Abstract
The present study attempts to cultivate Porphyridium purpureum under different scale-up conditions for further development and commercialization of microalgae-derived PUFAs such as ARA and EPA. Different temperatures (25, 30, and 35 °C) and light intensities (70, 165, and 280 μmol/m2s) were applied to the 50 L pilot-scale cultivation of P. purpureum in ASW. The cultivation under the light intensity of 280 μmol/m2s at 35 °C obtained biomass concentration up to 9.52 g/L, total fatty acid content to 56.82 mg/g, and ARA content to 22.29 mg/g. While the maximum EPA content of 7.00 mg/g was achieved under the light intensity of 280 μmol/m2s at 25 °C and the highest ratio of UFAs to TFAs of 74.66% was also obtained in this trial. Both biomass concentration and TFAs content were improved by increasing light intensity and temperature. Moreover, the ratio of ARA to EPA was enhanced by increasing cultivation temperature under the light intensity of 280 μmol/m2s. In contrast with flask culture, the conversion of linoleic acid (C18:2) to ARA was enhanced in scale-up culture, leading to more ARA content. Phosphate limitation enhanced the synthesis of lipid and LPUFAs. Moreover, the biomass concentration and biosynthesis of palmitic acid were preferred by sufficient C (NaHCO3).
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are acknowledged as a kind of promising non-food biomass for fuel and biochemical production owing to the high photosynthetic efficiency, by which light, carbon dioxide, and other inorganic nutrients can be converted to lipids, vitamins, carbohydrates, proteins, and pigments [1,2,3]. In addition, microalgae deserve on-going studies on account of their high lipid (fatty acid) content, fast growth, direct carbon dioxide mitigation, and year-round cultivation over a wide range of habitats including non-arable land [4]. Recently, much devotion and endeavor has been concentrated upon the cultivation and application development of microalgae including biofuel production [5], wastewater treatment [6, 7], animal feed, and high value-added eatable, and pharmaceutical substances such as polyunsaturated fatty acids (PUFAs), proteins, vitamins, and pigments [8]. Porphyridium purpureum, a kind of unicellular red microalgae, can produce a variety of active substances such as protein, exopolysaccharides, lipid, and pigments, among which lipid is composed of abundant unsaturated fatty acids, especially ARA and EPA [9,10,11].
Attempts on photobioreactors have been conducted for scale-up cultivation of different microalgal species. An attached cultivation mode was designed by Lutzu et al. [12] for polysaccharides production by P. cruentum, which provided convenience and feasibility for algal harvesting. A carrier model was set up by Wang et al. [13] for high-density cultivation of P. cruentum in a 42 L internal airlift loop photobioreactor. Androga et al. [14] explored the dynamic modeling of temperature change in outdoor operating tubular photobioreactors. Clarence et al. [15] reported the effect of conditions such as temperature, detention period, light intensity, and salinity on the growth rate and overall light energy conversion efficiency of P. cruentum. There also went a report on the daily cyclic variation of oxygen generation rates, carbon consumption rates, photosynthetic activities, growth rates, and biochemical composition of the biomass in a pilot plant continuous outdoor cultivation of P. cruentum [16]. Both light regime and photosynthetic efficiency were analyzed in characteristic examples of the state-of-the-art pilot-scale photobioreactors and productivity in this study was determined by the light regime inside the bioreactors [17]. Singh et al. [18] set up a process of outdoor mass cultures of Porphyridium sp. in flat plate glass reactors, in which cells constantly secreted some amorphous mucilaginous materials that form a capsule around themselves. This phenomenon made the cells masses together and adheres easily on the wall of vessels, which prevents further growth of algal cells [19, 20].
Studies focused on design of photobioreactors, however, little information was discovered about the effect of light, temperature, and nutrition on the scale-up culture of the red algae P. purpureum. The present study attempted to cultivate the red microalgae P. purpureum under different conditions for further contribution to the development and commercialization of microalgae-derived PUFAs such as ARA and EPA. The influence of nutrients (sodium bicarbonate and phosphate) on the yields of TFAs, ARA, and EPA was also investigated and presented.
Materials and methods
Microalgal cultivation
Microalgae P . purpureum CoE1 applied in the present study was screened and maintained by the authors’ research group. Prior to the scale-up cultivation, this algal species was prepared by shake flask cultivation.
All the chemicals (analytical reagents grade) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and fatty acid methyl ester standards from Sigma-Aldrich Inc. The cells grew in the artificial sea water medium (ASW) [21]. Stock culture was maintained in the above medium under the condition of light intensity of 165 μmol/m2s (24 h/day), temperature of 25 ± 0.5 °C, and aeration rate of 3 L/min (24 h/day).
Porphyridium purpureum was cultivated in 100 × 50 × 50 cm photoreactor (total volume of 250 L) within 50 L ASW under the pre-set light intensity and culture temperature. Aeration was supplied by bubble stone placed in the bottom of the pool, which was connected to an air pump with an aeration rate of 300 L/min. The inoculum size was 20%. To avoid the foam phenomenon, 10 g defoamer (LG-601) was added to the medium, which could be removed by biomass washing. Each cultivation run with different conditions was conducted with two replications. The experiment under different light intensities (70, 165, and 280 μmol/m2s) and temperature (25, 30, and 35 °C) was set as 70-25, 165-25, 280-25, etc. for short.
Growth analysis
The algal biomass concentration was determined by a correlation between the absorbance at 680 nm with a Shimadzu UV-1750 spectrophotometer and dry weight of biomass [biomass concentration (g/L) = 1.6711 OD680nm 0.0831, R 2 = 0.999]. This calibration curve was obtained from the dry biomass weight determined by weighing the cells after washing for two times with distilled water followed by drying in an 80 °C oven overnight until a constant weight was achieved. In addition, the biomass concentration was also determined by dry weight method (centrifugation and lyophilization) for reference.
Lipid extraction
Compared with various lipid extraction methods [22], lipid extraction in this study was performed using a modified Folch method, employing a 2:1 chloroform/methanol (v/v) extraction at 35 °C using an ultrasonic bath for 45 min [23, 24]. Freeze-dried algal cells of 0.1 g were treated with 5 mL of solvent mixture. After sonication, 1.7 mL of water was added to the samples, centrifuged at 6000 rpm for 10 min, and the lipid contained in chloroform layer was recovered. The procedure was repeated with fresh chloroform until the chloroform phase was almost transparent and colorless. Subsequently, the chloroform layers were collected, distilled and the crude lipid was blow-dried under nitrogen at 60 °C for subsequent analysis. The lipid was finally quantified gravimetrically and the content was calculated by the following equation:
Fatty acid analysis
The lipid collected above was esterified in 1 mol/L KOH–CH3OH solvent at 70 °C for 40 min, and simultaneously, the fatty acid methyl esters (FAMEs) generated were extracted with hexane. The qualitative and quantitative analysis of FAMEs was conducted with a Thermofisher Trace 1300 ISQ LT GC–MS instrument with a TR-5MS column (30.0 m × 250 μm × 0.25 μm). The following temperature program was used in the analysis: 313 K (1 min)—20 K/min—503 K (1 min)—3 K/min—543 K (2 min). The carrier gas was He with a flow rate of 60.0 mL/min and the split ratio was 1:50. The mass spectrometer contained electron impact ionization (EI) with an electron energy of 70 eV and an emission current of 25 μA. The amount of FAMEs was measured using an external standard method and was calculated as below:
Results and discussion
Effect of light intensity and temperature on biomass production and total lipid accumulation
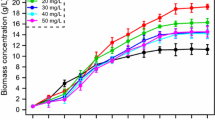
The effect of light intensity on biomass was investigated at 25 °C under light intensity of 70, 165, and 280 μmol/m2s (Fig. 1). Once the cells were cultivated under the conditions of temperature of 25 °C and light intensity of 70 μmol/m2s, decrease of the biomass concentration from the 4th to 6th days occurred, namely, 2.46 g/L in the 4th day dropped to 1.00 g/L in the 6th day. It was steady in the value of 1.1–1.3 g/L until harvested. Presumably, the cells could not grow smoothly under extremely low light intensity. While the biomass accumulated gradually under the 165-25 case and it shared a rapid growth after inoculation under the 280-25 case. Finally, the biomass concentrations of the two cases were up to 5.73 and 6.05 g/L, respectively. Though there was a tiny difference in biomass concentration of the two cases, the 165-25 case took a long period to the stationary phase. Obviously, the cells began an exponential growth without detention and then entered the stationary phase after a constant growth process in the 280-30 and 280-35 cases. The biomass concentrations of the two cases above were enhanced to 7.86 and 9.52 g/L, respectively. It was worth mentioning that the biomass harvested in the 165-30 case was almost the same as that in the 280-25 case.
Biomass concentration of P. purpureum under different light intensities and temperatures: (violet closed squares) 70 μmol/m2s, 25 °C; (dark cyan closed circles) 165 μmol/m2s, 25 °C; (pink closed rhombuses) 280 μmol/m2s, 25 °C; (blue closed five-pointed stars) 165 μmol/m2s, 30 °C; (olive closed up-triangulars) 280 μmol/m2s, 30 °C; (orange closed positive pentagons) 280 μmol/m2s, 35 °C. Cultivation conditions: pH 7.6, 300 L/min rate of sterile air
Overall, the algal biomass accumulation started to behave differently after inoculation and almost stopped on the 18th day in all cases (Fig. 1). The maximum biomass concentration of 9.52 g/L was obtained in the 280-35 case, suggesting that light intensity and temperature showed significant impact on the growth of P. purpureum [25,26,27]. In brief, moderately high light intensity temperature made for the rapid growth of P. purpureum. Meanwhile, light and temperature of a middle level prolonged the growth period with a lag phase. P. purpureum, a kind of autotrophic microalgae, could synthesize and accumulate chemicals by photosynthesis, which was affected and regulated by light intensity, temperature, CO2 and other conditions. Light was absorbed by the cells in the form of photons, so algal cell growth was promoted by moderately high light intensity. In addition, temperature mainly affected enzyme activity and ATP synthesis process, and perhaps, the enzyme activity was enhanced by properly high temperature.
The lipid contents of P. purpureum obtained in different cultivation conditions are concluded in Fig. 2. In general, the lipid accumulation declined markedly with the increase of light intensity and temperature. A highest lipid content of nearly 15 wt% of biomass was received in ASW under the lowest light intensity (70 μmol/m2s) and temperature (25 °C). It was assumed that excessive photo-assimilates could be stored in the form of proteins and polysaccharides by converting the excess light to chemical energy as an approach to avoid photo-oxidative damage [28, 29]. Furthermore, based on the following fatty acid analysis, the cells were apt to synthesize more neutral lipid to maintain basic life activities under low light and preferred to accumulate more functional lipid especially phospholipid under high light.
Lipid content of P. purpureum under different light intensities and temperatures: (violet column) 70 μmol/m2s, 25 °C; (cyan column) 165 μmol/m2s, 25 °C; (LT magenta column) 280 μmol/m2s, 25 °C; (yellow column) 165 μmol/m2s, 30 °C; (green column) 280 μmol/m2s, 30 °C; (orange column) 280 μmol/m2s, 35 °C. Cultivation conditions: pH 7.6, 300 L/min rate of sterile air
Effect of light intensity and temperature on fatty acid accumulation
With regard to fatty acid composition, it was deduced from Fig. 3 that cultures in different conditions provided similar categories of FAs, mostly including palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), ARA, and EPA [11]. Combined with Table 1, the TFA content was gradually improved by the increasing temperature and light intensity. The 280 μmol/m2s-35 °C trial provided the maximum TFAs of 56.72 mg/g and the maximum ARA content of 22.29 mg/g, implying that high temperature and intensity facilitated the synthesis of TFAs and ARA [27], while the contents of major FAs were also enhanced, especially the characteristic ones such as palmitic acid (C16:0), ARA, and EPA.
Fatty acid content of P. purpureum under different light intensities and various temperatures: (orange columns) C16:0; (green columns) C18:2; (violet columns) C18:1; (yellow columns) C18:0; (magenta columns) ARA; (cyan columns) EPA. Light intensity and temperature conditions: a 70 μmol/m2s, 25 °C; b 165 μmol/m2s, 25 °C; c 280 μmol/m2s, 25 °C; d 165 μmol/m2s, 30 °C; e 280 μmol/m2s, 30 °C; f 280 μmol/m2s, 35 °C. Other conditions: pH 7.6, 300 L/min rate of sterile air
Firstly, the algae P. purpureum, a kind of autotrophic organisms, could hardly grow in dark or under very low light intensity, let alone synthesize and accumulate lipid and fatty acids. Secondly, the fatty acids existed in the form of lipids, especially in the photosynthesis organs and chloroplast membrane structure. The synthesis of chloroplasts was greatly influenced by light and temperature. Moreover, NADPH, which was involved in the anabolism of lipid and fatty acids, could be merely formed on the thylakoid membrane of chloroplasts in the photoreaction stage of photosynthesis [30]. At length, high content of fatty acids resulted from high light intensity and appropriate temperature.
As shown in Table 2, the contents of UFAs were improved gradually by increasing light intensity and temperature with several exceptions. While the UFAs/TFAs played a fluctuating variation, namely, the ratio was first enhanced at 25 °C under increasing light intensity, and then, it decreased with gradient temperature under 280 μmol/m2s light intensity. The largest ratio of 74.66% was obtained in the 280-25 case, indicating that high light intensity and moderate temperature led to high ratio of UFAs to TFAs, which might be attributed to better expression of acetyl-CoA carboxylase (ACCase) and desaturase.
Effect of light intensity and temperature on ARA and EPA accumulation and ARA/EPA
The impact of light intensity and temperature on ARA and EPA content and ARA/EPA is presented in Figs. 3 and 4. Though the ARA content in 70-25 case (Fig. 3a) was badly lower than that of other tests, which was most likely to be on account of the shortage of photons, the EPA content was gradually improved. The contents of EPA in 25 °C under all light intensities (Fig. 3a–c) were somewhat higher than those of other temperature cases (Fig. 3d–f). On the other hand, ARA content was enhanced by increasing temperature and light intensity and the maximum of ARA content was 22.29 mg/g, gained in the 280 μmol/m2s-35 °C case (Fig. 3f), which was much higher than Su et al.'s results under optimal conditions [31]. Hence, it was speculated that scale-up cultivation enhanced ARA accumulation. While the maximum of EPA content (7.00 mg/g) was achieved in the 280 μmol/m2s-25 °C case (Fig. 3c).
Ratio of ARA to EPA of P. purpureum under different conditions: (violet closed squares) 70 μmol/m2s, 25 °C; (orange closed circles) 165 μmol/m2s, 25 °C; (pink up-closed triangulars) 280 μmol/m2s, 25 °C; (olive right-closed triangulars) 165 μmol/m2s, 30 °C; (dark cyan closed rhombuses) 280 μmol/m2s, 30 °C; (dark yellow closed five-pointed stars) 280 μmol/m2s, 35 °C. Cultivation conditions: pH 7.6, 300 L/min rate of sterile air
As shown in Fig. 4, the ARA/EPA ratio emerged a sharp fall from the 6th to 10th days, maintained to the 14th day, and dropped to about 0.6 until harvested under 70-25 case, implying that the conversion of ARA to EPA was accelerated by low light intensity and temperature [11, 32]. Interestingly, the ARA/EPA ratio in 165-25, 280-25, and 165-30 cases generally remained at a level of about 2.0 with a tiny fluctuation. On the contrary, ARA/EPA was advanced to about 5.4 by increasing culture temperature, which resulted from the prosperity of ARA with essential constant of EPA [33]. Both ARA and EPA contents shared a slightly reduce at the 16th–18th days due to their poor anti-oxidation properties. At length, the ARA content was notably enhanced by increasing light intensity and temperature, while ARA/EPA was promoted by increasing temperature under high light intensity. Therefore, it could be deduced that the synthetic pathways of fatty acids were pushed to stay at ARA rather than EPA by high light intensity and temperature, which might be due to the decreased activity of ∆17 desaturase by increasing temperature under high light intensity.
The ARA and EPA yields at different temperatures (25, 30, and 35 °C) under high light intensity (280 μmol/m2s) are presented in Fig. 5. Obviously, the ARA yield rose along with culture time and almost stopped at the 14th day in all cases. The maximum ARA yields at different temperatures (25, 30, and 35 °C) were 75.60, 113.77, and 191.85 mg/L, respectively, suggesting that the ARA accumulation mainly occurred in the late growth phase. The maximum ARA yield (191.85 mg/L) was distinctly higher than that (159.74 mg/L) of flask culture, which was attributed to not only the higher ARA content but also the larger biomass concentration in scale-up cultivation. It was proven that the scale-up cultivation was conducive to the accumulation of ARA. On the contrary, the EPA yield was hardly impressive. The maximum EPA yields of increasing temperature (25, 30, and 35 °C) were 37.69, 21.64, and 42.74 mg/L, respectively, implying that the scale-up cultivation showed none preference to EPA accumulation. Probably, the high yield of ARA was premised on sacrificing EPA synthesis.
Fatty acid and ARA synthesis
In algal cells, the synthesis of short-chain fatty acids is similar to that in higher plants, animals, fungi, and bacterial cells [34, 35]. It is catalyzed by two enzymes: ACCase and fatty acid synthase (FAS). Malonyl-CoA–ACP is obligatory during the fatty acid elongation (FAE) and FAs catalyze this reaction to form C16:0–ACP and C18:0–ACP. The synthesis of linoleic acid C18:2 (9, 12) is catalyzed by ∆9, ∆12 desaturases, respectively. EPA is synthesized via fatty acid elongation and multiple desaturation in both omega-3 and omega-6 pathways [36, 37]. In the omega-3 pathway, C18:2 is catalyzed by Δ6 desaturase to produce C18:3 (6, 9, 12), and C20:3 (8, 11, 14) is formed via FAE. The synthesis of ARA (5, 8, 11, 14) is catalyzed by Δ5 desaturase and EPA is formed by Δ17 desaturase. While in the omega-6 pathway, C18:2 was catalyzed by Δ15 desaturase to produce C18:3 (9, 12, 15), and then, C18:4 (6, 9, 12, 15) is synthesized by Δ6 desaturase. C20:4 (8, 11, 14, 17) is formed via FAE and EPA (5, 8, 11, 14, 17) synthesis is catalyzed by Δ5 desaturase [38, 39]. At length, ARA and EPA synthesis are accomplished with desaturase catalysis and multi-step FAE. EPA is the final product of elongation and desaturation in P. purpureum.
Comparative cultivation with 1 L and 50 L scale
Cultivation in 2 L glass flasks containing 1 L ASW was conducted under the light intensity of 165 μmol/m2s at 25 °C with an air flow of 3 L/min [31, 40] and the 50 L scale-up cultivation was set as 280-25 case. The comparative analysis of the fatty acid between 1 L flask and 50 L scale-up cultivation is represented in Fig. 6, which illustrated that ARA content (% in TFAs) obtained in scale-up cultivation was almost twice as much as that in flask cultivation, while the palmitic acid (C16:0) was significantly lower than that of flask cultivation. Simultaneously, the C18:2 and C18:0 contents in scale-up culture were notably lower than that of flask culture. However, the content of C18:1 was slightly larger than that of flask cultivation. In addition, the metabolic final product EPA content in scale-up cultivation was also higher than that in flask ones. According to the synthesis route, the scale-up cultivation promoted the conversion from C16 to C18 to C20, especially from C16:0 to C18 and from C18:2 to ARA, which could be referred that the activation of ∆6 and ∆5 desaturases was strengthened and better expressed [39].
Contrast of fatty acid content of P. purpureum under different culture scales: (LT yellow columns) fatty acid content of 1 L scale; (LT cyan columns) fatty acid content of 50 L scale. Cultivation conditions: 1 L culture conditions: 165 μmol/m2s, 25 °C, pH 7.6, 3 L/min rate of sterile air; 50 L culture conditions: 280 μmol/m2s, 25 °C, pH 7.6, 300 L/min rate of sterile air
The fatty acid accumulation mainly occurred in stationary phase, during which, perhaps, the limited nutrient availability accelerated the biosynthesis of lipids (fatty acids) in scale-up cultivation. Given that fatty acids mainly existed in the membrane structure, membrane fluidity was reinforced by LPUFAs, especially ARA and EPA to adapt the high light conditions, while the ACCase, a key enzyme in the biosynthesis of fatty acids, was also strengthened by high light intensity and temperature. Moreover, the photo-inhibition impact, as well as self-shading effect [41], could be suppressed by the openness of cultivation space under high light intensity. Because of the large ventilator capacity, the adherence phenomenon was also restrained by high circulation velocity of ASW. The algal cells could hardly gather to form blocks, which prevented the continuous growth. Perhaps, the heat and mass transfer conditions would be the potential reason. At length, the ARA accumulation was enhanced by scale-up cultivation.
Effect of sodium bicarbonate (NaHCO3) and phosphate (KH2PO4) on algal growth
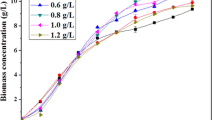
The effect of nutrient of C and P on P. purpureum was investigated under two cases: the nutrient of N-1 case was NaHCO3 (0.8 g/L) and KH2PO4 (0.035 g/L) and that of N-2 case was NaHCO3 (0.04 g/L) and KH2PO4 (0.035 g/L). Figure 7 illustrates that sufficient sodium bicarbonate possessed an obvious promotion on algal growth. The biomass concentrations of two cases were 10.02 g/L (N-1) and 6.97 g/L (N-2), respectively, demonstrating that carbon could obviously promote biomass accumulation of P. purpureum in 50 L scale-up cultivation with the limited introduction of phosphate (0.035 g/L). Perhaps, further enhancement of growth might be relied on engineering issues, involving availability of light, utilization of nutrient, medium circulation rate, etc.
Effect of sodium bicarbonate (NaHCO3) and phosphate (KH2PO4) on lipid/fatty acids
As shown in Table 3, the lipid contents of those two patterns were both higher than that of 280-35 case, which could be speculated that phosphate limitation was conducive to the accumulation of lipid [28], while the lipid content of the N-2 case was apparently 20–67% higher than that of N-1 case. Possibly, the adequate carbon resource, utilized in the form of HCO3 − by algae, would conceal the impact of phosphate limitation on lipid synthesis. Meanwhile, concerning that the sufficient phosphate is a positive factor for the synthesis of protein, phosphate limitation would enhance the synthesis of lipid in accordance with the performance of the study by Su et al. [28].
The effect of N-1 and N-2 patterns on fatty acids is shown in Fig. 8. Among the main fatty acids, the content of palmitic acid (C16:0) in N-1 case was higher than that in N-2 case, suggesting that the accumulation of palmitic acid, the substrate of the LPUFAs, was enhanced by adequate carbon resource (NaHCO3). Its content exceeded that of ARA (Fig. 8a). On the contrary, the ARA content in N-2 case was marginally larger than that of palmitic acid. Curiously, the ARA and TFAs were not remarkably enhanced under the two phosphate limitation conditions, which was inconsistent with our previous work in the flask cultivation. Perhaps, the effect of phosphate limitation was neutralized by scale-up and light, heat, and mass transfer issues. However, the EPA content showed a slight increase, improving the PUFAs (mainly for ARA and EPA) accumulation. In addition, the UFA-to-TFA ratio of N-2 case (see the additional document) was about 10% higher than that of 280-35 case, implying that phosphate limitation was still in favor of UFA synthesis. Interestingly, the maximum ARA content of the two cases in scale-up cultivation was also about 22.00 mg/g, the same as that in 280-35 case. The fatty acid yield of N-2 case was distinctly lower than that of N-1 case (Fig. 9a) owing to the lower biomass concentration of N-2 case (Fig. 7). Finally, the ARA content was doubled by scale-up cultivation compared with flask culture, and the maximum ARA yield (217.93 mg/L) was remarkably higher than that (159.74 mg/L) of flask culture [28], attributing to biomass prosperity in scale-up cultivation. Although the biomass concentration obtained in this study was relatively high, improvement of biomass is still an urgent problem in scale-up cultivation.
Fatty acid content of P. purpureum under different nutrients: (orange columns) C16:0; (green columns) C18:2; (violet columns) C18:1; (yellow columns) C18:0; (magenta columns) ARA; (cyan columns) EPA. Nutrient conditions: a 0.8 g/L NaHCO3 and 0.035 g/L KH2PO4; b 0.04 g/L NaHCO3 and 0.035 g/L KH2PO4. Cultivation conditions: 280 μmol/m2s, 35 °C, pH 7.6, 300 L/min rate of sterile air
Fatty acid yield of P. purpureum under different nutrients: (LT yellow columns) TFA yield; (LT magenta columns) ARA yield; (LT cyan columns) EPA yield. Nutrient conditions: a 0.8 g/L NaHCO3 and 0.035 g/L KH2PO4; b 0.04 g/L NaHCO3 and 0.035 g/L KH2PO4. Cultivation conditions: 280 μmol/m2s, 35 °C, pH7.6, 300 L/min rate of sterile air
Conclusions
In summary, the scale-up cultivation of P. purpureum was carried out under the various light intensities at increasing temperatures. The algal biomass, TFAs, and ARA were improved by increasing light intensity and temperature. Moreover, the ratio of ARA to EPA was enhanced by increasing cultivation temperature under the light intensity of 280 μmol/m2s. Most importantly, compared with the flask cultivation, the conversion pathway from C18:2 to ARA in scale-up cultivation was enhanced obviously, leading to significant ARA accumulation. Phosphate limitation was confirmed to enhance the synthesis of lipid and LPUFAs. Further research should be figured out an economic and efficient way of harvesting, which would save much time, labor as well as energy. In addition, the lipid and fatty acid metabolic pathways deserved continuous study for the production of LPUFAs, which could be utilized to guide the commercialization of LPUFAs.
References
Guo X, Su G, Li Z, Chang J, Zeng X, Sun Y, Lu Y, Lin L (2015) Light intensity and N/P nutrient affect the accumulation of lipid and unsaturated fatty acids by Chlorella sp. Bioresour Technol 191:385–390
Ramana K, Xavier J, Sharma R (2017) Recent trends in pharmaceutical biotechnology. Pharm Biotechnol Curr Res 1:1–10
Chen WH, Lin BJ, Huang MY, Chang JS (2015) Thermochemical conversion of microalgal biomass into biofuels: a review. Bioresour Technol 184:314–327
Juntila DJ, Bautista MA, Monotilla W (2015) Biomass and lipid production of a local isolate Chlorella sorokiniana under mixotrophic growth conditions. Bioresour Technol 191:395–398
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Zeng X, Guo X, Su G, Danquah MK, Zhang S, Lu Y, Sun Y, Lin L (2015) Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renew Sustain Energy Rev 42:1385–1392
Gao F, Nan J, Zhang X (2017) Simulating a cyclic activated sludge system by employing a modified ASM3 model for wastewater treatment. Bioprocess Biosyst Eng 40:877–890
Fábregas J, García D, Morales E, Domínguez A, Otero A (1998) Renewal rate of semicontinuous cultures of the microalga Porphyridium cruentum modifies phycoerythrin, exopolysaccharide and fatty acid productivity. J Ferment Bioeng 86:477–481
Zhao W, Wang Y, Jiang X, Huang J, Chen B (2009) Effect of different fatty acid extraction methods and growth condition on Porphyridium cruentum AA and EPA contnets. Sci Technol Food Ind 30:168–171
Fuentes MMR, Fernández GGA, Pérez JAS, Guerrero JLG (2000) Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem 70:345–353
Cohen Z (1999) Chemicals from microalgae. CRC Press, London
Lutzu GA, Zhang L, Zhang Z, Liu T (2017) Feasibility of attached cultivation for polysaccharides production by Porphyridium cruentum. Bioprocess Biosyst Eng 40:73–83
Wang C, Wang Y, Tian Z (2003) High density culture of Porphyridium cruentum in a 42 L internal airlift loop photobioreactor. Chin J Bioprocess Eng 3:564–569
Androga DD, Uyar B, Koku H, Eroglu I (2017) Dynamic modeling of temperature change in outdoor operated tubular photobioreactors. Bioprocess Biosyst Eng 40:1017–1031
Golueke CG, Oswald WJ (1962) Mass culture of Porphyridium cruentum. Appl Microbiol 10:102–107
Fuentes MR, Sánchez JG, Sevilla JF, Fernández FA, Pérez JS, Grima EM (1999) Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: quantitative analysis of the daily cyclic variation of culture parameters. J Biotechnol 70:271–288
Janssen M, Tramper J, Mur LR, Wijffels RH (2003) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up and future prospects. Biotechnol Bioeng 81:193–210
Singh S, Arad S, Richmond A (2000) Extracellular polysaccharide production in outdoor mass cultures of Porphyridium sp. in flat plate glass reactors. J Appl Phycol 12:269–275
Ramus J (1972) The production of extracellular polysaccharide by the unicellular red alga Porphyridium aerugineum. J Phycol 8:97–111
Wang C, Wen S, Ouyang F (1999) Optimization of culture conditions of Porphyridium cruentum. Eng Chem Metall 20:272–277
Jones RF, Speer HL, Kury W (1963) Studies on the Growth of the Red Alga Porphyridium cruentum. Physiol Plant 16:636–643
Li Y, Ghasemi Naghdi F, Garg S, Adarme Vega TC, Thurecht KJ, Ghafor WA, Tannock S, Schenk PM (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb Cell Factor 13:14
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Biller P, Ross AB (2014) Pyrolysis GC–MS as a novel analysis technique to determine the biochemical composition of microalgae. Algal Res 6:91–97
Jahn W, Steinbiss J, Zetsche K (1984) Light intensity adaptation of the phycobiliprotein content of the red alga Porphyridium. Planta 161:536–539
You T, Barnett SM (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Su G, Jiao K, Li Z, Guo X, Chang J, Ndikubwimana T, Sun Y, Zeng X, Lu Y, Lin L (2016) Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 39:1129–1136
Chen T, Liu J, Guo B, Ma X, Sun P, Liu B, Chen F (2015) Light attenuates lipid accumulation while enhancing cell proliferation and starch synthesis in the glucose-fed oleaginous microalga Chlorella zofingiensis. Sci Rep 5:14936
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4:12–21
Su G, Jiao K, Chang J, Li Z, Guo X, Sun Y, Zeng X, Lu Y, Lin L (2016) Enhancing total fatty acids and arachidonic acid production by the red microalgae Porphyridium purpureum. Bioresour Bioprocess 3:1–9
Durmaz Y, Monteiro M, Bandarra N, Gökpinar Ş, Işik O (2007) The effect of low temperature on fatty acid composition and tocopherols of the red microalgae Porphyridium cruentum. J Appl Phycol 19:223–227
Cohen Z, Vonshak A, Richmond A (1988) Effect of environmental conditions on fatty acid composition of the red alga Porphyridium cruentum: correlation to grwoth rate. J Phycol 24:328–332
Haines TH (1973) Sulfolipids and halosulfolipids—lipids and biomembranes of eukaryotic microorganisms—Chapter 4. Academic Press, Cambridge
Stumpf PK (1984) Chapter 6 fatty acid biosynthesis in higher plants. Elsevier B.V., Amsterdam
Khozin I, Adlerstein D, Bigongo C, Heimer YM, Cohen Z (1997) Elucidation of the biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum (II. studies with radiolabeled precursors). Plant Physiol 114:223–230
Shiran D, Khozin I, Heimer YM, Cohen Z (1996) Biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum. I: the use of externally supplied fatty acids. Lipids 31:1277–1282
Khozin I, Adlerstein D, Bigogno C, Cohen Z (1997) Elucidation of the biosynthesis of eicosapentaenoic acid (EPA) in the microalga Porphyridium Cruentum. In: Williams JP, Khan MU, Lem NW (eds) Physiology, biochemistry and molecular biology of plant lipids. Springer, Dordrecht
Sato N, Moriyama T, Mori N, Toyoshima M (2017) Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World J Microbiol Biotechnol 33:74
Su G, Guo X, Chang J, Liu X, Zeng X, Sun Y, Lin L (2015) Effect of 6-benzylaminopurine (6-BA) on cell growth and fatty acids biosynthesis of microglage Porphyridium Purpureum. J XM Univ (Nat Sci) 54:790–795
Imaizumi Y, Nagao N, Yusoff FM, Taguchi S, Toda T (2014) Estimation of optimum specific light intensity per cell on a high-cell-density continuous culture of Chlorella zofingiensis not limited by nutrients or CO2. Bioresour Technol 162:53–59
Acknowledgements
This work was supported by the special fund for Fujian Ocean High-Tech Industry Development (No. 2013015), China, Research Program from the Science and Technology Bureau of Xiamen City in China (3502Z20151254), and the Fundamental Research Funds for the Central Universities (20720160077).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors have declared no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, J., Le, K., Song, X. et al. Scale-up cultivation enhanced arachidonic acid accumulation by red microalgae Porphyridium purpureum . Bioprocess Biosyst Eng 40, 1763–1773 (2017). https://doi.org/10.1007/s00449-017-1831-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1831-x