Abstract
Lipid accumulation in oleaginous yeast is generally induced by nitrogen starvation, while oxygen saturation can influence biomass growth. Systematic shake flask studies that help in identifying the right nitrogen source and relate its uptake kinetics to lipid biosynthesis under varying oxygen saturation conditions are very essential for addressing the bioprocessing-related issues, which are envisaged to occur in the fermenter scale production. In the present study, lipid bioaccumulation by P. guilliermondii at varying C:N ratios and oxygen transfer conditions (assessed in terms of kLa) was investigated in shake flasks using a pre-optimized N-source and a two-stage inoculum formulated in a hybrid medium. A maximum lipid concentration of 10.8 ± 0.5 g L−1 was obtained in shake flask study at the optimal condition with an initial C:N and kLa of 60:1 and 0.6 min−1, respectively, at a biomass specific growth rate of 0.11 h−1. Translating these optimal shake flask conditions to a 3.7 L stirred tank reactor resulted in biomass and lipid concentrations of 16.74 ± 0.8 and 8 ± 0.4 g L−1. The fatty acid methyl ester (FAME) profile of lipids obtained by gas chromatography was found to be suitable for biodiesel application. We strongly believe that the rationalistic approach-based design of experiments adopted in the study would help in achieving high cell density with improved lipid accumulation and also minimize the efforts towards process optimization during bioreactor level operations, consequently reducing the research and development-associated costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The depletion of non-renewable fossil fuels over the last few decades has prompted the world scientific community to find alternative renewable fuel sources that can meet the challenges of ever increasing global demand for fuels. Here, the major challenge in the endeavor towards complete phasing-out of fossil fuel with biofuels is the identification of the right feedstock for biofuel production. Although various feedstocks such as lignocellulosic, micro and macro algae, yeast, and bacteria have been extensively investigated for biofuel production, single cell oil (SCO) production from oleaginous microbes has particularly gained increased attention, because of their short life cycle, ease of culturing, non-dependence on climatic conditions and higher productivity (up to 10× that of plant oils) [1, 2]. Also, SCOs primarily comprises of triglycerides (TAGs), the fatty acid composition of which is very much similar to that obtained from plant oils.
Oleaginous yeasts have been known to accumulate lipids up to 70% of their dry cell weight (DCW). In attempts to improve the yield and productivity of lipids, investigations related to batch and continuous modes of cultivation of oleaginous yeasts have been carried out under different nutrient limiting conditions like nitrogen, phosphorus, magnesium, and iron [3,4,5]. Both single and double nutrient limitations have been studied to understand their role in lipid synthesis [6, 7]. Among various nutrient limiting strategies adopted, nitrogen limitation has been found to influence lipid accumulation in yeast profoundly mainly because of shift in its metabolic flux. During nitrogen limitation, the major event is cessation of cell growth upon reaching the stationary phase, triggering lipid accumulation through the consumption of excess carbon source in the medium, and this trend has been invariably observed by a number of researchers [8, 9]. Apparently, the simple and obvious approach to impose nitrogen limitation on cells is growing the cells at high initial C:N molar ratio. However, the initial amounts of carbon and nitrogen sources required for medium preparation are determined by the desired final yield of biomass/lipid with respect to the fed substrate (YX/S or YP/S). To demonstrate this, in a recent work, lipid accumulation in Lipomyces starkeyi was reported to be 10 g L−1 (55% w/w) when grown in media with initial C:N of 72 (60 g L−1 glucose, 0.36 g L−1 urea and 0.64 g L−1 NH4Cl), which decreased by about 50–60% when grown in initial C:N ratio of 24, suggesting that monitoring the media C:N helped understand the conditions at which lipid accumulation is initiated [10]. Process optimization is very important for understanding the biological processes and identifying the right culture conditions. Since performing a large number of experiments in a bioreactor is energy and time-intensive, the only practical way is to do the optimization studies in shake flask and scale up to reactor level.
Several enzymes and metabolites are known to be responsible for channeling the carbon source for lipid synthesis rather than carbohydrate production in oleaginous yeasts. A series of events follow nitrogen depletion in the media such as decline in adenosine monophosphate (AMP) level, deactivation of isocitrate dehydrogenase, transport of citrate from mitochondria to cytoplasm and expression of ATP citrate lyase (ACP). ACP is one of the key enzymes found in oleaginous species, which splits the citrate to contribute to the acetyl coenzyme A pool for further channelization to the fatty acid synthesis (FAS) pathway [2, 11].
Only a few studies investigated the influence of oxygen/aeration on lipid accumulation by oleaginous yeasts. Different lipogenic strains behave differently to oxygen stress. While cell growth of most strains increases with increasing dissolved oxygen concentration, the effects on lipid accumulation varies widely. Cell growth of Rhodotorula glutinis almost doubled when aeration rate was increased from 1.5 to 2.5 vvm in a 5 L airlift reactor. Even though the cellular lipid content (% DCW) is little low at higher aeration rate, it is still preferred because of higher productivity (g L−1 h−1) [12].
While many reports have studied the effect of single or double nutrient limitation on lipid accumulation by yeast [4, 6, 8], few works have discussed about the changes in physiological behavior and metabolic flux of the organism. Reports studying the influence of oxygen transfer rate on lipid synthesis are scarce [13], a major factor affecting lipid biosynthetic pathway, and thus requires a detailed investigation. Thus, the present work aims to understand the influence of nitrogen limitation on lipid yield by oleaginous yeast and to obtain insight about the mechanism of lipid accumulation by studying its physiological behavior under stress condition. The study also aims at finding the most desirable volumetric oxygen transfer rate (kLa) for maximal growth and lipid accumulation and reproducing the results in a 3.7 L reactor. The lipid profile of the yeast was revealed by gas chromatographic analysis to determine its suitability as a biodiesel feedstock.
Materials and methods
Chemicals and media
All the chemicals used for media preparation and solvents were procured from Merck, India.
Strain and culture conditions
The yeast strain Pichia guilliermondii, isolated from a Kharagpur based oil mill and identified by 18s rRNA sequencing was used in all the studies. It was maintained in nutrient agar slants and revived in MGYP growth media [1] at 28 °C under constant shaking condition of 180 rpm.
MGYP media was used as both the primary and secondary inoculation media. An overnight grown culture of OD600 10 and 15 was used inoculate the secondary and production media, respectively. The production media used in the initial set of experiments was 100 mL Reader media ((NH4)2SO4, 3 g L−1; KH2PO4, 1 g L−1; K2HPO4. 3H2O, 0.16 g L−1; MgSO4. 7H2O, 0.7 g L−1; NaCl, 0.5 g L−1; Ca(NO3)2. 4H2O, 0.4 g L−1) with 50 g L−1 crude glycerol as C-source in 500 mL flask. Temperature and shaking condition were maintained for all the stages are similar to the primary inoculum preparation.
Shake flask studies
Inoculum optimization
Secondary inoculation media was optimized using three different combinations MGYP, Reader and Hybrid media and the growth profile of each was noted. Hybrid media was composed of 75% Reader and 25% MGYP media. MGYP was used as the primary inoculation for all the experiments. All the experiments were carried out with 25 mL media in 250 mL volume flask. 10% v/v inoculum was added in each flask.
Optimization of production media
Media optimization was done to determine the nitrogen source and the optimal C:N ratio for improved biomass and lipid yield. The different combinations of N-source tested were (1) ammonium chloride (NH4Cl), (2) sodium nitrate (NaNO3) and (3) both (NH4Cl + NaNO3). The best N-source was chosen for further studies. Nitrogen limitation experiments were studied by varying the initial C:N ratio of the media from 20 to 120. Carbon and nitrogen consumption as well as product formation were measured for all the runs. All the experiments were carried in triplicate.
Effect of oxygen transfer rate (OTR) on lipid accumulation
The effect of OTR on biomass growth and lipid productivity was investigated by varying the medium volume (50, 100, 150 and 200 mL) in 500 mL flask. Agitation rate was maintained at the optimal rate of 150 rpm (data not shown). KLa for different conditions were calculated based on the following empirical equation:
where N = agitation frequency (min− 1), VL = working volume (mL), d0 = shaking diameter (cm), d = inner shake flask diameter (cm).
Rangarajan et al. [15] has successfully used this equation to understand the relationship between kLa and lipopeptide production.
Fermenter study
A 3.7 L bioreactor (Bioengineering, Switzerland) with 2 L working volume was used to validate the results obtained from the shake flask studies with the optimal conditions. Air flow rate was maintained at 200 L h−1 (1.6 vvm) with an agitation rate of 350 rpm. kLa was calculated by the dynamic method and was maintained similar to that obtained in shake flask by means of trial and error by adjusting the agitation and air flow rate. MGYP and hybrid media was used as primary and secondary inoculation media, respectively. An overnight grown culture of P. guilliermondii with an OD600 of 15 was used as inoculum to the reactor at 10% v/v. Dissolved oxygen was measured during the entire batch by a pre-calibrated DO probe (Mettler Toledo).
Analytical methods
Estimation of ammonium, nitrate, glycerol and biomass
Ammonium and nitrate concentrations were measured throughout the studies by ammonium test strips, MQuant™ (Merck, Germany). Glycerol consumption was measured spectrophotometrically by a colorimetric method developed by Kuhn et al. [15]. Biomass growth was monitored by measuring the OD600 values in a UV–Vis spectrophotometer and the corresponding DCW was calculated from the standard curve of OD vs biomass concentration.
Lipid analysis
Fluorescent microscopy was used to qualitatively determine the lipid content after nile red staining of the cells [1]. Quantitatively lipid concentration was calculated by extraction of lipids in lipid extractor followed by gravimetric analysis [16].
Transesterification and FAME analysis
The lipid samples (72 h) were transesterified according to the earlier method [1]. The FAME profile was obtained by gas chromatography following the method of Dineshkumar et al. [17].
Results and discussion
Shake flask studies
Inoculum optimization
A two-stage inoculum has been found to be the most preferred. It introduces metabolic growth factors to the production media, which are essential for the balanced growth of cells. This helps to avoid the unproductive lag phase, as the two-stage inoculum does not impose any additional metabolic burden [18]. Our preliminary studies have indicated that MGYP medium being used both as primary and secondary inoculum (10% v/v) (Case 1), resulted in very good cell density (11 ± 0.85 g L−1) (Fig. 1), with a lipid concentration and productivity of 4.5 ± 0.22 g L−1 and 1.5 ± 0.09 g L−1 d−1, respectively, in the production medium (Reader medium).The culture reached log phase within 24 h and continued till 60th h (Fig. 1). Residual glycerol and ammonia was found to be 10 and 0.04 g L− 1, respectively, at the end of 96 h. The lipid time course profile revealed that the cells started accumulating smaller amount of lipids from the beginning of log phase, which increased in the late log- to stationary phase. However, since the main purpose of this work was to study the influence of C:N ratio on lipid yield, the presence of unconsumed complex substrates in the inoculum (10% v/v) would result in incorrect estimations of yield of biomass/lipid for the given carbon and nitrogen sources (i.e., glycerol, calcium nitrate and ammonium chloride) and also pose difficulty in monitoring the C:N in the production media.
Since the secondary inoculation media, MGYP, contains considerable amount of organic nitrogen, it can influence the uptake of ammonium and nitrate present in the production media. Ammonium being more readily consumable than nitrate, the latter must be reduced to ammonium before consumption. After utilization of the inorganic nitrogen sources in the media, the cells start converting the organic nitrogen (from peptone and yeast extract) to the easily consumable form NH4+. Also, nitrate was reduced to NH4+. Thus, the ammonium pool was getting replenished and the culture did not experience any nitrogen limitation which was requisite for the future nutrient limitation studies. So, there was a need to design the secondary inoculum media such that it would not influence the NH4+ profile in the production media. Figure 2 shows the different media combinations used for optimization study.
Two different media, Reader (case 2) and Hybrid (case 3) were tested as the secondary inoculum media and the NH4+ profile was studied in the respective production media. When Reader media was used as the secondary inoculum media, the lag phase was found to be very long and biomass density very low to serve as an inoculum for production media (Fig. 3). So, a hybrid media comprising the contents of both Reader’s medium and MGYP medium was formulated to study the growth curve and NH4+ profile in production media. Figure 3 shows that the performance of hybrid medium was better than Reader’s medium, but little lesser than the MGYP, as evident from the profile of biomass growth. So, the hybrid media was chosen as inoculum media for further studies, since it has lesser loading of complex carbon and nitrogen sources, which can result in better interpretation of the results, particularly the effect of nitrogen starvation on lipid yield.
Selection of suitable N-source
To optimize the lipid production, it is important to identify the nitrogen source (NH4+, NO3−, NO2, etc.) which can be easily utilized by the yeast. Various studies have reported different nitrogen sources and studied the respective growth profile of yeast [19]. Similar growth profiles were observed for C. albidus when grown using different nitrogen sources [20], but the lipid accumulating tendency varied significantly. In the present study, different inorganic N-sources (NH4Cl and NaNO3) were tested separately as well as in combination at a C:N ratio of 30:1 to understand the nitrogen uptake mechanism of the micro organism. Although, organic nitrogen sources (yeast extract, peptone, urea) have been proved to be better N-sources for both biomass and lipid production, it is difficult to monitor the concentration of carbon and nitrogen in a complex media during the course of fermentation. Also, the use of yeast extract is economically not viable. So, our study was restricted to the use of inorganic N-sources only. When ammonium chloride was used as the sole nitrogen source, the cells could utilize it efficiently and the concentration of ammonium ions (NH4+) dropped to 0.05 g L−1 at end of 72 h (Fig. 4a), whereas when sodium nitrate was used, the biomass growth was very less (only 3.5 g L−1) and around 30% of nitrate still remained in the media at the end of the batch (Fig. 4b). The lipid yield obtained with sodium nitrate (0.2 g L−1) is significantly low as compared to that obtained with ammonium chloride (4.5 g L−1). We have recorded almost similar lipid yield when grown with ammonium chloride and ammonium sulfate as nitrogen sources (Fig. 4c). Although lipid yield in reader media was comparable with ammonium chloride media, the media did not experience nitrogen limitation. We found ammonium chloride was utilized faster than ammonium sulfate (Refer to Online Resource 1). 0.1 g L−1 of nitrate remained in the media at the end of 96 h (Fig. 4c). Ammonium was utilized more efficiently than nitrate, as it can be directly converted to amino acids and was chosen as the suitable nitrogen source for our future studies. Initial ammonium concentration should be sufficiently high enough to support cell mass growth till a desired level of YX/S is reached. Once stationary phase is reached, reduction in number of cells shifts the lipid biosynthetic pathways to accumulate more neutral lipid than polar phospholipids required for cell membrane formation [21].
Although pH of culture media is considered to be a critical parameter influencing lipid accumulation in oleaginous yeast, for some species, the growth and lipid synthesis are not affected even at very low pH condition [10]. The growth profile of P. guilliermondii when grown under controlled pH (at 6.8) did not show any deviation when cultured without pH control (data not shown). The pH of culture dropped to a value less than 2 within 24 h of cultivation indicating that cells can sustain growth at the acidic condition and still continue to synthesize lipid. This stands out to be a major advantage of the species, since bacterial contamination could be easily eliminated. Also, since ammonium is the major nitrogen source in corn steep hydrolysate, ammonium chloride can be easily replaced by the same in industrial scale bioproduction [19, 22].
Effect of initial C:N on lipid accumulation
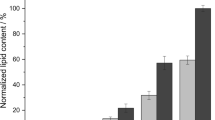
Imposing nitrogen limitation was found to be the most efficient strategy for the induction of lipid accumulation in yeast. The carbon source in the medium is initially channelized for the growth of cells and directed towards lipid accumulation after the depletion of nitrogen source. The duration of growth phase and transition to lipid storage phase depends on the initial C:N ratio of the media [23]. So, it is of utmost importance to determine the optimal initial C:N of the media. To understand the relationship between nitrogen limitation and lipid accumulation and how it affects the metabolic characteristics of P. guilliermondii, the yeast was grown in media with different initial C:N ratios of 20, 40, 60, 80,100 and 120. Figure 5 shows the biomass growth and lipid accumulation along with ammonium and glycerol consumption under different media combinations. Flasks with an initial C:N of 20 showed ammonium consumption of 68% (1.25 g L−1 initial ammonium concentration) at the end of 96 h of cultivation; while the glycerol consumption was 74% (initial glycerol concentration was 50 g L−1) during this period (Fig. 5a). The C:N ratio for this media combination dropped over time and the cells did not experience nitrogen limitation (Refer to Online Resource 1). The final lipid concentration was found to be 2 ± 0.1 g L−1 with a productivity of 0.67 g L−1 d−1 (Table 1). The lipid concentration (5 ± 0.26 g L−1) increased significantly with initial media C:N of 40 (Fig. 5b). As the initial C:N ratio of the media was increased (up to 120), the nitrogen concentration in the media dropped more rapidly as compared to carbon concentration resulting in increase of media C:N ratio over the cultivation time (additional information in Online resource 1) indicating the cells were under nitrogen stress. A maximum lipid concentration and productivity of 5.4 ± 0.28 g L−1 (45% of DCW) and 1.83 g L−1 d−1, respectively, were achieved with an initial C:N of 60 (Fig. 5c). Cultures grown with higher initial C:N (≥ 80) reached the log phase within 15 h and decline phase in 60 h. This is due to the limited availability of nitrogen in the media that the cells stopped proliferating and entered the stationary phase faster (Fig. 5d–f). The lipid yield for these cultures was measured at 50 h (the point of maximum biomass density). Although the lipid content in the cells increased with further increase of the initial C:N of the media, the lipid yield is less because of reduced biomass growth (Fig. 5f). All the results were further verified (qualitatively) by observing the cells under fluorescence microscope (inset Fig. 5). The microscopic images reconfirms that the lipid accumulation inside the cells increased up till a certain stress level (C:N = 60:1) and reduced thereafter. Similar results were obtained with Rhodococcus opacus when cultivated with 2 g L−1 glucose and varying (NH4)2SO4. The lipid content in the cells increased from 50.21 ± 3.02 to 65.89 ± 3.11%, as the concentration of (NH4)2SO4 was reduced from 2 to 0.01 g L−1 [24]. Table 1 summarizes the maximum biomass, lipid concentration, biomass (YX/S) and lipid yield (YP/S) as well as lipid productivity achieved for different media combinations. The YX/S and YP/S values increased consistently with increasing stress condition; however, it does not represent the actual scenario, since the substrate consumption was less in medias with initial C:N of 80, 100 and 120. Even though the YX/S (0.31) and YP/S (0.13) values were little low for media with C:N 60, the lipid concentration (5.4 ± 0.28) and productivity (1.83) was highest in this media and thus chosen as the optimal condition for further studies.
When the nitrogen source in the medium gets exhausted, the cell division ceases and the excess carbon is channelized towards formation of storage lipid in oleaginous yeasts. As the nitrogen level drops, the enzyme AMP deaminase gets activated and the level of AMP decreases, which in turn inhibits the enzyme isocitrate dehydrogenase. Citrate is funneled out of mitochondria and flux through TCA cycle is reduced. The citrate is then cleaved by ATP citrate lyase (ACL) and the produced acetyl CoA is directed towards formation of lipid bodies [2, 10].
Effect of oxygen transfer rate on biomass growth and lipid accumulation
As yeast biomass growth and in turn the lipid accumulation is a direct function of the oxygen transfer rate in the medium, investigations concerning the same in shake flasks will give good insight about the aerobic growth process and minimize the number of experiments required to be carried out in bioreactor studies [10, 12].
Pichia guilliermondii is a facultative aerobic organism and thus it is expected that cell growth rate will increase with increase in dissolved oxygen (DO) concentration. A combined increase in agitation and aeration rate can improve the DO level in the media and consequently the kLa. To identify the optimal combination of aeration and agitation for improved biomass and lipid production, shake flask experiments were conducted with the previously determined optimal conditions but with different medium volumes. kLa for different flasks were calculated according to Eq. 1, which was actually calculated based on the agitation rate, medium volume, shaking diameter of shaker and flasks. It was observed that the increase in headspace in the flasks (as a result of decrease in the medium volume) from 200 to 50 mL (operated at 150 rpm) resulted in almost doubled biomass growth (Fig. 6; Table 2) with the specific growth rate increasing from 0.09 to 0.11 h−1. A maximum lipid concentration of 10.8 g L−1 (53% of DCW) was achieved for 50 mL filling flask. The regression line of the kLa versus the cell growth rate is a reasonable fit with a R2 value of 0.95 (Fig. 7). The high kLa in the flasks with less filling is indicative of the high oxygen transfer rate (OTR). The higher OTR not only resulted in higher dry cell weight, but also increased the lipid content of the cells from 40% (w/w) in 200 mL filling to 58% (w/w) in 50 mL filling (Table 2). The rate of depletion of ammonium concentration in flask with 50 mL filling compared to that in 200 mL filling explains prevailed nitrogen limiting conditions (refer Online Resource 1) in the former and hence enhanced lipid accumulation. Yen and Liu [12] reported that cell growth rate of R. glutinis almost doubled in a 5 L airlift bioreactor when the aeration rate was increased from 1 to 2.5 vvm suggesting the dependence of cell growth on the OTR or kLa.
Validation of optimal condition in reactor
The optimal shake flask conditions (C:N = 60 and NH4Cl as N-source) were maintained in a 3.7 L (2 L working volume) reactor to reproduce the biomass and lipid profiles obtained earlier (Sect. 3.1.4). The aim was to grow the yeast in a similar environment as in shake flask. By trial and error method (data not shown), a kLa of 0.6 min−1 was achieved in reactor by maintaining the agitation and aeration rate at 400 rpm and 0.75 vvm (150 lph), respectively. A maximum biomass density of 16.74 g L−1 was achieved at 72 h, while the lipid concentration was around 8 g L−1 (Fig. 8), which were lower than obtained in shake flask cultures. A possible reason may be due to some inhibitory effects by antifoaming agent (silicone oil), which was added at regular intervals to prevent foam formation, a common occurrence when reactor is run at high agitation and air flow rates. Lower biomass yields obtained in bioreactor may be attributed to the difference in agitation and aeration patterns leading to mass transfer limitation. Oxygen is sparingly soluble in culture media and the transition from surface aeration in shake flask to submerged aeration in reactor might have taken toll on the biomass growth because of possible oxygen limitation, which may further be complicated by high shear stress and nutrient limitation due to high culture density. However, we observed that the broth was not viscous or dense enough to consider shear stress as a limiting parameter. Though nitrogen limitation was induced to increase lipid production after achieving good biomass growth, glycerol (carbon source) uptake profile showed the presence of residual carbon substrate after each batch, thereby ruling out glycerol limiting condition.
Some possible ways to improve the lipid production in reactor may be supplementing certain non-metabolizable oil or introducing some pathway intermediate. Further optimization in terms of feeding strategy or use of two-stage cultures (biomass growth phase and lipid accumulation phase) is required to improve the lipid productivity in reactor. Table 3 shows stepwise increment in biomass and lipid yield upon optimization. An overall 39 and 77% improvement in terms of biomass and lipid yield was observed in the reactor as compared to the initial study (Table 3). Relative filling is not applicable in reactor because of submerged aeration in it as compared to surface aeration in shake flask.
Lipid properties
Determination of fatty acid (FA) composition of lipid is important to realize its application potential as a biodiesel feedstock. The FA composition of lipids produced by P. guilliermondii when grown in different nitrogen limited conditions is summarized in Table 4. The profiles are almost similar with slight variations. The lipid profiles were similar to that obtained from other oleaginous yeasts [9, 26, 27] and was majorly composed of oleic (C 18:1), palmitic (C 16:0), stearic (C 18:0) and palmitoleic (C 16:1) acid (Table 3). Other FAs were present in minor amounts and the total percentage of other FA is less than 30%. The most desirable composition of biodiesel is the presence of large amount of the mono unsaturated oleic acid and trace amount of poly unsaturated FAs (PUFA) [19]. We found percentage of PUFA to be less than 10% for all combinations. The % saturation of lipids varied from 29 to 50% for media with different initial C:N ratio. The variation in fatty acid profile can be attributed by the difference in growth rates, which is a direct function of the change in the media nitrogen concentration. It is difficult to establish the exact co-relationship between nitrogen starvation, aeration rate and fatty acid profile in a particular yeast strain. Most strains behave differently to changes in culture condition such as change in limiting substrate, pH, and temperature [28]. Generally, a higher or almost equal ratio of saturation:unsaturation is preferred for lipids to qualify for biodiesel feedstock [29]. This is because a higher unsaturation leads to oxidative unstability of the fuel. Overall, the lipid profiles are very much similar to that of other vegetable oils such as soyabean, jatropha oil [30] indicating that the lipids produced under nitrogen limited condition can be used as an alternative feedstock to diesel (Table 4).
Concluding remarks
In the present study, attempts were made at enhancing lipid concentration of an indigenously isolated oleaginous yeast strain, P. guilliermondii by facilitating oxygen transfer and uptake, while simultaneously limiting nitrogen availability for higher production of biodiesel as an alternative fuel. With this aim, the optimal nitrogen source and growth conditions were found to be ammonium chloride with a C:N ratio of 60:1 and a kLa of 0.6 min−1, which resulted in a biomass and lipid concentrations of 20.35 ± 1 and 10.8 ± 0.5 g L−1, respectively, in shake flask studies. The results motivated us to replicate the same in a 3.7 L reactor. Although the optimal conditions were replicated in the reactor by controlling the agitation and aeration rate to get the desired kLa, the yields were little lower than that of shake flask due to experimental limitations. A maximum of 16.74 ± 0.8 and 8 ± 0.4 g L−1 could be achieved for biomass and lipid concentration, respectively, in the reactor. The % saturation of the lipids as calculated from its FAME profile conformed to the requirements for its application as a feedstock for biodiesel.
Abbreviations
- FAME:
-
Fatty acid methyl esters
- TAG:
-
Triglyceride
- MGYP:
-
Malt extract glucose yeast extract peptone
- k L a :
-
Volumetric oxygen transfer rate
References
Chopra J, Dineshkumar R, Bhaumik M, Dhanarajan G, Kumar R, Sen R (2016) Integrated in situ transesterification for improved biodiesel production from oleaginous yeast: a value proposition for possible industrial implication. RSC Adv 6(74):70364–70373
Sitepu IR, Garay LA, Sestric R, Levin D, Block DE, German JB, Boundy-Mills KL (2014) Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol Adv 32(7):1336–1360
Li Y, Han F, Xu H, Mu J, Chen D, Feng B, Zeng H (2014) Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour Technol 174:24–32
Wu S, Hu C, Jin G, Zhao X, Zhao ZK (2010) Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour Technol 01(15):6124–6129
Wu S, Zhao X, Shen H, Wang Q, Zhao ZK (2011) Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour Technol 102(2):1803–1807
Álvarez R, Acevedo F (2012) Increase in lipids production by Pichia angusta DL-1 utilizing the chemostat under double limitation of heterologous nutrients. Biochem Eng J 67:83–87
Bellou S, Triantaphyllidou IE, Mizerakis P, Aggelis G (2016) High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J Biotechnol 234:116–126
Beligon V, Poughon L, Christophe G, Lebert A, Larroche C, Fontanille P (2015) Improvement and modeling of culture parameters to enhance biomass and lipid production by the oleaginous yeast Cryptococcus curvatus grown on acetate. Bioresour Technol 192:582–591
Sestric R, Munch G, Cicek N, Sparling R, Levin DB (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient-limited conditions. Bioresour Technol 164:41–46
Calvey CH, Su YK, Willis LB, McGee M, Jeffries TW (2016) Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi. Bioresour Technol 200:780–788
Zhang H, Zhang L, Chen H, Chen YQ, Chen W, Song Y et al (2014) Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP: citrate lyase from Mus musculus. J Biotechnol 192 Pt A:78–84
Yen HW, Liu YX (2014) Application of airlift bioreactor for the cultivation of aerobic oleaginous yeast Rhodotorula glutinis with different aeration rates. J Biosci Bioeng 118(2):195–198
Yen HW, Zhang Z (2011) Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J Biosci Bioeng 112(1):71–74
Rangarajan V, Dhanarajan G, Sen R (2015) Bioprocess design for selective enhancement of fengycin production by a marine isolate Bacillus megaterium. Biochem Eng J 99:147–155
Kuhn J, Müller H, Salzig D, Czermak P (2015) A rapid method for an offline glycerol determination during microbial fermentation. Electron J Biotechnol 18(3):252–255
De Bhowmick G, Vegesna N, Sen R (2015) Process design for augmentation and spectrofluorometric quantification of neutral lipid by judicious doping of pathway intermediate in the culture of marine Chlorella variabilis for biodiesel application. Bioresour Technol 198:781–789
Dineshkumar R, Dash SK, Sen R (2015) Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv 5:73381–73394
Lin J, Li S, Sun M, Zhang C, Yang W, Zhang Z et al (2014) Microbial lipid production by oleaginous yeast ind-xylose solution using a two-stage culture mode. RSC Adv 4:34944–34949
Sitepu IR, Sestric R, Ignatia L, Levin D, German JB, Gillies LA et al (2013) Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour Technol 144:360–369
Hansson L, Dostálek M (1986) Effect of culture conditions on fatty acid composition in lipids produced by the yeast Cryptococcus albidus var. albidus. J Am Oil Chem Soc 63:1179–1184
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sustain Energy Rev 55:1–16
Kumar R, Dhanarajan G, Bhaumik M, Chopra J, Sen R (2017) Performance evaluation of a yeast biorefinery as a sustainable model for co-production of biomass, bioemulsifier, lipid, biodiesel and animal-feed components using inexpensive raw materials. Sustain Energy Fuel 1(4):923–931
Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48(6):375–387
Cortes MALRM, de Carvalho CCCR (2015) Effect of carbon sources on lipid accumulation in Rhodococcus cells. Biochem Eng J 94:100–105
Nambou K, Zhao C, Wei L, Chen J, Imanaka T, Hua Q (2014) Designing of a “cheap to run” fermentation platform for an enhanced production of single cell oil from Yarrowia lipolytica DSM3286 as a potential feedstock for biodiesel. Bioresour Technol 173:324–333
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48(6):1146–1151
Rakicka M, Lazar Z, Dulermo T, Fickers P, Nicaud JM (2015) Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels 8:104
Hall MJ, Ratledge C (1977) Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose under various conditions in a one and two-stage continuous culture. Appl Env Microbiol 33:577–584
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sustainable Energ Rev 16(1):143–169
Kumar G, Singh V, Kumar D (2017) Ultrasonic-assisted continuous methanolysis of Jatropha curcas oil in the appearance of biodiesel used as an intermediate solvent. Ultrason Sonochem 39:384–391
Acknowledgements
JC is thankful to Ministry of New and Renewable Energy, Government of India for her fellowship and contingency grants. The authors gratefully acknowledge Department of Biotechnology, Government of India (Project Grant No. BT/PR6909/PBD/26/391/2013, 21/03/2014) for the financial support. JC is also thankful to Mr. Ravi Ranjan Kumar, Ms. Moumita Bhaumik and Mr. Lakshmi Kanta Dolai for their timely help during reactor operation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chopra, J., Sen, R. Process optimization involving critical evaluation of oxygen transfer, oxygen uptake and nitrogen limitation for enhanced biomass and lipid production by oleaginous yeast for biofuel application. Bioprocess Biosyst Eng 41, 1103–1113 (2018). https://doi.org/10.1007/s00449-018-1939-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1939-7