Abstract
Polypropylene was modified to contain chitosan and evaluate its ability to generate Lactobacillus casei biofilms and their lactic acid production. Biofilm formation was carried out in either rich or minimal media. The chitosan-modified polypropylene harbored ~ 37% more cells than the control polypropylene. The biofilms from the chitosan-modified polypropylene grown in rich medium produced ~ 2 times more lactic acid after 72 h of incubation than the control suspended cells. There was no significant difference in the production of lactic acid after 72 h by L. casei biofilms on the chitosan-modified polypropylene grown in minimal media as compared with cells in suspension after 48 h and 72 h of incubation. Infrared spectroscopy confirmed higher deposition of nutrients and biomass on the chitosan-modified polypropylene as compared to the chitosan-free polypropylene. Electron and atomic force microscopy confirmed thicker biofilms when rich media were used to grow them as compared to minimal medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different microorganisms have the ability to produce through specific biochemical pathways a variety of compounds such as organic acids, alcohols, enzymes, polymers and antimicrobials. With the right environmental conditions (gas composition, temperature, and pH) and substrate availability (nutrient types and concentrations), it is possible to make use of the microbial cells as suspended suspended cells in growth medium to produce value-added compounds at the industrial scales [1]. Through the immobilization of cells on solid supports in the forms of biofilms, it is possible to increase substantially the productivity of metabolites as compared to suspended cells in the growth media [2]. Biofilm formation relies on several factors such as the affinity between the microbial cells and the surface characteristics of the solid support, which in turn depends on the hydrophobicity, hydrophilicity, and the surface energy the cells and the supports may share, the surface charge of the support, as well as its porosity and roughness [3]. Cells are able to deposit and attach on the surface of a solid support mainly through electrostatic interactions. They can also be covalently bound through the reaction between organic functional groups within their structure and the solid support with the aid of bioconjugation techniques [4, 5]. Another approach for cell immobilization that does not involve covalent bond formation is the entrapment of cells within the matrices of porous materials [2].
In general, the preferred method for cell immobilization is passive immobilization, which involves the deposition of cells on the outer surface of the solid support through electrostatic interactions, because it is less detrimental against the integrity and viability of the microorganisms. In contrast, active immobilization involves the above-mentioned phenomena of microbial entrapment within porous matrices and covalent crosslinking between the cells and the solid support, which may negatively impact cell viability [1].
In spite of the mentioned benefits of the use of active biofilm immobilization for the production of metabolites, their practical application has been halted by the poor stability the supports (frequently made from fragile materials such as gels of polysaccharides and proteins) may have [6,7,8,9,10], and the decay in cell viability [8, 11, 12] which may be a result of the toxicity of the substances (crosslinkers or supports) used for their attachment. Thus, there is a need to develop suitable supports for biofilm immobilization that are stable and able to retain cell viability.
The intrinsic chemical properties of a wide range of materials can be tailored effectively through a variety of polymer modification techniques [13, 14]. One potential approach to make materials suitable for cell immobilization is reactive blending or reactive extrusion. Polymer blending is a frequent step in the plastic industry, with diverse applications in transportation, electronics, appliances and packaging [15]. Different polymers may not be miscible or compatible for blending, but they can be modified through the incorporation of functional reactive groups within their molecular structure to become miscible between each other. Another major benefit of these techniques when they are applied to modify the surface of materials is the retention of the functional physical, thermal and mechanical properties of the original bulk material [16,17,18,19]. One suitable option to modify commonly used plastics to enhance biofilm formation is chitosan. Chitosan has been extensively tested for cell immobilization, demonstrating effectiveness for diverse types of mammalian as well as bacteria cells [20,21,22]. Chitosan is intrinsically hydrophilic, which makes it ideal for the formation biofilms of lactic acid bacteria [1, 23].
Lactic acid is an organic acid produced by multiple strains of Lactobacilli with a variety of applications in the food, pharmaceutical, and textile industries, among others. Moreover, given its chemical structure, that makes it suitable to undergo chemical modifications, it can be used for the synthesis of other useful compounds, such as propylene oxide, propylene glycol, acrylic acid, 2,3-pentanedione, lactate ester, and the biodegradable plastic polylactic acid (PLA), which has similar properties as the petroleum-derived plastics [24].
Therefore, this study was undertaken to develop a modified plastic support made with polypropylene and chitosan through reactive blending and evaluate its performance to harbor Lactobacillus casei biofilms for the production of lactic acid.
Materials and methods
Materials
d-Glucose, K2HPO4, agar, acetic acid (95%), acetone, absolute ethanol, isopropanol, glutaraldehyde (25%), hydrochloric acid (1 N), and Na2HPO4 were from Fisher Scientific (Pittsburgh, PA). KH2PO4 and NaCH3COO were from VWR (Philadelphia, PA). MgSO4∙7H2O, MnSO4∙H2O, yeast extract (YE), and lactate standard were from Alfa Aesar (Thermo Fisher Scientific, Waltham, MA). Isotactic polypropylene (PP) and methyl vinyl ether/maleic anhydride copolymer (MVE) were from Scientific Polymer Products (Ontario, NY). Man, Rogosa and Sharpe (MRS) broth, ribitol, acetonitrile, pyridine, methoxamine hydrochloride, N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA), and low molecular weight chitosan (50–190 kDa) were from Sigma-Aldrich (St. Louis, MO). Glass beads (500–750 μm) were from Acros Organics (Fair Lawn, NJ). Peptone was from Oxoid (Thermo Fisher Scientific, Waltham, MA). Anhydrous calcium sulfate was from Drierite Co. LTD (Xenia, OH). Tween® 80 was from MP Biomedicals (Solon, OH). Polybond 7200, a maleic anhydride grafted PP (PP-g-MA) was kindly provided by Dr. John Yun from SI group, Inc. (Niskayuna, NY).
Biofilm solid support preparation
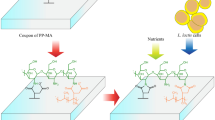
The fabrication of the modified PP support to form L. casei biofilms was based on our recent work [16]. A mixture of PP (50% w/w) and PP-g-MA (50% w/w) pellets was introduced into a bench-top Laboratory Mixing Extruder (LME) with a 1/8 in orifice strand die (Dynisco, Franklin, MA) at 180 °C and 30 rpm. The strands of the resulting polymer blend (referred to as PP-MA, a bulk polypropylene with MA groups provided by PP-g-MA) were turned into pellets with the aid of a chopper and the pellets where hot-pressed at 200 °C and 60 MPa, to obtain films with a thickness of 0.3 ± 0.03 mm. The PP-MA films were cut into 2.0 × 2.0 cm coupons and cleaned in an ultrasonic water bath by immersing them first in acetone and then in deionized (DI) water (2 cycles of 10 min for each solvent at 40 kHz). After cleaning, coupons were dried overnight under anhydrous calcium sulfate (relative humidity of < 30%). Then, the coupons were spin coated at 2000 rpm for 1 min on both sides first with 0.3 mL of MVE in acetone (1 mg/mL) and then with 0.3 mL of chitosan in 1% acetic acid (15 mg/mL) using an anti-corrosion spin coater (VTC-200P-110, MTI Corporation, Richmond, CA). After spin coating, coupons were again allowed to dry in anhydrous calcium sulfate for 30 min and then heat-cured for 1 h at 185 °C. These coupons were referred to as PP-MVE-CHI. Figure 1 shows a depiction of the preparation of PP-MVE-CHI followed the deposition of nutrients and cells (explained later).
Lactobacillus casei biofilm formation on solid support
Lactobacillus casei (12A), a wild type Lactobacillus strain [25, 26], was obtained from the culture collection of the department of Nutrition, Dietetics and Food Sciences at Utah State University (Logan, UT). A loopful of frozen stock in 25% glycerol (− 80 °C) was inoculated by streaking onto MRS agar. The MRS agar plates were incubated for 24 h at 37 °C and an individual colony was inoculated into 9 mL of sterile MRS broth. The inoculated MRS broth was incubated for 24 h at 37 °C and a loopful was inoculated onto new MRS agar. These MRS agar plates were incubated for 24 h at the same temperature and the resulting plates with L. casei colonies were stored at 4 °C not more than for 3 weeks.
All bacterial growth and fermentations were performed under static conditions. To prepare suspensions for biofilm formation, a single L. casei colony from MRS agar was first inoculated into 9 mL of MRS broth and incubated at 37 °C for 24 h. Then, a 10% dilution (v/v) of this broth was prepared with sterile lactic acid fermentation rich medium, which composed of D-Glucose (20 g/L), YE (4 g/L), KH2PO4 (0.5 g/L), K2HPO4 (0.5 g/L), sodium acetate (1 g/L), MgSO4∙7H2O (0.6 g/L), and MnSO4∙H2O (0.018 g/L) and incubated for 24 h at 37 °C (for simplicity, this medium will be referred to as just rich medium) [23, 27, 28]. In parallel, as prepared PP-MA and PP-MVE-CHI coupons were individually immersed in 50 mL sterile conical tubes with 20 mL of rich medium for initial nutrient deposition on their surface also for 24 h at 37 °C (the coupons completely immersed in the media, for their both surfaces to be exposed). After the 24 h of incubation, the inoculated rich media were centrifuged in 2 cycles at 1949×g for 10 min per cycle and the supernatant was replaced with 20 mL of new sterile rich medium after each centrifugation. A 1% dilution of this suspension was prepared with new sterile rich medium, to have an initial inoculum of ~ 7 log(CFU/mL), and the PP-MA and PP-MVE-CHI coupons that had been subjected to 24 h of immersion in sterile rich medium were individually transferred into 20 mL of this ~ 7 log (CFU/mL) L. casei suspension for initial cell attachment, which was followed by 48 h of incubation at 37 °C. After these 48 h of incubation for initial cell deposition, the PP-MA and PP-MVE-CHI coupons were subjected to 5 cycles of repeated batch fermentations for biofilm growth (48 h per cycle) in which the medium was replaced at the end of each cycle with new and sterile medium (20 mL per coupon). In the initial cell deposition and in the repeated batch fermentations, the coupons were immersed in 50 mL sterile conical tubes with 20 mL of the corresponding medium for total surface exposure. Two types of fermentation media were used in these cycles: rich medium and minimal medium (with the same ingredients of rich medium with the exception of YE). The use of minimal medium was explored to test the possibility of biofilm development with fewer ingredients. This procedure was conducted in at least 3 independent replicates.
Determination of cell density on solid support
To determine the number of cells attached on the PP-MA and PP-MVE-CHI coupons after the 5 cycles of repeated batch fermentations in either rich or minimal media, a published method was followed [23]. Briefly, the coupons were first transferred to 20 mL of sterile peptone water (PW, 0.1% w/v peptone in distilled water) and vortexed vigorously to remove loose bacteria for 5 s. Then, the coupons were aseptically transferred to 10 mL of sterile PW with 5 g of sterile glass beads to be vigorously vortexed in three 30 s cycles to remove the attached cells. Serial dilutions in sterile PW were prepared from the vortexed suspension and 100 μL of each dilution were inoculated onto MRS agar, which was followed by incubation at 37 °C for 48 h, and colony enumeration. The results were expressed as log(CFU/cm2), taking into account the total surface area of a single coupon (8 cm2). The support (PP-MA or PP-MVE-CHI) with the highest cell density was chosen to test the production of lactic acid, as explained below.
Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR)
The surface chemistry of PP-MA and PP-MVE-CHI coupons was analyzed as reported previously [16,17,18]. The coupons were analyzed with an IRTracer-100 infrared spectrometer (Shimadzu Corporation, Japan) equipped with a diamond ATR crystal (Quest Single Reflection ATR Accessory, Specac Limited, UK). Multiple spots on samples from independent replicates were analyzed through Happ-Genzel apodization (4 cm−1 resolution, 32 scans per spot). Spectra analysis was done with the software KnowItAll (Biorad Laboratories, Philadelphia, PA). PP-MA and PP-MVE-CHI coupons were analyzed as prepared and after the biofilm growth in the 5 repeated batch fermentations, in either rich or minimal media. For the latter case, individual coupons were first vigorously vortexed for 5 s in 20 mL of sterile DI water to remove loose bacteria and then allowed to dry under anhydrous calcium sulfate until the excess humidity had evaporated, to be then analyzed through ATR-FTIR as explained above.
Contact angle goniometry
Static contact angle (θs) was measured by applying a droplet of DI water (1 μL) on the surface of the as prepared PP-MA and PP-MVE-CHI coupons, using a VCA Optima digital contact angle instrument (AST Products, Billerica, MA). Measurements were performed from at least 3 independently prepared coupons of either PP-MA or PP-MVE-CHI.
Scanning electron microscopy (SEM)
After completion of the 5 cycles of repeated batch fermentations in either rich or minimal media, the supports (PP-MA or PP-MVE-CHI) with higher cell density were subjected to SEM analysis as follows. Single coupons were first immersed for 1 h at room temperature in 20 mL of 2% glutaraldehyde in sterile Sorensen’s buffer (133 mM Na2HPO4 and 133 mM KH2PO4 in DI water, pH 7.0). Then, the coupons were rinsed 3 times in 20 mL of sterile Sorensen’s buffer (10 min per rinse) to shake off loose bacteria. This was followed by gentle rinses (immersions) in aqueous ethanol solutions as follows: 2 rinses in 20 mL of 70% (v/v) absolute ethanol in DI water (5 min per rinse), 1 rinse in 20 mL of 90% (v/v) absolute ethanol in DI water (5 min), 1 rinse in 20 mL of 95% (v/v) absolute ethanol in DI water (5 min), and 3 rinses in 20 mL of absolute ethanol (10 min per rinse). Coupons were then subjected to Critical Point Drying (CPD) and sputter-coating with a 10 nm layer of gold and palladium using a rotary sputter coater system EMS150R ES (Electron Microscopy Sciences, Hatfield, PA). SEM analysis was done with a FEI Quanta FEG 650 (Field Electron and Ion Company, Hillsboro, OR) under low vacuum at an accelerating voltage of 10 kV.
Atomic force microscopy (AFM)
As with SEM analysis, after completion of the 5 cycles of repeated batch fermentations in either rich or minimal media, the type of supports (PP-MA or PP-MVE-CHI) with higher cell density were subjected to AFM analysis in the same way as for SEM analysis. After sputter-coating with gold and palladium, AFM images of the coupons were obtained with a Nanoscope III Bioscope (Digital Instruments, Inc., NY) under taping mode. Budget Sensors-Tap 300AL-G cantilevers with a tip radius of curvature < 10 nm, 125 μm length, 30 μm width, 4 μm thickness and a 40 N/m force constant were used. Images were captured at 256 × 256 pixel resolution and 1 Hz over a range of scan sizes and angles. Bacteria dimensions and mean surface roughness were calculated with the Digital Instruments software (v5.21).
Lactic acid fermentation and analysis
After completion of the 5 cycles of repeated batch fermentations for biofilm growth in rich and minimal media and the cell density determination, the type of support (PP-MA or PP-MVE-CHI) with higher cell density was subjected to a single cycle of fermentation to challenge the ability of its L. casei biofilms to produce lactic acid. After the 5 repeated batch fermentations, multiple coupons were transferred individually to 20 mL of sterile rich media and incubated for up to 72 h at 37 °C. Media samples were collected over time for lactic acid analysis, which was conducted through gas chromatography–mass spectrometry (GC–MS) as follows [29], using 20 mL volumes of rich media with suspended L. casei cells as controls (initial inoculum of ~ 7 log (CFU/mL)). At different time points during fermentation (0, 24, 48 and 72 h), the fermentation media were collected and separated from the coupons as needed. Then, the medium was centrifuged at 3800 × g and 4 °C for 15 min. The supernatant was decanted and stored at – 20 °C until analysis. Media samples were diluted 10% (v/v) with DI and 50 µL was aliquoted into a 1.5 mL polypropylene microcentrifuge tube and spiked with 10 µL of 200 ppm ribitol as an internal standard. Metabolites were extracted in 1 mL of an acetonitrile, isopropanol, and DI water mixture (3:3:2, v/v/v). The mixture was vortexed for 5 min, and then centrifuged at 14,000 × g for 5 min. Next, 450 µL of the supernatant was removed and evaporated to dryness in a rotary evaporator. For quantification, a seven-point standard curve was made by serial 50% dilutions (v/v) of 100 mM lactate in sterile rich media, and the standards were prepared according to the same procedure as the samples. After samples and standards were dried, 10 µL of methoxamine hydrochloride in pyridine (20 mg/mL) was added and samples were shaken at 30 °C for 1.5 h. Next, 80 µL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) was added to each sample, and tubes were shaken for an additional 30 min. Samples were then transferred to 100 µL glass inserts in gas chromatography vials and analyzed by GC–MS. The GC–MS analysis was conducted using a Shimadzu QP2010 (Shimadzu Corporation, Columbia, MD) equipped with a ZB-5MSi column (30 m × 0.25 mm internal diameter × 0.25 µm film thickness; Phenomenex, Torrance, CA) fitted with a 5 m inactivated silica guard column. One µL of sample was injected and split (25:1) in the injection port which was held at 250 °C. The column was held at 60 °C for one minute, and then ramped at 10 °C/min to 325 °C and held for 1 min. The interface temperature was held at 290 °C, and the detector at 230 °C. Helium was used as the carrier gas, and the flow rate was 36.7 cm/s. Analytes were ionized by electron impact, and ions from 50 to 600 m/z were scanned 5 times per second. The detector was held at 1.2 kV. Lactic acid concentrations in rich media were expressed as millimoles per L (mM). At least 3 replicates were performed for this evaluation.
Statistical analysis
When appropriate, significance between treatments was determined through Analysis of Variance (ANOVA) and Tukey’s pairwise comparisons using Minitab® (Minitab Inc., State College, PA). Linear or nonlinear regression analyses were done using SigmaPlot® (Systat Software, San Jose, CA). Significance was set at α = 0.05.
Results
Biofilm solid support cell density and surface characterization
When biofilm formation took place in the repeated batch fermentations with rich media, L. casei cell population on the PP-MVE-CHI coupons (6.1 ± 0.3 log(CFU/cm2)) was on average 37% higher than the cell population on PP-MA (5.8 ± 0.4 log(CFU/cm2)). When minimal media were employed, the cells were not culturable, even if the inoculated MRS plates were incubated for more than 48 h at 37 °C. Even though the cell density between the PP-MA and PP-MVE-CHI subjected to the repeated batch fermentations in rich media was not significant (P > 0.05), PP-MVE-CHI was selected for the microscopy evaluations and lactic acid fermentation evaluations as shown below, given its apparent ability to bind more nutrients and cells.
The values of static contact angle (θs) for as prepared PP-MA and PP-MVE-CHI were 105.0 ± 3.0° and 88.0 ± 1.0°, respectively. This indicates that PP-MA is hydrophobic and PP-MVE-CHI is slightly hydrophilic (θs < 90°).
Figure 2 shows the ATR-FTIR spectra of the as prepared PP-MA and PP-MVE-CHI coupons, and the PP-MA and PP-MVE-CHI coupons after the 5 repeated batch fermentations in rich medium (RM) and minimal medium (MM). The as prepared PP-MA coupons show the characteristic carbonyl group C=O at 1780 cm−1, and the carboxylic acids group –COO– at 1720 cm−1 given by the maleic anhydride rings. The as prepared PP-MVE-CHI coupons show the vibration of the carbonyl groups in the imide bonds (1755–1680 cm−1), which corroborates covalent bond formation between MVE and chitosan. After the 5 cycles of repeated batch fermentations in either rich or minimal media, the formation and/or concentration increase of amides can be seen from the corresponding C=O and N–H functional groups at 1755–1680 cm−1 and 1570–1515 cm−1, respectively [16, 30]. These bands present higher absorbance from coupons exposed to rich media, which suggests higher concentration of cells and nutrients, and the concentration of these organic entities is higher in the surface of PP-MVE-CHI. Therefore, due to its confirmed ability to harbor more cells and nutrients, PP-MVE-CHI was selected for the microscopy evaluations and lactic acid fermentation evaluations, which results are shown later in the text.
Scanning electron microscopy and atomic force microscopy
Figures 3, 4, 5, 6 show L. casei biofilms formed on PP-MVE-CHI after the 5 repeated batch fermentations in either minimal or rich media. For the former case, the biofilms did not cover the area of the support completely, and the biofilms presented themselves in clusters of bacteria with a thickness of a few cells. When rich medium was used, a thick layer of biofilm developed with multiple cells, and it covered most of the support’s surface. This was confirmed by AFM analysis (Fig. 7), showing that when minimal medium was employed, the biofilms formed as thin layers of bacteria with a few cells on the top of each other, while dense multicellular layers developed when rich medium was employed.
Lactic acid fermentation and analysis
Table 1 shows the lactic acid production over time by the control L. casei suspended cells and the PP-MVE-CHI coupons with L. casei biofilms formed in either minimal or rich media after the 5 repeated batch fermentations (treatments that share the same superscript letter within Table 1 are not significantly different, P > 0.05). After 24 h of incubation, the biofilms from PP-MVE-CHI grown in rich media were able to produce a significant (P < 0.05) concentration of lactic acid (6.2 ± 0.6 g/L), amount ~ 2.8 times higher on average than the control suspended cells at the same time of incubation (2.2 ± 0.2 g/L). The trend continued for these 2 treatments throughout the fermentation period; after 48 and 72 h of incubation, the PP-MVE-CHI coupons with L. casei biofilms formed in rich media produced on average ~ 1.7 and ~ 1.74 times more lactic acid than the control (suspended cells), respectively, giving a final average yield based on the initial glucose concentration (20 g/L) of 41%, substantially higher than the yield obtained by the control suspended cells (24% on average). For the case of the biofilms from PP-MVE-CHI grown in minimal media, no detectable levels of lactic acid were measured until 48 h, and after 72 h the amount produced (4.0 ± 0.4 g/L) was statistically the same given by the control after 48 and 72 h of incubation (P > 0.05). Figure 8 shows the lactic acid production over time from the different treatments.
Lactic acid production by L. casei biofilm on PP-MVE-CHI, formed in either rich media ( ) or minimal media (
) or minimal media ( ), and the suspended cells controls (○). Lactic acid was measured through GC–MS using MSTFA derivatives as outlined in Materials and methods
), and the suspended cells controls (○). Lactic acid was measured through GC–MS using MSTFA derivatives as outlined in Materials and methods
Discussion
The higher cell density found in PP-MVE-CHI as compared to PP-MA may be attributed to its higher hydrophilic character (lower θs). The chemical composition of the cell membrane of lactic acid bacteria make them prone to attach to hydrophilic surfaces [1, 23]. In addition, hydrophilic surfaces and materials are prone to experiencing fouling of organic matter, such as proteins and sugars [31]. This agrees with the apparent higher deposition of L. lactis cells and nutrients on PP-MVE-CHI as compared to PP-MA. The high concentration of cells and organic matter was also confirmed by the higher levels of absorbance by the organic functional groups (mainly amides) that can be assigned to proteins (Fig. 2, C=O and N–H bands at 1755–1680 cm−1 and 1570–1515 cm−1, respectively) and that are also an infrared feature of lactic acid bacteria [32].
Even though L. casei biofilms formed under repeated batch fermentations in minimal media were not culturable, their formation was confirmed through SEM and AFM on the surface of PP-MVE-CHI (Figs. 3,4,5,67). It has been reported that some species of nonsporeformer bacteria can enter a phase known as viable but nonculturable (VBNC), in which they are able to drastically reduce their metabolic rates as a survival response to marginal availability of nutrients and a harsh environment (such as extreme pH, temperature, and gas composition), but retain viability [33]. Eventually, and under the right conditions for growth, VBNC cells can resuscitate and reproduce. This phenomenon was demonstrated in our study in L. casei biofilms on PP-MVE-CHI (grown with minimal media) by the substantial increase in lactic acid concentration after 72 h of fermentation in rich medium, which was statistically the same given by the control L. casei suspended cells after 48 and 72 h (P > 0.05). Comparable results were obtained in a study in which a composite of polypropylene and biopolymers (such as yeast extract, bovine albumin, soybean flour, and soybean hulls) was used to harbor L. casei biofilms [23]. In the cited work, the maximum lactic acid concentration obtained was 7.6 g/L. The highest production of lactic acid was given at every time point by L. casei biofilms on PP-MVE-CHI grown with rich media. This latter treatment was able to provide a lactic acid concentration ~ 100% higher than the control L. casei suspended cells. This suggests the potential of this concept of cell immobilization to increase substantially the production of metabolites in industrial fermentations.
Conclusions
In this study, it was demonstrated that surface modification with organic compounds (specifically polycations like chitosan) of commonly used polymers can enhance the formation of biofilms able to produce almost 2 times more (8.2 ± 1.2 g/L, 72 h of incubation) metabolite as compared to control suspended cells (4.7 ± 0.1 g/L, 72 h of incubation). In addition, the use of minimal media may be considered as a practical and cost-effective approach for the development of biofilms, as it was demonstrated in this study that those biofilms can remain viable and able to produce metabolites after exposure to suitable media. Infrared spectroscopy confirmed that the surface modification of polypropylene with chitosan enhanced nutrient and biomass deposition, and microscopy analyses also confirmed that with the use of rich media biofilms are thicker and have higher cell density. This work suggests that with this approach, the productivity of fermentation processes can be substantially improved.
References
Ercan D, Pongtharangkul T, Demirci A, Pometto AL III (2015) Applications of biofilm reactors for production of value-added products by microbial fermentation. In: Anthony LP, Ali D (eds) Biofilms in the food environment. Wiley, pp 255–283
Verbelen PJ, De Schutter DP, Delvaux F et al (2006) Immobilized yeast cell systems for continuous fermentation applications. Biotechnol Lett 28:1515–1525. https://doi.org/10.1007/s10529-006-9132-5
Klein J, Ziehr H (1990) Immobilization of microbial cells by adsorption. J Biotechnol 16:1–15. https://doi.org/10.1016/0168-1656(90)90061-f
Oliveira R (1997) Understanding adhesion: a means for preventing fouling. Exp Therm Fluid Sci 14:316–322. https://doi.org/10.1016/S0894-1777(96)00134-3
Hermanson GT (2008) Bioconjugate techniques, 2nd edn. Academic Press, Boston
Genari AN, Passos FV, Passos FML (2003) Configuration of a bioreactor for milk lactose hydrolysis. J Dairy Sci 86:2783–2789. https://doi.org/10.3168/jds.S0022-0302(03)73875-2
Krasaekoopt W, Bhandari B, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J 13:3–13. https://doi.org/10.1016/S0958-6946(02)00155-3
Lamboley L, St-Gelais D, Champagne CP, Lamoureux M (2003) Growth and morphology of thermophilic dairy starters in alginate beads. J Gen Appl Microbiol 49:205–214
Lemay MJ, Champagne CP, Gariépy C, Saucier L (2002) A comparison of the effect of meat formulation on the heat resistance of free or encapsulated cultures of Lactobacillus sakei. J Food Sci 67:3428–3434. https://doi.org/10.1111/j.1365-2621.2002.tb09601.x
Champagne CP, Lee BH, Saucier L (2010) Immobilization of cells and enzymes for fermented dairy or meat products. In: Zuidam NJ, Nedović V (eds) Encapsulation technologies for active food ingredients and food processing. Springer, New York, pp 345–365
Champagne CP, Gardner NJ, Lacroix C (2007) Fermentation technologies for the production of exopolysaccharide-synthesizing Lactobacillus rhamnosus concentrated cultures. Electron J Biotechnol 10:211–220
Morin N, Bernier-Cardou M, Champagne CP (1992) Production of Lactococcus lactis biomass by immobilized cell technology. J Ind Microbiol 9:131–135. https://doi.org/10.1007/BF01569745
Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32:698–725
Bastarrachea LJ, Denis-Rohr A, Goddard JM (2015) Antimicrobial food equipment coatings: applications and challenges. Annu Rev Food Sci Technol 6:97–118. https://doi.org/10.1146/annurev-food-022814-015453
Backer WE, Hu G (2001) Introduction. In: Baker WE, Scott CE, Hu G (eds) Reactive polymer blending. Cincinnati, OH, pp 2–12
Gagon AT, Britt DW, Bastarrachea LJ (2020) Antimicrobial light-activated polypropylene modified with chitosan: characterization and reusability. J Agric Food Chem 68:13076–13082. https://doi.org/10.1021/acs.jafc.9b06009
Gagon AT, Britt DW, Bastarrachea LJ (2020) Zein-modified antimicrobial polypropylene: characterization and reusability upon UV-A light exposure. LWT 121:108983. https://doi.org/10.1016/j.lwt.2019.108983
Bastarrachea LJ (2019) Antimicrobial polypropylene with ε-poly(lysine): effectiveness under UV-A light and food storage applications. LWT 102:276–283. https://doi.org/10.1016/j.lwt.2018.12.047
Bastarrachea LJ, Goddard JM (2016) Self-healing antimicrobial polymer coating with efficacy in the presence of organic matter. Appl Surf Sci 378:479–488. https://doi.org/10.1016/j.apsusc.2016.03.198
Machluf M (2005) Protein therapeutic delivery using encapsulated cell platform. In: Nedović V, Willaert R (eds) Applications of cell immobilisation biotechnology. Springer, Netherlands, pp 197–210
Nedović V, Willaert R, Leskošek-Čukalović I et al (2005) Beer production using immobilized cells. In: Nedović V, Willaert R (eds) Applications of cell immobilization biotechnology. Springer, Netherlands, pp 259–273
Willaert Ronnie G, Baron Gino V (1996) Gel eentrapment and micro-encapsulation: methods, applications and engineering principles. Rev Chem Eng 12:1–205. https://doi.org/10.1515/REVCE.1996.12.1-2.1
Ho KL, Pometto AL 3rd, Hinz PN et al (1997) Ingredient selection for plastic composite supports for L-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. rhamnosus. Appl Environ Microbiol 63:2516–2523. https://doi.org/10.1128/AEM.63.7.2516-2523.1997
Malinconico M, Vink ETH, Cain A (2018) Applications of poly(lactic acid) in commodities and specialties. Adv Polym Sci 282:35–50
Vinay-Lara E, Wang S, Bai L et al (2016) Lactobacillus casei as a biocatalyst for biofuel production. J Ind Microbiol Biotechnol 43:1205–1213. https://doi.org/10.1007/s10295-016-1797-8
Kandler O, Weiss N (1986) Genus Lactobacillus. In: Sneath H, Mair N, Sharpe M, Holt J (eds) Bergey’s manual of systematic bacteriology. Williams and Wilkins, Baltimore, pp 1063–1065
Demirci A, Pometto AL, Johnson KE (1993) Evaluation of biofilm reactor solid support for mixed-culture lactic acid production. Appl Microbiol Biotechnol 38:728–733. https://doi.org/10.1007/BF00167135
Demirci A, Pometto AL (1995) Repeated-batch fermentation in biofilm reactors with plastic-composite supports for lactic acid production. Appl Microbiol Biotechnol 43:585–589. https://doi.org/10.1007/BF00164758
Fiehn O (2016) Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr Protoc Mol Biol 114:30.4.1-30.4.32. https://doi.org/10.1002/0471142727.mb3004s114
Smith BC (1998) Infrared spectral interpretation: a systematic approach. Taylor & Francis
Barish JA, Goddard JM (2013) Anti-fouling surface modified stainless steel for food processing. Food Bioprod Process 91:352–361. https://doi.org/10.1016/j.fbp.2013.01.003
Santos MI, Gerbino E, Tymczyszyn E, Gomez-Zavaglia A (2015) Applications of infrared and raman spectroscopies to probiotic investigation. Foods (Basel, Switzerland) 4:283–305. https://doi.org/10.3390/foods4030283
Liu J, Deng Y, Li L et al (2018) Discovery and control of culturable and viable but non-culturable cells of a distinctive Lactobacillus harbinensis strain from spoiled beer. Sci Rep 8:11446. https://doi.org/10.1038/s41598-018-28949-y
Acknowledgements
Authors would like to thank Dr. John Yun from SI group, Inc. for providing Polybond 7200 and Dr. FenAnn Shen from the Microscopy Core Facility at Utah State University (Logan, UT) for assistance in the Scanning Electron Microscopy analyses. This work was supported by the Utah Agricultural Experiment Station UTA01377 and approved as Journal Paper 9500.
Funding
Utah Agricultural Experiment Station project UTA01377.
Author information
Authors and Affiliations
Contributions
LJB designed and executed experiments, authors DWB and REW aided in surface and metabolite analytical techniques, author AD provided additional assistance in experimental design and results interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bastarrachea, L.J., Britt, D.W., Ward, R.E. et al. Development of bioactive solid support for immobilized Lactobacillus casei biofilms and the production of lactic acid. Bioprocess Biosyst Eng 45, 217–226 (2022). https://doi.org/10.1007/s00449-021-02654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02654-z