Abstract
Herein, we systematically reported the capability of T. harzianum RY44 for decolorization of Mordant orange-1. The fungi strains were isolated from the Universiti Teknologi Malaysia tropical rain forest. For initial screening, the decolorization was conducted using 50 strains of the fungi for 20 days incubation time and the best performance was selected. Then, the decolorization capability and fungal biomass were evaluated using different dye concentrations, namely, 0, 50, 75 and 100 ppm. Effects of the carbon sources (fructose, glucose, and galactose), nitrogen sources (ammonium nitrate, ammonium sulfate and yeast extract), surfactant (tween 80), aromatic compounds (benzoic acid, catechol and salicylic acid), and pH on the decolorization efficiency were examined. This study has found that the employed carbon sources, nitrogen sources, and aromatic compounds strongly enhance the decolorization efficiency. In addition, increasing the surfactant volume and pH generally decreased the decolorization efficiencies from 19.5 to 9.0% and 81.7 to 60.5%, respectively. In the mechanism philosophy, the present work has found that Mordant orange-1 were initially degraded by T. harzianum RY44 to benzoic acid and finally transformed into salicylic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic dyes have been widely used for various industries such as pharmaceuticals, cosmetics, foods, leathers, papers, and textiles for coloring their final products [1]. The dyes can be classified as an hazardous material and their direct discharge into the water bodies can damage the environment [2]. Presence of the dyes in the water system can diminish photosynthetic activities because of their ability to cut light access into the deeper layers resulting in lower water quality. In addition, their fate also can be toxic to the aquatic biota [3, 4]. Moreover, the contamination of the dyes in drinking water at even a concentration of 1.0 mg L−1 is unsafe to be consumed [5]. Therefore, there is a growing body of literature that recognizes the importance of decolorization of the dyes from water [5,6,7,8,9,10,11,12].
Several methods to degrade dyes such as ozonation, photochemical membrane filtration, and ion exchange have been proposed and implemented [5]. The above-mentioned methods have been proven to have the capability to degrade the dyes depending on the employed dye types and procedures. However, these methods have several drawbacks such as the creation of a large amount of sludge that can continue leaching pollutants into the environment. In addition, these methods are costly and has the potential to produce hazardous materials [13]. For a comprehensive overview, the advantages and disadvantages of the aforementioned methods for decolorization of the dyes are summarized in Table 1 [5]. Therefore, to improve the decolorization philosophy, the use of microorganisms is recently highly considered because it is environmentally friendly, has a low cost, and the potential to produce less sludge compared to the above-mentioned methods [1, 14].
Various microorganisms such as bacteria [15, 16], yeast [17, 18], algae [19], actinomycetes [20], and fungi [12, 21] have been used for decolorization of synthetic dyes. Although many decolorization methods are possible, most studies on the dye decolorization have focused on the bacteria and fungi. Bacteria demonstrated to have the capability to degrade the dyes because of their high activity, extensive distribution, and strong adaptability [22]. An apparent weakness using bacteria, however, is that the activity of bacteria is inhibited when they are employed for decolorization of products such as aromatic amines [23]. Otherwise, fungi were proven to degrade the considered complex organic compounds through catalysis with the extracellular ligninolytic enzymes such as laccase, manganese peroxidase, and lignin peroxidase [24]. In addition, an efficient system can be proposed when the fungi are employed due to their large surface area and easy solid–liquid separation, [25].
Dye decolorization mechanism by fungi can be divided into two categories namely the biosorption and biodegradation [24]. The mechanism of the biosorption which involves physio-chemical interactions such as adsorption, deposition, and ion exchange is commonly devoted for dead cells. Contrary to this, the biodegradation which involves the production of lignin-modifying enzymes, laccase, manganese peroxidase, and lignin peroxidase is frequently adopted for living cells. The relative contributions of the above products for the decolorization of the dyes may be diverse according to the fungi types. Therefore, evaluation of other new fungal strains to degrade the dyes efficiently is an interesting study. Mordant orange-1 is a type of azo dyes characterized by the presence of at least one azo bond (–N=N–) bearing aromatic rings and has high photolytic stability and resistance towards major oxidizing agents to avoid degradation of dyes [26]. It is widely used for coloring many industrial materials such as textiles, leathers, plastics, foods, and pharmaceuticals. By these facts, it is also potentially possible that the dye can be hugely released to the environment through the water effluent from the washing machine. Therefore, the presence of the dye in the water environment becomes a serious problem, since it is also reported to be toxic to aquatic biota and the human health by contaminating the water. Moreover, it was also reported to cause inflammation when in contact with human skin. Therefore, a reliable approach to remove this dye particularly from water is crucially needed.
Considering the aforementioned research necessity, the aim of the present work is to investigate the capability of newly isolated strain T. harzianum RY44 for decolorization of Mordant orange-1. This work is highly beneficial to investigate and clarify another possible mechanism of the fungi for decolorization of this dye efficiently. In addition, the effects of carbon, nitrogen, surfactants, aromatic compounds, and pH on the decolorization capability and fugal biomass are also evaluated. Previously, decolorization of Mordant orange-1 has been investigated using two different bacteria namely Halobacillus Trueperi MO-22 and Marinobacter algicola MO-17 [27, 28]. The use of fungi in this work is due to their excretion of extracellular enzymes that are proven to degrade, though possibly not completely, structures that are difficult for bacteria to handle [29]. In addition, most of the fungi are robust organisms that are more tolerant to high concentrations of pollutants compared to bacteria. Although the use of white rot fungi to degrade some dyes is also possible, the present work provides an alternative approach by employing the filamentous fungi that may have a different mechanism compared to the white rot fungi. Therefore, the findings of this study provide a framework using the presently employed fungi for future dye degradation development.
Materials and methods
Materials
In the present work, an azo dye, Mordant orange-1 was purchased from Sigma Aldrich (St. Louis, USA). The properties of the dye are presented in Table 2. In addition, fructose, galactose, glucose, malt extract agar, and other chemicals were obtained from Merck (Darmstadt, Germany).
Microorganism and culture condition
The cultivation, screening, and decolorization in solid medium experiment were conducted in a petri dish (90-mm in the diameter) with 20 mL agar medium containing (g L−1): glucose (20), malt extract agar (20), chloramphenicol (anti bacteria) (0.04), and ammonium nitrate (10). All medium and equipments employed in this study was previously sterilized using autoclave at a temperature of 120 °C for 25 min. In the preparation, each plate was first inoculated with one fungus having a size of 3 mm in diameter and about 1 g of weight followed by incubation for 20 days. The decolorization by the potential fungus was examined at a Mordant orange-1 concentration of 50 ppm. The growth and zone of color changes were measured daily. For this initial screening, the decolorization of Mordant orange-1 was conducted in the solid medium using 50 strains of the potential fungi. This examination was performed in duplicate for each culture.
Identification and characterization of fungi
Identification was conducted on the petri dishes containing the potato dextrose agar (PDA) and malt extract agar (MEA). The basis of 18S ribosomal RNA (rRNA) variation was employed to identify RY 44 strain. Extracted DNA genome was amplified using the universal primers which are NSI and NS8. Polymerase chain reaction (PCR) was conducted according to the previous procedure [11]. Amplification products were cloned into pGEM-T Easy (Promega). Furthermore, the results were sent to 1st BASE Laboratory Sdn. Bhd for sequencing. The DNA sequence was then read and edited using BioEdit and compared with other 18S rRNA gene sequences obtained from the National Center for Biotechnology Information (NCBI) GenBank database.
Biomass determination
Fungal biomass was determined by filtering 20 mL culture broth through Whatman no. 1 filter paper. It was then dried at a temperature of 105 °C and kept in desiccators until constant weight was achieved. Therefore, the fungal biomass was expressed in grams per liter.
Decolorization by the fungi RY44 on liquid medium
Decolorization of Mordant orange-1 was carried out using a 100-ml Erlenmeyer flask. The flask contains 20 mL nutrient media and dyestuff. Next, 3-mm plug (about 1 g) cut from the pure fungal culture was added into the flask. For comparison purpose, a flask without fungi was prepared as a control case. This study was carried out using different dye concentrations ranging from 50 to 100 ppm. Then, the effects of other parameters such as carbon sources, nitrogen sources, surfactant, aromatic compounds, and pH on the decolorization performance were examined as listed in Table 3. Specifically, the effects of surfactant and pH were examined using different mixtures varying from 0.1 to 1.5 mL and 3 to 6, respectively. In this investigation, the decolorization was performed for 15–30 days at room temperature and at agitation speed of 120 rpm.
Then, the mixtures were sampled periodically, centrifuged at 4000×g for 20 min at a temperature of 15 °C. The supernatant was used to determine the rate of decolorization spectrophotometrically by measuring its absorbance in the UV–Vis Spectrometer DR 5000. The percentage of decolorization was calculated using the following formula:
where D is the decolorization, C o is the absorbance of the dye as the initial experiment and C is the absorbance of the dye after decolorization.
Inspection of metabolites
The biodegradation products of Mordant orange-1 were inspected using samples of cell culture collected during and after decolorization. For GC-MS analysis, extract cultures were previously prepared by condensing using rotary evaporator and passed through a column. The GC-MS system (Agilent 5975E FID GC-MS) equipped with DB-1 capillary column was employed for this inspection. It was operated at an injector temperature of 260 °C with an oven temperature of 80 °C for 1 min, increased up to 160 °C with 18 °C min−1, raised up to 320 °C with 20 °C min−1, and then held at 320 °C for 15 min. In addition, it was then operated in full scan of m/z 50–500.
Characterization
For FTIR observation, residue obtained after evaporation of solvent extract was mixed with the potassium bromide with a ratio of 1:100 to produce the specimen in a pellet form. The pellet was prepared by the hydraulic pressing (Specac model) at 10 tons in a pressure. The FTIR spectrometer (PerkinElmer Frontier-GPOB model) equipped with the PerkinElmer Spectrum software was employed for this characterization. It used the OptKBr (7800–400 cm−1) as a beam splitter and MIR TGS (15,000–370 cm−1) as a detector. Then, the characterization was carried out using a spectrum wavelength in the range of 650–4000 cm−1 at a resolution of 4 cm−1 and accumulation of 10 scans. In addition, surface morphology was characterized by means of the field emission scanning electron microscopy (FESEM ZEISS Supra 35VP). For this investigation, the samples were collected before and after decolorization.
Results and discussion
Screening the decolorization capability of fungi on solid medium
Table 4 provides the summary of certain fungal strains employed in this study for decolorization of the dye. It was apparent from the table that certain strains showed a good ability for dye colorization. From 9 to 14 days incubation, this study found that there were 16 strains that revealed very fast decolorization of Mordant orange-1. Specifically, this study found only five strains namely fungi RY 06, 36, 40, 42 and 44 having colorization capability more than 60%.
Therefore, this study considered fungi RY44 for decolorizing Mordant orange-1 for further investigation due to their performance. This work observed that fungi RY 44 decolorized Mordant orange-1 from its color to very light yellow white. In this inspection showed by the mycelia growth and decolorization rate, a good advantage of the screening using solid medium is that the data can be measured and monitored daily by visual without the requirement for complicated instruments. In addition, the solid medium commonly contains carbon and nitrogen, so that it also supports the dye decolorization.
Characteristic and identification of fungi RY44
RY44 fungus considered in this work was isolated from the tropical rain forest. Description of the fungi are branched and hyaline. Their flask-shaped projection is phialide. The conidia are generally green, smooth or roughened, ranging in shape from globose to ellipsoidal and produced in slimy heads. They can be fruiting in row and tiers on fallen hardwood trunks and are very common in the soil. The colony is originally hyaline darkening to white with green tufts in most species. Texture of the colony is wooly. Based on the macroscopic morphological characteristic and the phylogenetic position, RY44 is classified as T. harzianum (see Fig. 1).
Decolorization in liquid medium
Decolorization of Mordant orange-1 by T. harzianum RY44 was examined for different dye concentrations namely 0, 50, 75 and 100 ppm. The effect of dye concentration on percentage of the decolorization and fungal biomass is shown in Fig. 2. This study revealed that for all the different concentrations, the decolorization and fungal biomass increased with the rise of incubation time from 15 to 30 days. That increasing is due to the increase in cell wall mass when the culture age was increased [30]. Maximum decolorization obtained in this work was 83%, achieved when T. harzianum RY44 were applied for 50 ppm of the dye concentration. In general, increasing the dye concentration ranging from 50 to 100 revealed to decrease the decolorization capability from 83 to 53%.
In addition, this study also confirmed that the fungal biomass decreased from 1287 mg L−1 in the control (0 ppm) to 934, 885, and 754 mg L−1 for the dye concentration of 50, 75 and 100 ppm, respectively. These results are in line with the previous study by Yang and McGarrahan [31] who also found that the decolorization efficiency of C.I. Reactive Black 5 by Debaryomyces polymorphus decreased from 100 to 80% when the dye concentration was increased from 200 mg L−1 to 400 mg L−1. By estimating the biomass of fungal obtained from different dye concentrations, the results from this study can be used to evaluate the effect of dye toxicity on the fungal growth. Moreover, this study revealed that T. harzianum RY44 has the potential to survive and produce maximum biomass in highly toxic environmental conditions.
Effect of carbon sources
To investigate the effect of carbon sources on the decolorization of Mordant orange-1 by T. harzianum RY44, several carbon sources such as fructose, galactose, and glucose were added to the culture liquid medium. Figure 3 shows the effect of these carbon sources on the decolorization and fungal biomass obtained for 15 and 30 d incubation. T. harzianum RY44 decolorized 58, 68.2 and 67.4% of Mordant orange-1 with fungal biomass of 1530, 1310, and 2430 mg L−1 using fructose, galactose, and glucose for 15 days, respectively. In addition, for 30 days incubation, T. harzianum RY44 decolorized 71.9, 75.6, and 83.6% of Mordant orange-1 with fungal biomass of 2735, 1985, and 2875 mg L−1 when fructose, galactose, and glucose were employed, respectively.
Obviously, this study found that the highest percentage of decolorization was achieved by additional glucose for 15 and 30 days. In addition, the addition of glucose in the liquid medium also improved the fungal biomass. These results are in agreement with the previous study conducted by Stajić et al. [32] and Hadibarata et al. [33]. Their study found that increasing the incubation time and addition of carbon sources gave a high enzyme production and decolorization rate. This is related to the fact that the decolorization is mainly due to the extracellular enzyme activity [34]. Moreover, the enzyme formation can also be induced by various carbohydrates and their derivatives, including lactose, sophorose, xylobiose, d-xylose, and l-sorbose [35, 36]. Alternatively, another related study reported that the production of the main enzymes filamentous fungi related to degradation of lignocellulosic biomass is transcriptionally regulated and carbon source dependent [37].
Effect of nitrogen sources
The effects of nitrogen sources such as ammonium nitrate, ammonium sulfate, and yeast extract on the decolorization and fungal biomass are shown in Fig. 4. For 15 days incubation time, this study found that T. harzianum RY44 has capability to decolorize 75.0, 59.3, and 67.3% of the dye with fungal biomass of 5303, 2430, and 2430 mg L−1 using additional yeast extract, ammonium sulfate, and ammonium nitrate, respectively. Then, 98.0, 66.9 and 83.9% decolorizations of Mordant orange-1 with fungal biomass of 6705, 2960, and 2875 mg L−1 were achieved by the addition of the yeast extract, ammonium sulfate, and ammonium nitrate for 30 days, respectively.
In general, the present work found that the highest percentage of the decolorization was obtained when yeast extract was employed. The results clearly indicate that decolorization of Mordant orange-1 by T. harzianum RY44 is greatly affected by the addition of the nitrogen sources. These findings are consistent with those of Carliell et al. [38] who observed that the metabolism of yeast extract is highly beneficial to the regeneration of NADH that acts as the electron donor for the reduction of azo bonds. Also, the availability of nutrients, including a sufficient nitrogen source, is an important factor for the growth and development of many fungal species.
Effect of surfactant
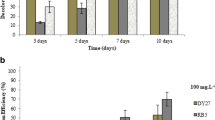
Surfactant is also considered to affect the decolorization and fungal biomass. For this purpose, its effect on the Mordant orange-1 decolorization was examined by applying Tween 80 at different volumes such as 0.1, 0.5, 1.0, and 1.5 ml. The use of this surfactant is due to their internal property to enhance the decolorization performance compared to another surfactant type such as Tween 20 [33]. Figure 5 shows the effects of the surfactant on the decolorization and mass of fungal biomass. The present work found that the percentage of decolorization of Mordant orange-1 at 0.1, 0.5, 1.0, and 1.5 ml of Tween 80 are 17.5, 8.9, 6.6, and 4.1% with fungal biomass of 2365, 3535, 5320, and 5950 mg L−1 for 15 days, respectively. For 30 days incubation time, their colorizations become 19.5, 11.3, 10.2 and 9.0% with biomass 3515, 3930, 5650, and 7335 mg L−1, for different volumes of 0.1, 0.5, 1.0, and 1.5 mL, respectively. In general, this study exhibited that the presence of the surfactant in the liquid medium can decolorize below 20%. However, the mass of fungal biomass was increased for all considered volumes of surfactant. The presence of surfactant might affect the growth of fungi during chemical reaction. Furthermore, these results confirmed that the addition of the surfactant was not effective for decolorization of Mordant orange-1.
Effect of aromatic compounds
A previous study found that the presence of aromatic compounds such as benzoic acid, gallic acid, or tannic acid affected the growth and laccase production of fungi [39]. Therefore, this study considers three aromatic compounds, namely, benzoic acid, catechol, and salicylic acid to evaluate their effects on the dye decolorization. The effect of different aromatic compounds on Mordant orange-1 decolorization is shown in Fig. 6. For 15 days incubation by addition of benzoic acid, catechol, and salicylic acid, this study found that the percentages of the decolorization were 72.6, 56.53, and 78.3% with fungal biomass 660, 550, and 685 mg L−1, respectively. The best performance was obtained when the benzoic acid was employed by achieving 89.9% decolorization and fungal biomass of 1870 mg L−1 for 30 days incubation. There was a relationship between aromatic compounds and laccase production [40]. The basis of the laccase-mediator concept is the use of low molecular weight phenolic compounds oxidized by the enzyme to stabilize radicals, act as redox mediators, and oxidize other compounds [41].
Effect of pH
pH is one of the important parameters affecting the decolorization. Figure 7 shows the effect of pH on the decolorization and fungal biomass. It is apparent from this study that high decolorization was observed in acidic pH values. Specifically, this study found that about 73.8, 59.5, 63.4 and 49.2% with biomass of 1850, 1595, 1145 and 875 mg L−1 for 15 days incubation time were obtained when pH of the mixture was set to be 3, 4, 5, and 6, respectively. For 30 days incubation, pH at 3, 4, 5 and 6 were able to decolorize about 81.7, 65.6, 65.2 and 60.5% with biomass of 2055, 1965, 1375, and 1100 mg L−1, respectively. In general, T. harzianum RY44 showed remarkable ability for decolorization of Mordant orange-1 at pH 3 and achieved for 15 and 30 days incubation time. In addition, results from this study revealed that T. harzianum RY44 can survive in extremely acidic environments. The high decolorization observed at acidic pH values can be attributed to the interaction of negatively charged dye molecules and positively charged binding sites on the decolorization surface [42].
Surface morphology
Figure 8a shows the morphological structure of T. harzianum RY44 before decolorization of Mordant orange-1. It is apparent from this figure that T. harzianum RY44 has a perforated surface structure and rough surface in nature. For comparison purpose, Fig. 8b demonstrates the surface morphology of these fungi after decolorization. Obviously, there are significant changes of the surface morphology in terms of their structure and roughness. After decolorization, their surface morphology becomes smoother and has an unperforated structure. This is because Mordant orange-1 was adsorbed by surface of the fungi and then it filled their pores.
FTIR characteristics
FTIR spectrum of Mordant orange-1 compared with degraded product is depicted in Fig. 9a, b. In case of control dye Mordant orange-1, several prominent peaks were observed around 665 to 860 cm−1 due to =C–H stretching. For a comparison purpose, noticeable change in these peaks has been defined for the FTIR spectrum of degraded product, which are less intensify or disappear in the region. In addition, azo-bond vibration (N=N) identified at a peak of 1528 cm−1 for control dye shifts to 1520 cm−1 and becomes less intense after decolorization. In addition, prominent peaks observed at regions of 1172–1464 cm−1 due to C–O, N–O, and –N=N–, respectively, also become less intense or disappear after decolorization. Moreover, several peaks at 1033, 2939, and 3707 cm−1 attributed to C–O, C–H, and O–H stretching, respectively, exhibit more intensity compared to the control dye. Considerable difference between the FTIR spectra of the control dye and degraded product obtained after decolorization by T. harzianum RY44 confirmed the biodegradation of Mordant orange-1. These results are also confirmed by the previous studies such as decolorization of congo red dye by Alternaria alternata CMERI F6 [43].
Proposed pathway for decolorization mechanisms
The decolorization of Mordant orange-1 was previously investigated using Marinobacter algicola MO-17 [28]. Their study found that 76–78% of the dye can be removed by employing the bacteria type. Since fungi have been well-known to have a better performance compared to bacteria, this study then considers the use of T. harzianum RY44, which also has different decolorization mechanisms compared to the use of bacteria. Therefore, GC-MS was employed for identification of the presence of these intermediates and the final transformation products of Mordant orange-1 after decoloration using T. harzianum RY44. Table 5 summarizes mass spectra analysis of the principal metabolites of Mordant orange-1 biodegradation. It is obviously from Table 5 that GC-MS chromatograms of samples from Mordant orange-1 biodegradation exhibits metabolite I with the molecular ion (M+) at m/z 194 and the substantial fragment ions at m/z 179, 121, 105, and 77. These characteristics can be correlated to the sequential losses of CH3, (CH3)3Si, OSi (CH3)3, and COOSi (CH3)3, respectively. The aforementioned mass spectral characteristics can be categorized as benzoic acid confirmed by a standard sample and database in the library of GC–MS. In addition, metabolite II had major spectrum at m/z 282 and the significant fragment ions at m/z 267, 209, and 193 attributed to the sequential losses of CH3, (CH3)3Si, and OSi (CH3)3, respectively. By comparing to the authentic compound, these characteristics can be related to salicylic acid confirmed by a standard sample and database in the library of GC–MS. These results indicated that enzymes secreted from T. harzianum RY44 have the capability to degrade Mordant orange-1 into benzoic acid and finally transformed into salicylic acid. Furthermore, a pathway of Mordant orange-1 biodegradation by T. harzianum RY44 was proposed in Fig. 10. In general, this study has confirmed that T. harzianum RY44 can be used for decolorization of Mordant orange-1.
Conclusions
This study aims to investigate capability of T. harzianum RY44 to decolorize Mordant orange-1. It was found that the carbon sources, nitrogen sources, aromatic compounds, and pH greatly affected the decolorization and fungal biomass. On the contrary, the presence of surfactant did not significantly affect the decolorization of Mordant orange-1 using T. harzianum RY44. The maximum decolorization and fungal biomass can be achieved when the glucose, yeast extract, benzoic acid, and pH 3 were applied. In the decolorization mechanism, it is approved that Mordant orange-1 was degraded by T. harzianum RY44 to become benzoic acid and it was finally transformed into salicylic acid. In general, the present work successfully approved that T. harzianum RY44 was capable for decolorization of Mordant orange-1 depending on the environmental condition. This research provides a framework on the exploration of T. harzianum RY44 as a dye decolorization agent for future water treatment applications.
References
Chen K-C, Wu J-Y, Liou D-J, Hwang S-CJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68
de Souza SM, d. AGU, Bonilla KAS, de Souza AAU (2010) Removal of cod and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J Hazard Mater 179:35–42
Palmieri G, Cennamo G, Sannia G (2005) Remazol brilliant blue R decolourisation by the fungus Pleurotus ostreatus and its oxidative enzymatic system. Enzyme Microb Technol 36:17–24
Rigas F, Dritsa V (2006) Decolourisation of a polymeric dye by selected fungal strains in liquid cultures. Enzyme Microb Technol 39:120–124
Garg VK, Amita M, Kumar R, Gupta R (2004) Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: a timber industry waste. Dyes Pigm 63:243–250
Fang Z, Song HL, Cang N, Li XN (2013) Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour Technol 144:165–171
Cui R, Bai C, Jiang Y, Hu M, Li S, Zhai Q (2015) Well-defined bioarchitecture for immobilization of chloroperoxidase on magnetic nanoparticles and its application in dye decolorization. Chem Eng J 259:640–646
Tan L, He M, Song L, Fu X, Shi S (2016) Aerobic decolorization, degradation and detoxification of azo dyes by a newly isolated salt-tolerant yeast Scheffersomyces spartinae TLHS-SF1. Bioresour Technol 203:287–294
Adnan LA, Mohd Yusoff AR, Hadibarata T, Khudhair AB (2014) Biodegradation of bis-azo dye reactive black 5 by white-rot fungus Trametes gibbosa sp. WRF 3 and its metabolite characterization. Water Air Soil Pollut 225:1–11
Hadibarata T, Nor NM (2014) Decolorization and degradation mechanism of Amaranth by Polyporus sp. S133. Bioprocess Biosyst Eng 37:1879–1885
Hadibarata T, Yusoff ARM, Aris A, Salmiati, Hidayat T, Kristanti RA (2012) Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Pollut 223:1045–1054
Lai C-Y, Wu C-H, Meng C-T, Lin C-W (2017) Decolorization of azo dye and generation of electricity by microbial fuel cell with laccase-producing white-rot fungus on cathode. Appl Energy 188:392–398
Sarioglu M, Bali U, Bisgin T (2007) The removal of C.I. Basic Red 46 in a mixed methanogenic anaerobic culture. Dyes Pigm 74:223–229
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Ayed L, Bekir K, Achour S, Cheref A, Bakhrouf A (2017) Exploring bioaugmentation strategies for azo dye CI Reactive Violet 5 decolourization using bacterial mixture: dye response surface methodology. Water Environ J 31:80–89
Buntić AV, Pavlović MD, Antonović DG, Šiler-Marinković SS, Dimitrijević-Branković SI (2017) A treatment of wastewater containing basic dyes by the use of new strain Streptomyces microflavus CKS6. J Clean Prod 148:347–354
Song L, Shao Y, Ning S, Tan L (2017) Performance of a newly isolated salt-tolerant yeast strain Pichia occidentalis G1 for degrading and detoxifying azo dyes. Bioresour Technol 233:21–29
Singh A, Manju, Rani S, Bishnoi NR (2012) Malachite green dye decolorization on immobilized dead yeast cells employing sequential design of experiments. Ecol Eng 47:291–296
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeterior Biodegradation 63:699–704
Badis A, Ferradji FZ, Boucherit A, Fodil D, Boutoumi H (2010) Removal of natural humic acids by decolorizing Actinomycetes isolated from different soils (Algeria) for application in water purification. Desalination 259:216–222
Kristanti RA, Kamisan MKA, Hadibarata T (2016) Treatability of methylene blue solution by adsorption process using Neobalanocarpus hepmii and Capsicum annuum. Water Air Soil Pollut 227:1–7
dos Santos AB, Cervantes FJ, van Lier JB (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385
Qu Y, Shi S, Ma F, Yan B (2010) Decolorization of reactive dark blue k-r by the synergism of fungus and bacterium using response surface methodology. Bioresour Technol 101:8016–8023
Sen SK, Raut S, Bandyopadhyay P, Raut S (2016) Fungal decolouration and degradation of azo dyes: A review. Fungal Biol Rev 30:112–133
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
Hsueh C-C, Chen B-Y, Yen C-Y (2009) Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas hydrophila. J Hazard Mater 167:995–1001
Shertate R, Thorat P (2017) Biotransformation of a textile azo dye Mordant Orange 1 by Halobacillus Trueperi Mo-22 in marine condition. Int J Pharm Phytopharm Res 3:268–276
Shertate R, Thorat P (2012) Decolorization of mordant orange-1 by Marinobacter algicola MO-17. Online J Biol Sci 12:1–5
Forss J, Welander U (2009) Decolourization of reactive azo dyes with microorganisms growing on soft wood chips. Int Biodeterior Biodegradation 63:752–758
Zhou JL, Banks CJ (1993) Mechanism of humic acid colour removal from natural waters by fungal biomass biosorption. Chemosphere 27:607–620
Yang C-L, McGarrahan J (2005) Electrochemical coagulation for textile effluent decolorization. J Hazard Mater 127:40–47
Stajić M, Persky L, Friesem D, Hadar Y, Wasser SP, Nevo E, Vukojević J (2006) Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzyme Microb Technol 38:65–73
Hadibarata T, Yusoff ARM, Kristanti RA (2012) Decolorization and metabolism of anthraquionone-type dye by laccase of white-rot fungi Polyporus sp. s133. Water Air Soil Pollut 223:933–941
Kaal EEJ, Field JA, Joyce TW (1995) Increasing ligninolytic enzyme activities in several white-rot Basidiomycetes by nitrogen-sufficient media. Bioresour Technol 53:133–139
Olsson L, Christensen TMIE., Hansen KP, Palmqvist EA (2003) Influence of the carbon source on production of cellulases, hemicellulases and pectinases by T. reesei Rut C-30. Enzyme Microb Technol 33:612–619
Juhász T, Szengyel Z, Réczey K, Siika-Aho M, Viikari L (2005) Characterization of cellulases and hemicellulases produced by T. reesei on various carbon sources. Process Biochem 40:3519–3525
Foreman PK, Brown D, Dankmeyer L, Dean R, Diener S, Dunn-Coleman NS, Goedegebuur F, Houfek TD, England GJ, Kelley AS, Meerman HJ, Mitchell T, Mitchinson C, Olivares HA, Teunissen PJM, Yao J, Ward M (2003) Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus T. reesei. J Biol Chem 278:31988–31997
Carliell CM, Barclay SJ, Naidoo N, Buckley CA, Mulholland DA, Senior E (1995) Microbial decolourisation of a reactive azo dye under anaerobic conditions. Water SA 21:61–69
Bettin F, Montanari Q, Calloni R, Gaio TA, Silveira MM, Dillon AJP (2014) Additive effects of CuSO4 and aromatic compounds on laccase production by Pleurotus sajor-caju PS-2001 using sucrose as a carbon source. Braz J Chem Eng 31:335–346
Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martínez AT, Martínez MJ (2006) Laccase purification and characterization from Trametes trogii isolated in tunisia: Decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39:141–148
Camarero S, Ibarra D, Martínez MJ, Martínez ÁT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Cooke RC, Whipps JM (1993) Ecophysiology of fungi. Blackwell Scientific Publication, Oxford
Chakraborty S, Basak B, Dutta S, Bhunia B, Dey A (2013) Decolorization and biodegradation of congo red dye by a novel white rot fungus Alternaria alternata CMERI F6. Bioresour Technol 147:662–666
Acknowledgements
This work was financially supported by a Fundamental Research Grant Scheme from Ministry of Higher Education Malaysia (No. 4F813).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadibarata, T., Syafiuddin, A., Al-Dhabaan, F.A. et al. Biodegradation of Mordant orange-1 using newly isolated strain Trichoderma harzianum RY44 and its metabolite appraisal. Bioprocess Biosyst Eng 41, 621–632 (2018). https://doi.org/10.1007/s00449-018-1897-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1897-0