Abstract
The laccase has the ability to oxidize substituted phenols and the water is the sole byproduct, thus it has been employed to remove and/or modify the lignin in lignocellulosic material. A putative laccase gene, LacSM, from Sordaria macrospora k-hell was screened by a genome mining approach. Then, it was cloned and highly expressed in Escherichia coli. The molecular weight of recombinant LacSM was ~ 67 kDa. The optimal pH values for the LacSM oxidation of guaiacol, syringaldazine, 2,6-dimethoxyphenol, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) were 6, 7, 5, and 5, respectively. The optimal activity of laccase was observed at 60, 55, 55, and 50 °C for four respective substrates. LacSM remained stable at pH 5–8 and thermostable at 60 °C with guaiacol as the substrate. 1 mM K+, Na+, or Mn2+ ions slightly stimulated laccase activity. In addition, LacSM was moderately tolerant to the Cl− ion and showed an ability to remove and/or modify lignin. Thus, LacSM was a potential candidate for industrial applications, such as lignin degradation of lignocellulosic biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laccases (EC1.10.3.2, phenoloxidases) are multicopper oxidases with the ability to oxidize various organic and inorganic compounds, such as polyphenols, diphenols, and aromatic amines. Substrate oxidation is coupled to the four-electron reduction of dioxygen to water [1]. Laccases have been reported in bacteria, fungi, plants, and insects [2]. The physiological roles of laccases depend on their origin [3]. In plants, laccase induces monolignolic building blocks to generate radical polymerization, which results in lignin biosynthesis; laccase produced by fungi catalyzes the opposite process, and leads to lignin degradation. Laccase is assumed to be a “green” biotechnology since its byproduct is water. It was widely used industrially e.g., for paper pulp biobleaching, synthetic dye decolorization, and lignin degradation [4].
Lignocellulosic biomass is a renewable carbon-based energy source. The conversion of lignocellulose is impeded by its recalcitrant structure. The main components of lignocellulose are lignin, hemicellulose, and cellulose. Lignin interlaces with hemicellulose to cover the cellulose, which physically blocks the cellulases to contact the cellulose, and/or deactivates cellulases via binding [5]. Thus, lignin must be removed from carbohydrates under suitable conditions. Lignin is a three-dimensional polymer formed by phenylpropanoid subunits, and its degradation is difficult. Bacterial and fungal laccases can degrade lignin [6, 7]. Fungal laccases have high redox potential and have attracted widespread attention. Basidiomycete laccase, from the genus Trametes, dissociates lignin from the whole lignocellulosic biomass, thus making cellulose accessible for hydrolysis [8]. Ascomycete laccase, from Myceliophthora thermophila, was used to pretreat Eucalyptus feedstock, which improved saccharification [7]. However, studies on phytopathogenic fungi laccases are relatively few, and fungal laccases with novel functions need to be identified to facilitate their industrial use [9].
In this study, a novel laccase from Sordaria macrospora k-hell (LacSM) was identified and the lacSM gene of S. macrospora k-hell was cloned. Then, the protein was expressed in Escherichia coli and purified for both biochemical and kinetic characterizations. The laccase of S. macrospora k-hell displayed the ability to degrade lignin.
Materials and methods
Strains, media, and chemicals
The pET-30a plasmid with the lacSM gene was amplified in E. coli Top10 (Invitrogen). The recombinant laccase was expressed in BL21 (DE3) (Novagen). The protocols and media used follow the manual of the Novagen pET system. The chemicals guaiacol, 2,6-dimethoxyphenol (2,6-DMP), syringaldazine (SGZ), and 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were obtained from Sigma Aldrich. All chemicals were of analytical grade and were used without further purification.
Cloning of LacSM and vector construction
Uncharacterized laccases were searched online (https://blast.ncbi.nlm.nih.gov/Blast.cgi), using a protein BLAST, with the laccase (Accession Number XP_003663741.1 in GenBank) from Myceliophthora thermophila as the search template. From the numerous homologs, a protein from S. macrospora k-hell (XP_024511624.1) was selected, with an identical amino acid sequence to that of the M. thermophila laccase by over 60%. The cleavage sites of signal peptide in this protein were identified using SignalP 4.1 (www.cbs.dtu.dk/services/SignalP/). The pI value and molecular weight were calculated via the ExPASy proteomics server (https://www.expasy.org).
The sequence of the lacSM gene was optimized by OptimumGene software for expression in BL21 (DE3) and was synthesized by GenScript Biotech Inc. (Nanjing, China). Using the synthesized gene as template, the putative laccase gene was amplified via polymerase chain reaction (PCR) with PSMF and PSMR primers. The PCR products were purified following the protocol of a gel extraction kit (HiPure Gel Pure DNA Mini Kit, Magen); then, the products were digested by Nco I and Hind III endonucleases, sequentially, ligated into the vector pPET-30a (which had been digested with enzymes Nco I and Hind III) to generate a plasmid with the gene lacSM. The resulting expression plasmid was denoted as pET-30a-LacSM.
Heterologous expression of LacSM in BL21 (DE3)
The expression plasmid pET-30a-LacSM was transformed into BL21 (DE3) cells. Transformants were grown on plates containing Luria–Bertani (LB) medium (1% yeast extract, 1% NaCl, and 2% peptone) supplemented with kanamycin (100 µg/L). Clones were selected and cultivated in a 50 mL flask containing 10 mL LB medium at 37 °C and 250 rpm. This culture was incubated for 16 h at 16 °C and 250 rpm for the expression of extracellular laccase. To explore the effect of Cu2+ exposure on the laccase yield, CuSO4 solution was added to the induction medium, and the final Cu2+ concentrations in the culture were 0, 0.2, 0.4, 0.5, and 0.8 mM.
Laccase activity assay
One unit of enzyme activity [U] refers to the amount of enzyme required to oxidize 1 µmol substrate per minute. Laccase activity was measured using a previously reported method [10]. Briefly, the activity of LacSM was determined at 60 °C by exploring the oxidation of 2 mM guaiacol at 465 nm (ɛ465 = 12,000 M−1 cm−1). The reaction system (1 mL) includes 2 mM guaiacol, 50 mM Na2HPO4/NaH2PO4 buffer (pH 6.0), and an appropriate concentration of LacSM. Other substrates, such as ABTS (1 mM, ɛ420 = 36,000 M−1 cm−1), 2, 6-dimethoxyphenol (2,6-DMP; 2 mM, ɛ468 = 49,600 M−1 cm−1), and syringaldazine (SGZ; 100 µM, ɛ525 = 65,000 M−1 cm−1) were used to characterize laccase activity at the, respectively, optimal temperature. All experiments were performed in triplicate.
Purification and characterization of LacSM
LB cultures were centrifuged at 6000× g, 4 °C for 5 min. Cell pellets were suspended in 50 mM Na2HPO4–NaH2PO4 lysis buffer, pH 7.5 and sonicated. Cell lysates were centrifuged at 4 °C and 30,000× g for 30 min. Recombinant LacSM was present in the supernatants, and purified by an ÄKTA-fast protein liquid chromatography (FPLC) system (GE Healthcare) equipped with a HisTrap FF column, which is immobilized metal affinity chromatography (IMAC). The LacSM purity was characterized by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein concentration of purified LacSM was measured by the Bradford assay, and bovine serum albumin was used as the standard. Purified LacSM was kept at 4 °C prior to use.
The activity of LacSM was measured in the range of temperature from 30° to 90 °C and the pH value from 3 to 10 with guaiacol, 2, 6-DMP, SGZ, or ABTS as substrates, respectively. The optimum temperature and pH values for LacSM oxidation were determined in the buffer (40 mM acetic acid, 40 mM H3PO4, and 40 mM H3BO3; NaOH was added to adjust the required pH). Residual activities of LacSM were measured in the same buffer with guaiacol as the substrate after the enzyme had been incubated at various pH values (5–8) at 30 °C for 24 h. LacSM was pre-incubated in the temperature range of 50–90 °C for 0–120 min. The thermostability of LacSM was determined by monitoring its residual activities using the substrate guaiacol.
The kinetic experiments of LacSM were performed at various concentrations of guaiacol (125–2000 μM), SGZ (3.125–200 μM), 2,6-DMP (62.5–4000 μM), and ABTS (62.5–1000 μM) substrates at their optimal pH and temperature. The kinetic parameters were determined by fitting the experimental data to Lineweaver–Burk plots.
Various metal ions might affect the enzyme activity. 1 mM of each metal ion was added to the reaction system, which was then incubated for 15 min at 4 °C. The residual activity was measured at standard assay conditions, using guaiacol as the substrate [11]. The effect of halide on the LacSM activity was studied by adding NaF at concentrations of 0–100 mM, and NaCl or NaBr at concentrations of 0–200 mM to the reaction mixture. The residual activity was recorded with guaiacol as the substrate, and the experimental procedure was the same as described above.
Treatment of lignin with LacSM
Lignin (1 g dry weight) was dissolved in 50 mM sodium dihydrogen phosphate. The sample was incubated at 50 °C and 200 rpm for 24 h in a 200 mL flask with laccase alone, laccase + NaOH, and water (as control), respectively. Laccase doses of 10 U g−1 were used. The final concentration of NaOH was 0.25 mol/L. After the treatment, samples were washed with water (1 L) and air-dried at 70 °C until complete dryness. The treated lignin samples were analyzed by Fourier-transform infrared (FTIR) spectroscopy.
Results and discussion
Cloning and expression of LacSM
Using a laccase sequence of Myceliophthora thermophila as the template, a putative laccase from S. macrospora k-hell was identified via protein Blast. The “uncharacterized protein SMAC_06098” of the fully sequenced genome of S. macrospora k-hell was cloned and expressed in E. coli. This uncharacterized putative laccase was denoted as LacSM. Its open reading frame contains 1854 nucleotides which can encode a protein of 618 amino acid length. The theoretical molecular weight and pI value of LacSM were predicted to be 67 kDa and 7.68, respectively. Multiple sequence alignment showed that the LacSM sequence conserved the histidine ligand coordinating copper active centers, similar to those found in other bacterial and fungal laccase sequences [4].
To express LacSM in E. coli, the expression plasmid pET-30a-LacSM, which carries the laccase gene, was constructed. The enzyme activity was monitored as response to time. The maximum activity of 239 U/L was found after incubating the culture for 16 h at 16 °C and 250 rpm. Furthermore, the effect of Cu2+ concentration on the laccase yield was also studied. No laccase activity could be detected without CuSO4 in the culture (data not shown), suggesting that copper played an important role for LacSM expression in E. coli. These findings were also observed for another laccase expression in Pichia pastoris [12, 13].
Characterization of recombinant LacSM
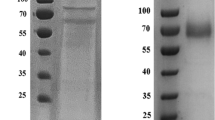
Recombinant LacSM was purified by immobilized Ni2+ affinity chromatography. SDS-PAGE analysis indicated that LacSM had a molecular weight of approximately 67 kDa (Fig. 1), which is consistent with the predicted theoretical molecular weight.
LacSM was active with substrates SGZ, ABTS, 2,6-DMP, and guaiacol. Judging from the enzyme activity versus pH, the optimal pH values for LacSM activity with substrates SGZ, ABTS, 2, 6-DMP, and guaiacol were 7, 5, 5, and 6, respectively (Fig. 2a). The optimum pH values of most fungal laccases ranged between 3.5 and 5 [9]. They displayed very low activity or were even deactivated when the pH value of the reaction system exceeded 7. In contrast, LacSM was active at a higher pH compared with the laccases of most fungi [14]. LacSM retained 30, 20, 15, and 30% of its maximum activity on substrates SGZ, ABTS, 2,6-DMP, and guaiacol at pH 8.0, respectively (Fig. 2a). Furthermore, LacSM remained stable in a broad pH range of 5–8 with guaiacol as the substrate, and retained more than 50% of its original activity after incubation for 24 h at 30 °C (Fig. 2b).
a Effects of pH on the activity. Relative activities of LacSM on guaiacol, ABTS, SGZ, and 2, 6-DMP in pH range of 3–9. b Stability of purified recombinant LacSM. Stability of LacSM with guaiacol used as the substrate over a pH range. Data points were measured in triplicate. Error bars represent the standard deviation
Judging from the enzyme activity versus temperature, the optimum reaction temperatures for LacSM oxidation of ABTS, SGZ, 2,6-DMP, and guaiacol were 50, 55, 55, and 60 °C, respectively (Fig. 3a). Similar results were observed for the laccases from both M. perniciosa and C. comatus [13, 14]. LacSM remained stable at 60 °C with guaiacol used as the substrate. After incubation for 120 min, 90% of the original activity was retained. At 50 °C, the enzymatic activity decreased to 70% after incubation for 80 min. When the temperature exceeded 60 °C, the enzymatic activity decreased significantly within 20 min, to below 50% of its original activity, and decreased slowly after 20 min (Fig. 3b).
a Effects of temperature on the activity. Optimal temperatures were determined with guaiacol, ABTS, SGZ, and 2, 6-DMP used as substrates at the temperature of 30–90 °C. b Stability of purified recombinant LacSM. Residual laccase activity of LacSM was determined after incubation at 50, 60, 70, 80 and 90 °C for 120 min with guaiacol used as the substrate. Values presented were measured in triplicate. Error bars represent the standard deviation
The kinetic properties of LacSM oxidation on guaiacol, ABTS, SGZ, and 2,6-DMP were characterized at the corresponding optimal temperature and pH. The kinetic parameters of LacSM oxidation on all four substrates were in the range reported for other fungal and bacterial laccases [15]. Among the four substrates, LacSM showed the highest catalytic efficiency (kcat/Km) on the oxidation of guaiacol (Table 1).
Metal ions and halides could both affect the laccase activity, which might limit their use in salt and/or halide-containing industrial processes [1]. Here, guaiacol was used as the substrate to study the effect of various metal ions on the resulting LacSM activity (Table 2). In the presence of Mg2+, Zn2+, or Fe2+, the activity of LacSM decreased. LacSM activity was most affected by Fe2+, and decreased to 10% of initial activity. Addition of 1 mM Zn2+ and Mg2+ resulted in the decrease of LacSM activity to 32% and 87%, respectively. However, addition of 1 mM K+, Na+, and Mn2+ enhanced the LacSM activity to 103%, 104%, and 109%, respectively. Metal ions affected the LacSM activity most likely by modifying amino acid residues in laccase [4]. These results show that LacSM was resistant to several metal ions. Similar observations were reported for other fungal and bacterial laccases [11, 13, 14].
LacSM activity was monitored in the presence of F−, Cl−, or Br− ions with different concentrations at pH 6 with guaiacol as the substrate. The enzymatic activity decreased to about 45% with 30 mM NaF, while about half of the LacSM activity was lost with the addition of 120 mM NaCl or 200 mM NaBr (Fig. 4). These results suggested that halides with small size inhibited the LacSM activity more efficiently. The halide with lower size accessed the trinuclear copper center of the laccase more easily, thus further inhibiting the laccase activity by interrupting the internal electron transfer [16]. The I50 value denotes the concentration of an effector that decreases half of the activity. The I50 value of Cl− for fungal laccases was reported to range between 0.4 and 1400 mM [1]. LacSM displayed an I50 value of about 120 mM, thus, was moderately tolerant to Cl− ions.
Lignin degradation by LacSM
To explore the ability of LacSM to degrade lignocellulosic biomass, lignin was purchased and treated either by LacSM alone or by LacSM + 1% NaOH. The samples were analyzed by FTIR spectroscopy. FTIR spectra that were either different or lacked bands reflected changes in the lignin structure, as shown in Fig. 5. The assignments of absorption bands are listed in Table 3. Compared with the control sample, four main absorption bands were found at 1024 cm−1, 1582 cm−1, 2900 cm−1, and 3335 cm−1 for the sample treated with LacSM. These corresponded to aromatic C–H in-plane deformation, stretching of aromatic groups, aliphatic C–H asymmetric stretching, and OH stretching [17], respectively. This indicated that the flexibility of groups in lignin samples treated with LacSM increased, and the lignin structure was modified by LacSM [7, 18]. Furthermore, the lignin sample was handled by LacSM and NaOH. The absorption intensity of four bands was enhanced, and two absorption bands (at 1024 cm−1 and 1582 cm−1) moved slightly to 1037 cm−1 and 1584 cm−1, respectively, compared with those treated with LacSM alone. This suggested that the structure of lignin was further modified due to the addition of NaOH. In addition, one more strong absorption band appeared at 1378 cm−1, which originated from the stretching of non-esterified phenolic OH groups. This indicated the breakage of the ester bond, and the amount of short side chains in lignin samples treated by LacSM + NaOH increased [17]. These results demonstrated that LacSM and LacSM + NaOH were efficient to modify the structure of lignin. In lignin samples treated with either LacSM or LacSM + NaOH, the amount of short side chains and the flexibility of groups increased, reflecting cleavage of the lignin unit sidechains. Thus, LacSM might potentially be useful for the pretreatment of lignocellulosic biomass. Previous studies have reported that bacterial and fungal laccases could be used to treat lignocellulosic biomass. For example, the laccase from M. thermophila removed 20% lignin polymer from ground eucalyptus wood. The polymer displayed a shorter side chain, and more lignin-derived compounds were released from the sample treated with laccase compared with the control assay [7]. The bacterial laccase from Amycolatopsis sp. 75iv3 (LacAS) was used for the delignification of steam-pretreated poplar. LacAS preferred to oxidize syringyl units and changed the distributions of interunit linkage. This resulted in a decrease of the amount of acid-soluble lignin by around 15%, while the amount of acid-precipitable polymeric lignin increased about sixfold [6].
Conclusions
A novel fungal laccase from S. macrospora k-hell was identified. Recombinant LacSM was expressed in E. coli and was characterized. The results showed that LacSM retained its oxidative capacity at neutral or alkaline pH and was moderately tolerant to Cl− ions. These observations indicated that LacSM could potentially be used in industrial biocatalytic applications. Furthermore, LacSM treatment of lignin material improved lignin degradation and/or modification. These results suggested LacSM as a potential candidate for the pretreatment of lignin degradation of lignocellulosic biomass.
References
Kittl R, Mueangtoom K, Gonaus C, Khazaneh ST, Sygmund C, Haltrich D, Ludwig R (2012) A chloride tolerant laccase from the plant pathogen ascomycete Botrytis aclada expressed at high levels in Pichia pastoris. J Biotechnol 157:304–314
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Dwivedi UN, Singha P, Pandeya VP, Kumara A (2011) Structure–function relationship among bacterial, fungal and plant laccases. J Mol Catal B-Enzym 68:117–128
Martín-Sampedro R, Rahikainen JL, Johansson LS, Marjamaa K, Laine J, Kruus K, Rojas OJ (2013) Preferential adsorption and activity of monocomponent cellulases on lignocellulose thin films with varying lignin content. Biomacromol 14:1231–1239
Singh R, Hu J, Regner MR, Round JW, Ralph J, Saddler JN, Eltis LD (2017) Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci Rep 7:42121
Rico A, Rencoret J, Del Río JC, Martínez AT, Gutiérrez A (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuels 7:6
Moilanen U, Kellock M, Várnai A, Andberg M, Viikari L (2014) Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol Biofuels 7:177
Santhanam N, Vivanco JM, Decker SR, Reardon KF (2011) Expression of industrially relevant laccases: prokaryotic style. Trends Biotechnol 29:480–489
Kiiskinen L-L, Viikari L, Kruus K (2002) Purification and characterisation of a novel laccase from the ascomycete Melanocarpus albomyces. Appl Microbiol Biotechnol 59:198–204
Gu C, Zheng F, Long L, Wang J, Ding S (2014) Engineering the expression and characterization of two novel laccase isoenzymes from Coprinus comatus in Pichia pastoris by fusing an additional ten amino acids tag at N-terminus. PLoS ONE 9:e93912
Kage-Nakadai E, Kobuna H, Funatsu O, Otori M, Gengyo-Ando K, Yoshina S, Hori S, Mitani S (2012) Cloning, sequence analysis, expression of Cyathus bulleri laccase in Pichia pastoris and characterization of recombinant laccase. BMC Biotechnol 12:75
Liu H, Cheng Y, Du B, Tong C, Liang S, Han S, Zheng S, Lin Y (2015) Overexpression of a novel thermostable and chloride-tolerant laccase from Thermus thermophilus SG05JP1716 in Pichia pastoris and its application in synthetic dye decolorization. PLoS ONE 10:0119833
Bao S, Zhen T, Ding D (2013) Heterologous expression and characterization of a novel laccase isoenzyme with dyes decolorization potential from Coprinus comatus. Mol Biol Rep 40:1927–1936
Ye M, Li G, Liang WQ, Liu YH (2010) Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression. Appl Microbiol Biotechnol 87:1023–1031
Xu F (1996) Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry 35:7608–7614
Canteria MHG, Renardb MGCC, Bourvellecb CL, Bureau S (2019) ATR-FTIR spectroscopy to determine cell wall composition: application on a large diversity of fruits and vegetables. Carbohydr Polym 212:186–196
Tejado A, Peña C, Labidi J, Echeverria JM, Mondragon I (2007) Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour Technol 98:1655–1663
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 41673074) to X. Yang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, X., Gu, C. & Lin, Y. A novel fungal laccase from Sordaria macrospora k-hell: expression, characterization, and application for lignin degradation. Bioprocess Biosyst Eng 43, 1133–1139 (2020). https://doi.org/10.1007/s00449-020-02309-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02309-5