.89 and 7.01 mg/gresin at regenerant HCl of 1%, 5% and 10%, respectively, which indicates 25% increase in regeneration efficiency of the resin for increase in HCl concentration from 1 to 10%. The low desorption at 1% HCl concentration could be explained by the insufficiency of accessible displacement ion for the available sorption sites, thus acting as limiting factor for displacement of organic acids. Ion exchange and hydrophobic adsorption are two known adsorption processes that occur in resin treatment [30, 35]. The latter occurs when pH of the solution is less than the pKa of the organic acid. At this condition, the acid is in its free form. Since all experiments in this study were conducted at pH > 6, which is greater than the pKa of all the organic acids in the feed solution, ion exchange was the predominant absorption process.
Effect of temperature on elution of organic acids
Temperature influences hydrophobic interactions in any chemical process [36]. To explore the behaviour of anionic resins under different temperature conditions, experiments were performed at 20, 30 and 40 °C, while maintaining flow rate at 0.42 BV/h. Figure 6 shows the observed elution profiles at various temperatures. The calculated separation efficacies at the afore-mentioned temperatures were 81.2, 73.9 and 76.4%, respectively. Overall, the organic acid sorption of Amberlite IRA958 Cl slightly increased with increase in operation temperature from 293 to 313 K. This was expected as viscosity of solution decreases at higher temperature, which enhances diffusion of the organic acids across the resin eternal pores and external boundary layer [29, 37]. Moreover, the equilibrium constant of most adsorption reactions increases with temperature. Nevertheless, the observed limited increase of sorption process with temperature is attributed to operation at pH > pKa since at this conditions, sorption mainly occurs by ion exchange as hydrophobic adsorption is not favoured [30]. Also striking is that though operations at 313 K presented enhanced absorption capacity for the resin, the separation efficiency reduced at high temperatures probably due to increase in axial dispersion. Furthermore, operation at room temperature (283 K) is desirable in terms of energy requirements.
Table 5 presents results of organic acid desorption at different experimental conditions. As can be seen, the total amount of organic acids desorbed from resin (\(\mathrm{mg}/{\mathrm{g}}_{\mathrm{resin}}\)), increased from 4.97 to 7.01 as the regenerant HCl concentration was increased from 1 to 10%. Although the flow rate affected the dispersion of separation peaks, the effect of flow rate was total acid desorption was insignificant.
In comparison with other studies, an investigation by [38] using reactive extraction with trioctylamine in 1-hexanol and direct crystallisation coupled with cation-exchange resins, obtained succinic acid yields of 73% and 79%, respectively. In another study by [39], highest succinic acid recovery of 75% from fermentation broth was obtained after direct vacuum distillation-crystallisation. In a similar study, one step recovery of succinic acid from fermentation broths by crystallisation achieved 70% succinic acid yield. Electrodialysis was used to concentrate and purify simulated fermentation broths in a study by [40], recovery efficiencies ranged between 50 and 60% on a total carboxylate basis. Combined desalination, electrodialysis and bipolar membrane electrodialysis was developed by Glassner and Datta [41] for succinic acid purification. A total purification yield of 60% was achieved. In the recovery of succinic acid from fermentation broth produced by Mannheimia succiniciproducens, using reactive extraction, vacuum distillation and the crystallisation processes the reported yield was 73.1% [42]. Salting-out extraction, consisting of organic solvents and acidic salts was used by Sun et al. (2014) for recovery and crystallisation of succinic acid from fermentation broths, A yield of greater than 65% was reported [43].
Conclusion
In this study, chromatographic purification of bio-based succinic acid (SA) from other carboxylic acids was studied using weak and strong base anionic exchange resins. The resins used include Amberlite IRA958 Cl Resin (Strong base anion exchange resin), Amberlite HPR 900 OH (Strong base anion exchange resin) and Amberlyst A21 (week base anion exchange Resin). The influence of process variables such as flow rate (0.18 BV/h, 0.42 BV/h and 0.84 BV/h), eluent concentration (1%, 5% and 10% HCl) and temperature (20, 30 and 40 °C) were investigated. The SA elution profile peaked at 0.9—1.2BV and was distinct from the other carboxylic acids. According to acid elution profiles, SA separation efficiency was 76.1%, 69.3% and 81.2% for Amberlyst A21, Amberlite HPR 900 OH and Amberlite IRA958 Cl, respectively. For regenerant HCl concentration of 1%, 5% and 10%, calculated succinic acid separation efficiencies were 80.3%, 81.2% and 70.7%, respectively. Notably, as the regenerant strength increased from 1 to 10%, the total amount of organic acids desorbed from the resin sharply increased. At operation temperatures of 20, 30 and 40 °C, separation efficacies were 81.2%, 73.9% and 76.4% respectively. Overall, over 81% SA separation efficiency from lactic acid was calculated. The insights from this study will be of great value in design of chromatographic separation systems for organic acids.
Data availability statement
The corresponding authors’ data supporting this study’s findings are available upon reasonable request.
References
Saxena RK, Saran S, Isar J, Kaushik R (2017) Production and applications of succinic acid. In: Pandey A, Negi S, Soccol CR (eds) Current developments in biotechnology and bioengineering. Elsevier, pp 601–630. https://doi.org/10.1016/B978-0-444-63662-1.00027-0
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates - The US department of energy’s “top 10” revisited. Green Chem 12:539–554. https://doi.org/10.1039/b922014c
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26:100–108. https://doi.org/10.1016/j.tibtech.2007.11.006
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552. https://doi.org/10.1007/s002530051431
Pateraki C, Patsalou M, Vlysidis A, Kopsahelis N, Webb C, Koutinas AA, Koutinas M (2016) Actinobacillus succinogenes: advances on succinic acid production and prospects for development of integrated biorefineries. Biochem Eng J 112:285–303. https://doi.org/10.1016/j.bej.2016.04.005
Zheng P, Dong JJ, Sun ZH, Ni Y, Fang L (2009) Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresour Technol 100:2425–2429. https://doi.org/10.1016/j.biortech.2008.11.043
Shen N, Zhang H, Qin Y, Wang Q, Zhu J, Li Y, Jiang MG, Huang R (2018) Efficient production of succinic acid from duckweed (Landoltia punctata) hydrolysate by Actinobacillus succinogenes GXAS137. Bioresour Technol 250:35–42. https://doi.org/10.1016/j.biortech.2017.09.208
Lee SJ, Song H, Lee SY (2006) Genome-based metabolic engineering of Mannheimia succiniproducens for succinic acid production. Appl Environ Microbiol 72:1939–1948. https://doi.org/10.1128/AEM.72.3.1939-1948.2006/SUPPL_FILE/AEM02630_05_SUPPLTABLE1_FIGLEG.DOC
Oh I, Lee H, Park C, SL-J of microbiology, undefined 2008, Succinic acid production by continuous fermentation process using Mannheimia succiniciproducens LPK7, Koreascience.or.Kr. (nd). https://www.koreascience.or.kr/article/JAKO200822179194515.page (Accessed Sep 10 2022).
Kumar H, Alén R (2015) Recovery of aliphatic low-molecular-mass carboxylic acids from hardwood kraft black liquor. Sep Purif Technol 142:293–298. https://doi.org/10.1016/j.seppur.2014.12.038
Cao X, Yun HS, Koo YM (2002) Recovery of L-(+)-lactic acid by anion exchange resin Amberlite IRA-400 in. Biochem Eng J. https://doi.org/10.1016/S1369-703X(02)00024-4
Kang SH, Chang YK (2005) Removal of organic acid salts from simulated fermentation broth containing succinate by nanofiltration. J Memb Sci 246:49–57. https://doi.org/10.1016/j.memsci.2004.08.014
Prochaska K, Antczak J, Regel-Rosocka M, Szczygiełda M (2018) Removal of succinic acid from fermentation broth by multistage process (membrane separation and reactive extraction). Sep Purif Technol 192:360–368. https://doi.org/10.1016/j.seppur.2017.10.043
Djas M, Henczka M (2018) Reactive extraction of carboxylic acids using organic solvents and supercritical fluids: a review. Sep Purif Technol 201:106–119. https://doi.org/10.1016/j.seppur.2018.02.010
Cheng KK, Zhao XB, Zeng J, Wu RC, Xu YZ, Liu DH, Zhang JA (2012) Downstream processing of biotechnological produced succinic acid. Appl Microbiol Biotechnol 95:841–850. https://doi.org/10.1007/s00253-012-4214-x
Nam HG, Park KM, Lim SS, Mun S (2011) Adsorption equilibria of succinic acid and lactic acid on amberchrom CG300C resin. J Chem Eng Data 56:464–471. https://doi.org/10.1021/je1008729
Käkölä J, Alén R, Pakkanen H, Matilainen R, Lahti K (2007) Quantitative determination of the main aliphatic carboxylic acids in wood kraft black liquors by high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1139:263–270. https://doi.org/10.1016/j.chroma.2006.11.033
Glód BK (1997) Ion exclusion chromatography: parameters influencing retention. Neurochem Res 22:1237–1248. https://doi.org/10.1023/A:1021933013492
Alén R, Sjöström E, Suominen S (2007) Application of ion-exclusion chromatography to alkaline pulping liquors; separation of hydroxy carboxylic acids from inorganic solids. J Chem Technol Biotechnol 51:225–233. https://doi.org/10.1002/jctb.280510208
Hellstén S, Heinonen J, Sainio T (2013) Size-exclusion chromatographic separation of hydroxy acids and sodium hydroxide in spent pulping liquor. Sep Purif Technol 118:234–241. https://doi.org/10.1016/j.seppur.2013.06.027
Close EJ, Salm JR, Bracewell DG, Sorensen E (2014) Modelling of industrial biopharmaceutical multicomponent chromatography. Chem Eng Res Des 92:1304–1314. https://doi.org/10.1016/j.cherd.2013.10.022
Blanc C, Theoleyre M, Lutin F, Pareau D, Stambouli M (2015) Purification of organic acids by chromatography : adsorption isotherms and impact of elution flow rate. Sep Purif Technol 141:105–112. https://doi.org/10.1016/j.seppur.2014.11.032
Zhang Y, Li Q, Zhang Y, Wang D, Xing J (2012) Optimization of succinic acid fermentation with Actinobacillus succinogenes by response surface methodology (RSM). J Zhejiang Univ Sci B 13:103–110. https://doi.org/10.1631/jzus.B1100134
Corona-González RI, Miramontes-Murillo R, Arriola-Guevara E, Guatemala-Morales G, Toriz G, Pelayo-Ortiz C (2014) Immobilization of Actinobacillus succinogenes by adhesion or entrapment for the production of succinic acid. Bioresour Technol 164:113–118. https://doi.org/10.1016/j.biortech.2014.04.081
Nobre C, Santos MJ, Dominguez A, Torres D, Rocha O, Peres AM, Rocha I, Ferreira EC, Teixeira JA, Rodrigues LR (2009) Comparison of adsorption equilibrium of fructose, glucose and sucrose on potassium gel-type and macroporous sodium ion-exchange resins. Anal Chim Acta 654:71–76. https://doi.org/10.1016/j.aca.2009.06.043
Stefansson M, Westerlund D (1996) Ligand-exchange chromatography of carbohydrates and glycoconjugates. J Chromatogr A 720:127–136. https://doi.org/10.1016/0021-9673(95)00276-6
Xiong Z, Zhao D, Harper WF (2007) Sorption and desorption of perchlorate with various classes of ion exchangers a comparative study in. Ind Eng Chem Res. https://doi.org/10.1021/ie0702025
Sharbatmaleki M, Batista JR (2012) Multi-cycle bioregeneration of spent perchlorate-containing macroporous selective anion-exchange resin. Water Res 46:21–32. https://doi.org/10.1016/j.watres.2011.10.012
Zhu Y, Gao N, Wang Q, Wei X (2015) Adsorption of perchlorate from aqueous solutions by anion exchange resins: effects of resin properties and solution chemistry colloids surfaces a. Physicochem Eng Asp 468:114–121. https://doi.org/10.1016/j.colsurfa.2014.11.062
Li Q, Xing J, Li W, Liu Q, Su Z (2009) separation of succinic acid from fermentation broth using weak alkaline anion exchange adsorbents. Ind Eng Chem Res 48:3595–3599. https://doi.org/10.1021/ie801304k
Vente JA, Bosch H, de Haan AB, Bussman PJT (2016) Sorption and separation of sugars with adsorbents based on reversible chemical interaction. Adsorpt Sci Technol. https://doi.org/10.1260/026361706781388987
Ghim YS, Chang HN (1982) Adsorption characteristics of glucose and fructose in ion-exchange resin columns. Ind Eng Chem Fundam 21:369–374. https://doi.org/10.1021/i100008a009
Lin SKC, Du C, Blaga AC, Camarut M, Webb C, Stevens CV, Soetaert W (2010) Novel resin-based vacuum distillation-crystallisation method for recovery of succinic acid crystals from fermentation broths. Green Chem 12:666–671. https://doi.org/10.1039/b913021g
Omwene PI, Sarihan ZBO, Karagunduz A, Keskinler B (2021) Bio-based succinic acid recovery by ion exchange resins integrated with nanofiltration/reverse osmosis preceded crystallization. Food Bioprod Process 129:1–9. https://doi.org/10.1016/J.FBP.2021.06.006
Tung LA, King CJ (1994) Sorption and extraction of lactic and succinic acids at pH > pKa1. 2. regeneration and process considerations. Ind Eng Chem Res 33:3224–3229. https://doi.org/10.1021/ie00036a042
Uemura Y, Moritake I, Kurihara S, Nonaka T (1999) Preparation of resins having various phosphonium groups and their adsorption and elution behavior for anionic surfactants. J Appl Polym Sci 72:371–378. https://doi.org/10.1002/(SICI)1097-4628(19990418)72:3%3c371::AID-APP7%3e3.0.CO;2-1
Sathishkumar M, Binupriya AR, Kavitha D, Selvakumar R, Jayabalan R, Choi JG, Yun SE (2009) Adsorption potential of maize cob carbon for 2,4-dichlorophenol removal from aqueous solutions: Equilibrium, kinetics and thermodynamics modeling. Chem Eng J 147:265–271. https://doi.org/10.1016/j.cej.2008.07.020
Alexandri M, Vlysidis A, Papapostolou H, Tverezovskaya O, Tverezovskiy V, Kookos IK, Koutinas A (2019) Downstream separation and purification of succinic acid from fermentation broths using spent sulphite liquor as feedstock. Sep Purif Technol 209:666–675. https://doi.org/10.1016/J.SEPPUR.2018.08.061
Luque R, Lin C, Du C, Macquarrie D, A.K.-G. chemistry, undefined 2009, Chemical transformations of succinic acid recovered from fermentation broths by a novel direct vacuum distillation-crystallisation method, Pubs.Rsc.Org. (nd). https://pubs.rsc.org/en/content/articlehtml/2009/gc/b813409j?casa_token=Xb-2BUsiwwcAAAAA:LBivLJobF64hA5D93XPoVQRPg4nwqRDCMLJ-HUcH0PVum6NXwA382aMlwOIhWtGInsDho-fgVxtMEg (Accessed Sept 12 2022).
Sosa PA, Roca C, Velizarov S (2016) Membrane assisted recovery and purification of bio-based succinic acid for improved process sustainability. J Memb Sci 501:236–247. https://doi.org/10.1016/J.MEMSCI.2015.12.018
Glassner D, 143,834 R Datta - US Patent 5, undefined 1992, Process for the production and purification of succinic acid, Google Patents. (nd.). https://patents.google.com/patent/US5143834A/en (accessed Sep 12 2022).
Huh YS, Jun YS, Hong YK, Song H, Lee SY, Hong WH (2006) Effective purification of succinic acid from fermentation broth produced by Mannheimia succiniciproducens. Process Biochem 41:1461–1465. https://doi.org/10.1016/J.PROCBIO.2006.01.020
Sun Y, Yan L, Fu H, Xiu Z (2014) Salting-out extraction and crystallization of succinic acid from fermentation broths. Process Biochem 49:506–511. https://doi.org/10.1016/j.procbio.2013.12.016
Acknowledgements
We wish to thank the Scientific and Technological Research Council of Turkey (TÜBITAK) for providing the financial support under project No. 115Y824
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omwene, P.I., Öcal, Z.B., Yağcıoğlu, M. et al. Novel chromatographic purification of succinic acid from whey fermentation broth by anionic exchange resins. Bioprocess Biosyst Eng 45, 2007–2017 (2022). https://doi.org/10.1007/s00449-022-02805-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02805-w