Abstract
Fossil fuels are still major energy sources, but the search for renewable energy sources has been encouraged. Bioethanol has been recognized as an alternative to fossil fuels and nowadays it represents more than 90% of the global biofuel production. Bioethanol production from raw sugar beet cossettes as a semi-solid substrate was studied. The study was carried out in the horizontal rotating tubular bioreactor (HRTB) with Saccharomyces cerevisiae as a microbial production strain. The impact of different combinations of HRTB operational parameters such as, rotation speed (5–15 min−1), rotation type [constant or interval (3–15 min h−1)] and working volume (ratio V W/V T = 0.2–0.7) on the bioethanol production was examined. In this study, the highest bioprocess efficiency parameters (\(Y_{{P_{1} /S}}\) = 0.47 g g−1, E = 87.36% and Pr = 0.618 g L−1 h−1) were observed at 0.20 V W/V T, interval rotation of 12 min h−1 and rotation speed of 15 min−1. It has to be pointed out that bioethanol production efficiency in the HRTB was on the similar level as observed by bioethanol production from the raw sugar beet juice. Naturally present microorganisms of sugar beet could have a significant impact on bioethanol production. Higher yeast inoculation rate could reduce contaminant activities and, consequently, the bioethanol production efficiency would be improved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global fossil fuels reserves decrease as energy requirements has been continuously grown. In the last two decades, the search for renewable, sustainable and environmentally-friendly energy sources has been encouraged around the world in order to satisfy energy demands and to prevent climate changes [1]. Bioethanol has been recognized worldwide as an alternative to petroleum-derived transport fuels and nowadays it represents more than 90% of the global biofuel production [2,3,4,5]. For the production of bioethanol, sugar (e.g. sugarcane or sugar beet) and starch (e.g. corn or wheat) containing raw materials as well as lignocellulosic raw materials can be used [6, 7]. Non-expensive and more abundant raw materials are desirable for industrial bioethanol production. Lignocellulosic raw materials are considered as an attractive feedstock for the production of bioethanol because of their availability in large quantities at relatively low cost, thus reducing competition with food but not necessarily with feed [7,8,9,10,11,12,13]. Microalgae and cyanobacteria are also considered as a feedstock for bioethanol production [14]. Degradation of lignocellulosic raw materials is complicated, energy-consuming and still an immature process [7, 15, 16]. In the future, the use of modern genetics and other tools will significantly reduce the cost of producing sugars from lignocellulosic raw materials [10, 17]. Currently, the participation of raw materials in the final bioethanol price is in the range of 40–70% [18]. This participation of raw materials in the bioethanol price can be reduced by the use of waste products from forestry, agriculture and industry [10, 19, 20]. Furthermore, it is also important that the bioethanol production will be located in the renewable raw materials production region because of the reduction of costs for raw materials collection, transportation and storage [21, 22].
In Europe industrial production of bioethanol is still based on the sugar and starch containing raw materials. Due to the often surplus of sugar beet as a raw material for sugar production it is possible that sugar factories redirect this sugar beet surplus into bioethanol production [23]. However, availability and transport of this raw material represent major cost when new cost effective production facilities are planned to be build up. Sugar production plants with current equipment are capable to utilize different intermediates of sugar beet processing (e.g. raw sugar juice with 15–18% sugar or concentrated sugar syrup with 65–67% sugar) as well as by-products (e.g. molasses ≈50% sugar) for bioethanol production [24, 25]. However, production of these intermediates involves energy demanding processes with main energy consuming operations like sugar extraction from sugar beet cossettes, sugar juice concentration by evaporation, ethanol distillation as well as distillers mash concentration and drying [26]. These processes significantly affect the energy input/output ratio and therefore they have direct impact on the ecological sustainability of bioethanol production. The development of integrated bioprocesses, in which simultaneous product synthesis and in situ product removal are combined, can result in more energy conserving and less expensive bioethanol production. So far integrated bioprocess has been successfully developed for acetone-butanol-ethanol (ABE) fermentation [27]. Another possible aspect to consider, as way of reducing production costs and increasing sustainability, is to replace liquid broth with semi-solid substrate. So far different types of substrate and microorganism in semi-solid bioethanol production were studied [28,29,30,31,32]. The promising idea for the reduction of bioethanol production cost is the use of semi-solid intermediates of sugar beet processing (e.g. cossettes or pulp) as a semi-solid substrate. Until now, investigation of bioethanol production in semi-solid bioprocesses with ensilage sugar beet pulp [33], mixed grape and sugar beet pomace [34] or mixed mash of sugar beet roots and potato tubers [21] were performed. For efficient and sustainable bioethanol production different types of bioreactors have to be used because of renewable feedstock’s diversity. Tubular bioreactors are one possibility due to the fact that mixing is more uniform in these bioreactors compared to the stirred tank bioreactors. Area-to-volume ratio is significantly higher in tubular bioreactors resulting in more efficient mass and heat transfer. This is important for bioprocesses with semi-solid or solid substrates, photoreactions (maximum exposure to light) and shear sensitive organisms. Due to the plug flow conditions the gradients of concentrations along the bioreactor are formed that is advantageous in the case of inhibition and/or repression kinetics. In these cases, high conversion efficiency and productivity can be simultaneously achieved. However, the major drawback of tubular bioreactors is relatively low oxygen supply capacity [35]. Currently, computational fluid dynamic (CFD) model is developing for description of simultaneous extraction and fermentation process in the sugar beet cossettes [36].
In this research, the possibility of using untreated raw sugar beet cossettes as a semi-solid substrate for bioethanol production in the new developed type of horizontal rotating tubular bioreactor (HRTB) was studied. Furthermore, the optimal operational conditions of HRTB (i.e. the rotation speed and the manner of bioreactor rotation as well as bioreactor working volume) for semi-solid bioethanol production were examined.
Materials and methods
Bioethanol production in a horizontal rotating tubular bioreactor
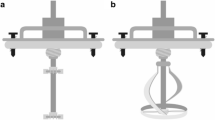
In this research, bioethanol production from raw sugar beet cossettes (as a semi-solid substrate) in the HRTB was studied. The HRTB is constructed as 0.6 m long and 0.25 m wide stainless steel tube with total volume of 30 L. The interior of HRTB contains two paddles (0.04 m height and 0.6 m length) fixed on the bioreactor wall in order to improve mixing and homogenization of bioreactor content. The bioreactor was placed on the bearings that enable rotation of the whole bioreactor. Furthermore, bioreactor is additionally equipped with a regulation system that enables continuous or interval rotation with different rotation/stagnation times. In this research, yeast Saccharomyces cerevisiae (yeast strain isolated from sugar factory; Culture collection of the Faculty of Food Technology and Biotechnology University of Zagreb; YSLZ-01) was used as a microbial production strain. The inoculum for HRTB inoculation was prepared in Erlenmeyer flasks (ratio medium/total volume = 0.4) on the raw sugar beet juice containing approximately 150 g L−1 of sugar with the addition of 1 g L−1 of NH4H2PO4 (as an additional source of phosphate and nitrogen) in order to support yeast growth and its physiological activity. The flasks were cultivated on a rotary shaker (rotation speed of 100 min−1) for 18 h at 28 °C.
The study of bioethanol production in the HRTB was also characterized by NH4H2PO4 addition (1 g kg−1 of raw sugar beet cossettes). Prior to the addition of raw sugar beet cossettes and yeast inoculation, the bioreactor was sterilized at 121 °C (2 bars) for 20 min. In this study, bioethanol production was examined by three different sets of HRTB operational conditions. In the first set of experiments bioethanol production was examined by continuous bioreactor rotation during bioprocess where speed varied from 5 to 15 min−1. The second set of experiments was characterized by using interval bioreactor rotation where bioreactor rotated minimum 3 and maximum 15 min per hour. Bioreactor rotation speed was also varied in the range of 5–15 min−1. In first two sets of experiments, HRTB was filled with 5 kg of unsterile raw sugar beet cossettes and inoculated with 1 L of yeast suspension (16.67% v/w raw sugar beet cossetets). In the third part of this research, the effect of different working volume [ratio between the working (V w) and total (V T) bioreactor volume] of HRTB on the bioethanol production was examined. In these experiments, the mass of raw sugar beet cossettes in the HRTB was changed in the range of 5–17.5 kg. The quantity of yeast suspension for these experiments was also enlarged so that initial inoculation rate was 16.67% v/w of raw sugar beet cossettes. The study of bioethanol production in the HRTB was performed at room temperature without pH value correction. The bioethanol production in the HRTB was monitored by sampling the liquid and the solid part (sugar beet cossettes) of the broth. After that, sugar beet cossettes were pressed by the hand juice press to obtain liquid samples for analytical purposes. The duration of bioethanol production in the HRTB by all combinations of operational parameters was determined by the ethanol concentration that has to reach approximately constant level in the period of at least 48 h. All experiments in the HRTB were at least repeated one time and the standard deviation of all measurements was in the range of experimental error (below 4.8%).
Analytical methods and bioprocess efficiency parameters
The yeast cell number (CFU mL−1) in the liquid phase of the broth was determined after cultivation on the diluted sugar beet juice (approximately 30 g L−1 sugars) medium with 20 g L−1 agar and 1.0 g L−1 NH4H2PO4 in Petri dishes at 28 °C for 48 h. During bioethanol production in the HTRB dry mass of sugar beet cossettes was monitored by gravimetrical method. Liquid samples of both sample types (liquid part of broth and liquid obtained by pressing sugar beet cossettes) were centrifuged for 15 min at 4500 min−1 (3629×g). Sediments obtained after centrifugation of the liquid part of the broth were used for determination of dry biomass concentration by gravimetrical method. The samples were dried at 105 °C until constant mass was obtained. Supernatants were used for substrate (glucose, fructose and sucrose) and metabolic products (ethanol, glycerol, acetate and lactate) determination by high performance liquid chromatography with Supelcogel™ C-610H column (Shimadzu CLASS-VP LC-10A VP , Shimadzu, Kyoto, Japan). Mobile phase was phosphoric acid solution (0.02 mol L−1) in demineralized water (conductivity <1 µS cm−1) and its flow rate was 0.5 mL min−1. Before chromatographic analysis the prepared supernatant solution was filtered through a nylon filter (0.22 µm) and subsequently degassed in ultrasonic bath for 20 min. Bioprocess efficiency parameters were determined by standard procedures. For estimation of these parameters data from liquid and solid part of broth were used and average values of these parameters are presented in Tables 1, 2 and 3. Bioethanol yield was determined by following equation:
where P and P 0 are ethanol concentration at the end and the beginning of bioprocess, respectively.
Bioethanol conversion coefficient (\(Y_{{P_{1} /S}}\)) was calculated by the following equation:
where ΔS = S 0 − S; S 0 and S are substrate concentration at the beginning and the end of bioprocess, respectively. Furthermore, Δm P,T is the total mass of the ethanol obtained in the HRTB, and Δm S,T is the total mass of consumed substrate in the HRTB.
Bioprocess efficiency (E) was estimated as a ratio between experimental (\(Y_{{P_{1} /S}}\)) and theoretical conversion coefficient (\(Y_{{P_{1} /S}}\))T:
where (\(Y_{{P_{1} /S}}\))T = 0.538 g g−1 is theoretical (stoichiometric) conversion coefficient of sucrose into ethanol.
Bioprocess productivity (Pr) was determined by the following equation:
where t is cultivation time and V w is working volume of the bioreactor.
Results and discussion
During this research bioethanol production from raw sugar beet cossetets in the new type of HRTB was studied by different bioreactor operational conditions. In our preliminary research, the minimal inoculum quantity (16.67% v/w raw sugar beet cossettes) that is required for successful bioethanol production with yeast S. cerevisiae in the HRTB was defined. Further increase of inoculum quantity in the HRTB was not related to the significant increase of bioethanol production efficiency (data not shown). From another point of view, the increase of inoculum quantity was also related to the increase of free water content in the broth, which consequently has an impact on the energy demand for ethanol separation by distillation. Therefore, in this research all experiments were performed by inoculation rate of 16.67% v/w of raw sugar beet cossettes that is related to the initial yeast concentration over 107 CFU mL−1 in the liquid part of the broth. In order to obtain an accurate view of bioethanol production under these conditions, it was necessary to take samples from the liquid as well as from the solid part (raw sugar beet cossettes) of the broth in the HRTB. In this research, some oscillations of monitored bioprocess parameters were observed as a consequence of analytical errors and heterogeneity of semi-solid substrate in the HRTB. Due to the fact that non-sterile raw sugar beet cossettes were used for bioethanol production, presence of acetate and lactate was also detected in the broth as a consequence of the activity of naturally present heterofermentative lactic acid bacteria (mostly Leuconostoc species especially Leuconostoc mesenteroides) of the sugar beet [37]. During this investigation HRTB rotation speed was changed in the range of 5–15 min−1. At higher HRTB rotation speeds (>15 min−1; data not shown) acetic acid concentration was considerably increased due to the higher activity of naturally present heterofermentative lactic acid bacteria (HFLAB) in these conditions. This phenomenon can be explained as a consequence of HFLAB metabolism shift from lactate and ethanol to the acetate production due to the pyruvate dehydrogenase activity that is directly related to the oxygen supply in the broth [38,39,40,41]. Although lactate concentration was monitored in the liquid as well as in the solid part of the broth during the entire research, its presence was detected only in negligible concentrations (below 0.15 g L−1). This effect additionally confirmed the impact of oxygen on the physiological activity of HFLAB. Furthermore, lactate as a non-fermentable substrate can be also easily aerobically consumed by yeast cells [42]. Glucose, fructose and sucrose concentrations were determined separately, but in all figures and tables their values were summarized and presented as a substrate concentration. Total dry biomass in the liquid part of the broth in the HRTB (Figs. 3, 6) was also monitored, but due to the presence of the debris of sugar beet cossettes in the liquid samples, significant oscillations of total dry biomass were observed. Direct determination of biomass concentration during semi-solid (or solid) bioprocesses is very difficult due to the impossibility of separating the microorganisms from the substrate (especially by filamentous fungi). Therefore, indirect methods for biomass determination were developed that include physical (e.g. scanning electron microscopy, epifluorescence microscopy, flow cytometry, effluent gas composition or light reflectance), chemical and biochemical [quantification of cell-specific components (e.g. ergosterol, glucosamine, chitin, nucleic acids or proteins) or other cell parameters’ determination (e.g. intracellular water and total carbon content, enzymatic or immunological activity or ATP levels, etc.) methods [43,44,45]. However, all these methods have their own advantages and disadvantages that have to be considered in the selection procedure of method for biomass determination during semi-solid (or solid) bioprocesses. In our case, yeast cell number was determined by Thoma-chamber counting and CFU determination. On the basis of obtained results, it was observed that significantly higher cell number oscillations were detected by Thoma-chamber counting as compared to the CFU determination. Therefore, it was decided that CFU determination will be used as an indicator of yeast growth although it has its own disadvantages (e.g. time consuming and accuracy level). Furthermore, the accuracy of this method was additionally lowered due to the fact that in some samples the presence of HFLAB was also observed (up to 105 CFU mL−1; data not shown) because of the use of non-sterile raw sugar beet cossettes.
Bioethanol production in the HRTB by continuous bioreactor rotation
In the first set of experiments, bioethanol production from raw sugar beet cossettes in the HRTB was studied by continuous bioreactor rotation (5–15 min−1) during bioprocess. For example, experimental data obtained during bioethanol production at continuous bioreactor rotation speed of 5 min−1 are presented in Figs. 1, 2 and 3. As it can be seen in Figs. 1 and 2, the difference between the concentration of fermentable sugars in the liquid and solid parts of the broth was insignificant, although a higher concentration of sugars was detected in the sugar beet cossettes. Diffusion of sugars is the limiting process, but once the sugar diffuses into the liquid part of the broth, it is easily and very quickly used by the microorganisms present. Microorganisms present in the HRTB utilize carbon source for their growth and products’ synthesis. Ethanol and glycerol are detected as main yeast metabolism products. However, metabolic products of naturally occurring HFLAB on the sugar beet are acetic acid and lactic acid [37, 41]. At the beginning of the bioprocess, small amounts of ethanol and glycerol were also detected as a consequence of their entry in the HRTB by inoculation. In the first part of fermentation (till the 17th hour), the pH value of the broth declined from 6.45 to 4.35 and remained almost unchanged till the end of the bioprocess (Fig. 3). In the first 40 h, the increase of total biomass concentration was observed. After that period, it declined and stayed almost unchanged due to accumulation of ethanol and acetic acid and their inhibitory effect on the yeast cells. During the entire bioprocess, lactic acid was not detected. On the basis of previous results, it is clear that naturally occurring contaminants have influence on the bioprocess performance efficiency [46]. Dry mass of sugar beet cossettes declined from 23.0 to 13.5% and it corresponds well with the utilized total sugar concentration during the bioprocess (Fig. 2). The bioethanol yield was \(Y_{{P_{1} }}\) = 39.84 g L−1. The conversion coefficient was \(Y_{{P_{1} /S}}\) = 0.27 g g−1 which is 50.20% of the theoretical value. Bioprocess productivity achieved in this experiment was Pr = 0.247 g L−1 h−1. Similar observations concerning substrate utilization and products’ synthesis were observed by other two experiments conducted at higher bioreactor rotation speeds (10 and 15 min−1). As it can be seen in Table 1, lower total bioethanol concentration and yield as well as the bioprocess productivity was observed in these experiments (\(Y_{{P_{1} }}\) = 31.03 g L−1, \(Y_{{P_{1} /S}}\) = 0.19 g g−1, Pr = 0.185 g L−1 h−1 at 10 min−1 and \(Y_{{P_{1} }}\) = 37.00 g L−1, \(Y_{{P_{1} /S}}\) = 0.26 g g−1, Pr = 0.196 g L−1 h−1 at 15 min−1). The increase of bioreactor rotation speed has significant impact on the bioprocess dynamics where higher rotations were related to the better oxygen transfer rate and less favourable conditions for bioethanol production in the HRTB [38,39,40,41].
Changes of substrate (S C, filled circle), ethanol (P 1, filled triangle), glycerol (P 2, open triangle), acetic acid (P 3, open square) concentrations in the solid part of the broth and cossettes dry mass (DM, open circle) during bioethanol production in the HRTB at continuous bioreactor rotation of 5 min−1 (V W/V T = 0.2)
Bioethanol production in the HRTB by interval bioreactor rotation
On the basis of previous experimental results the new experimental setup was established. In this experimental setup, the influence of interval HRTB rotation (3–15 min h−1) and different HRTB rotation speeds (5–15 min−1) on the bioethanol production was studied. At the beginning of this research, it was shown that interval rotation (3 min h−1 at rotation speed of 5 min−1) has a positive impact on bioethanol production. In these conditions, bioprocess efficiency parameters were considerably increased as compared to the previous experimental setup (Table 1). Bioethanol yield was \(Y_{{P_{1} }}\) = 60.78 g L−1, \(Y_{{P_{1} /S}}\) = 0.36 g g−1, bioprocess efficiency E = 66.90% and productivity Pr = 0.832 g L−1 h−1, respectively (Table 2). The positive impact on the bioprocess performance had also relatively low acetic acid concentration (1.19 g L−1) detected in these conditions. In further experiments, bioreactor rotation period in the rotation/stagnation ratio was increased. At interval rotation of 6 min h−1 and rotation of 5 min−1 higher \(Y_{{P_{1} /S}}\) = 0.44 g g−1 and E = 81.80%, but lower \(Y_{{P_{1} /S}}\) = 50.78 g L−1 and Pr = 0.682 g L−1 h−1 were observed compared to the experimental setup of 3 min h−1 and 5 min−1. Bioethanol production efficiency was also declined at interval rotation of 9 min h−1 and 5 min−1. Further increase of interval rotation at 12 min h−1 shows positive impact on the bioethanol production, but lower bioprocess efficiency parameters (except \(Y_{{P_{1} /S}}\) and E) were observed compared to the interval rotation of 3 min h−1 at rotation speed of 5 min−1. However, at interval rotation of 15 min h−1 considerable reduction of bioprocess efficiency parameters was detected as a consequence of intensive broth mixing that is related to the increased oxygen supply. In these conditions, HFLAB activity was increased and consequently higher acetate concentration (7.43 g L−1) was detected what has negative impact on bioethanol production [38,39,40,41] (Table 2). In Table 2, products of yeast and HFLAB metabolism are presented as conversion coefficients [ethanol (\(Y_{{P_{1} /S}}\)), glycerol (\(Y_{{P_{2} /S}}\)) and acetate (\(Y_{{P_{3} /S}}\))]. Lactate conversion coefficient is not presented due to the negligible lactate concentrations (below 0.15 g L−1).
In further research, bioreactor rotation speed was increased at 10 min−1 by the same rotation/stagnation time ratios (interval rotation). Bioethanol production at 3 min h−1 and 10 min−1 shows similar trends of bioprocess parameters changes as observed in previous experimental setup (rotation speed of 5 min−1). Therefore, similar values of bioethanol efficiency parameters were also observed (\(Y_{{P_{1} }}\) = 65.14 g L−1, \(Y_{{P_{1} /S}}\) = 0.40 g g−1, E = 74.30% and Pr = 0.552 g L−1 h−1). However, further increase of interval rotation (rotation/stagnation time ratio) did not result in improvement of bioethanol production. This observation is confirmed by the lower or similar levels of bioethanol production efficiency. The exception is the experiment at interval rotation of 9 min h−1 where bioprocess was finished in only 71 h and consequently higher bioprocess productivity was obtained (Pr = 0.812 g L−1 h−1). In the next part of this investigation bioethanol production was studied by the same interval rotations, but increased HRTB rotation speed (15 min−1). First experiment was done at interval rotation of 3 min h−1. The bioprocess ended in only 60.5 h which resulted in the highest bioprocess productivity (Pr = 0,934 g L−1 h−1) recorded in all experiments performed in the HRTB. Other bioprocess efficiency parameters were \(Y_{{P_{1} /S}}\) = 56.50 g L−1, \(Y_{{P_{1} /S}}\) = 0.35 g g−1 and E = 64.30%. Increase of interval rotation at 6 min h−1 resulted in higher acetic acid concentrations what indicates that favorable conditions for HFLAB activity were established. In this experiment, final acetic acid concentration in the liquid part of broth was 7.52 g L−1. Due to this phenomenon less substrate was converted into ethanol and consequently reduction of bioprocess efficiency was observed (\(Y_{{P_{1} }}\) = 47.14 g L−1, \(Y_{{P_{1} /S}}\) = 0.31 g g−1, E = 57.6%, and Pr = 0.327 g L−1 h−1). Slightly higher bioethanol concentration (52.10 g L−1) and considerable reduction of bioprocess time (only 86 h) resulted in higher values of bioprocess efficiency parameters at interval rotation of 9 min h−1 (15 min−1) compared to the bioprocess conducted at interval rotation of 6 min h−1 (15 min−1). Bioprocess productivity rose up to 0.606 g L−1 h−1, but only slightly increase of \(Y_{{P_{1} /S}}\) = 0.32 g g−1 and E = 58.70% was observed. In these conditions, lower acetic acid concentration (3.30 g L−1) was also detected. Bioethanol production at interval rotation of 12 min h−1 (15 min−1) lasted for 90 h, but even lower acetic acid concentration was detected (2.11 g L−1) compared to the bioethanol production at 6 min h−1 (15 min−1). Therefore, bioethanol production efficiency was enlarged (\(Y_{{P_{1} }}\) = 55.64 g L−1, \(Y_{{P_{1} /S}}\) = 0.47 g g−1, E = 88.10% and Pr = 0.618 g L−1 h−1). Changes of bioprocess parameters during this experiment are presented in Figs. 4, 5 and 6.
Changes of substrate (S L, filled circle), ethanol (P 1, filled triangle), glycerol (P 2, open triangle) and acetic acid (P 3, open square) concentrations in the liquid part of the broth during bioethanol production in the HRTB by interval rotation of 12 min h−1 and rotation speed of 15 min−1 (V W/V T = 0.2)
As it can be seen in Figs. 4 and 5, higher total sugars concentration was observed in the liquid (140 g L−1) than in the solid (122 g L−1) part of broth. This observation shows that mixing conditions (rotation speed and duration of interval rotation) and physiological characteristics of sugar beet cossettes (closely related to the sugar beet storage conditions) have significant impact on sugars release from the sugar beet cossettes [33]. Changes of bioethanol, glycerol and acetate concentrations are in agreement with substrate consumption. Concentrations of all three fermentation products were on the similar levels in the liquid and solid part of broth. Changes in the dry mass of cossettes (Fig. 5) correspond well with changes in substrate concentration, which indicate that released sugars (glucose, fructose and sucrose) were immediately used in the fermentation process. In first 20 h of bioprocess, pH value of liquid part of broth (Fig. 6) was reduced from 6.01 to 4.59 due to the acetic acid synthesis by HFLAB. As it can be seen in Fig. 6, increase of dry biomass was registered in the first 20 h of bioprocess and after that considerable oscillations of this parameter were observed. As mentioned earlier, this phenomenon is a consequence of the presence of cossettes debris in samples as well as the heterogeneity of cultivation broth. However, yeast cell number (log N) shows more stable trend during the bioprocess which indicates that it could be used as an indicator of yeast growth. It has to be pointed out that HFLAB was also detected during microbiological analysis (up to 105 CFU mL−1), which reduces reliability and accuracy of this determination. Bioethanol production was also studied at interval rotation of 15 min h−1 (15 min−1). In these conditions, increased concentrations of glycerol (13.85 g L−1) and acetic acid (6.74 g L−1) were observed as a consequence of intensive broth mixing. In enlarged oxygen supply and substrate concentration conditions, higher glycerol and acetate synthesis rates were present and therefore higher concentrations of both products were detected [38,39,40,41, 47,48,49]. On the basis of results present in Table 2, it can be concluded that the optimal conditions for bioethanol production on the raw sugar beet cossettes in the HRTB are interval rotation of 12 min h−1 and rotation speed of 15 min−1. This statement is based on the fact that the highest substrate conversion into ethanol (\(Y_{{P_{1} /S}}\) = 0.47 g g−1) and bioprocess efficiency (E = 87.36%) were obtained with relatively low acetate (2.11 g L−1) and glycerol (8.21 g L−1) concentration. Because of the fact that the economic feasibility of bioethanol production is based on the carbon source accessibility and price [19, 22] these operational parameters of HRTB were selected as optimal for bioethanol production. Furthermore, other two bioprocess efficiency parameters (\(Y_{{P_{1} }}\) and Pr) were also at acceptable levels for successful conduction of bioethanol production. Obtained bioprocess efficiency parameters are also in the range with bioethanol production from the raw sugar beet juice [24].
Changes of substrate (S C, filled circle), ethanol (P 1, filled triangle), glycerol (P 2, open triangle), acetic acid (P 3, open square) concentrations in the solid part of the broth and cossettes dry mass (DM, open circle) during bioethanol production in the HRTB by interval rotation of 12 min h−1 and rotation speed of 15 min−1 (V W/V T = 0.2)
Bioethanol production in the HRTB by different V W/V T ratio
In the last part of this research, the impact of ratio between working (V W) and total (V T) volume of HRTB on the bioethanol production was studied. However, it should be pointed out that oscillations in analytical results were observed due to the non-homogenous substrate in the HRTB. Furthermore, it was expected that the reduction of the HRTB free space would be related to reduced oxygen supply possibility for natural contaminants of raw sugar beet cossettes what could additionally support bioethanol production by yeast. In this investigation, bioethanol production was performed in the HRTB by using 5–17.5 kg of raw sugar beet cossettes (0.2–0.7 V W/V T) by interval rotation of 12 min h−1 and rotation speed of 15 min−1. In all experiments yeast inoculation rate was 16.67% v/w of raw sugar beet cossettes. On the basis of obtained results it can be generally pointed out that increase of HRTB working volume was related to the reduction of bioethanol production efficiency (Table 3).
In experiment with 0.4 V W/V T slightly lower bioethanol production efficiency was observed compared to the experiment with 0.2 V W/V T. In this experiment, bioprocess efficiency parameters have following values: \(Y_{{P_{1} }}\) = 48.97 g L−1, \(Y_{{P_{1} /S}}\) = 0.45 g g−1, E = 83.64% and Pr = 0.560 g L−1 h−1. In experiment with 0.5 V W/V T raw sugar beet cossettes contained increased concentration of HFLAB and therefore acetic acid concentration raised up very quickly (first 22 h) at 4.43 g L−1 and consequently pH value was lowered at 3.78. In these conditions, yeast activity was considerably slowed down and therefore bioethanol production efficiency was reduced (Table 3). This observation is the most obvious by the prolongation of bioprocess time that affects bioprocess productivity (Pr = 0.312 g L−1 h−1). In this experiment relatively high glycerol concentration (10.62 g L−1) was also observed as a consequence of nonadequate bioethanol production conditions [47,48,49]. In experiment with 0.6 V W/V T lower acetic acid concentration was detected (3.31 g L−1) and therefore bioethanol production efficiency parameters were increased: \(Y_{{P_{1} }}\) = 48.8 g L−1, \(Y_{{P_{1} /S}}\) = 0.37 g g−1, E = 68.77% and Pr = 0.548 g L−1 h−1. This observation shows that HFLAB were present at lower concentration compared to the previous experiment, but these conditions were also not favorable for bioethanol production. In experiment with 0.7 V W/V T acetic acid concentration was not considerably increased during bioprocess although its final concentration was 3.65 g L−1. Therefore, bioprocess efficiency parameters have following values: \(Y_{{P_{1} }}\) = 53.85 g L−1, \(Y_{{P_{1} /S}}\) = 0.37 g g−1, E = 68.77% and Pr = 0.552 g L−1 h−1. On the basis of results presented in Table 3 it is obvious that studied combinations of HRTB operational parameters could create conditions for efficient bioethanol production at V W/V T ratio up to 0.4. However, it is also important to point out that further optimization of HRTB operational parameters have to be performed in order to increase the efficiency of bioethanol production at V W/V T ratio higher than 0.5.
Conclusions
On the basis of these results it is clear that that bioreactor operational conditions (rotation speed, type of rotation and V W/V T ratio) have significant impact on the efficiency of bioethanol production. Furthermore, fine tuning of HRTB operational conditions has to be performed at V W/V T ratio higher than 0.5 in order to obtain higher bioethanol production efficiency. Naturally present microorganisms (mostly HFLAB) of sugar beet could have significant impact on the bioethanol production in the HRTB. Higher yeast inoculation rate could reduce the HFLAB activity and consequently the bioethanol production efficiency will be improved.
References
EC of European Parliament and of the Council (2009) Directive 2009/28/of 23 April 2009 on the promotion of the use of energy from renewable sources. Official Journal of the European Union L 140/64, 5.6
Demirbas A (2011) Production of fuels from crops. In: Speight JG (ed) The biofuels handbook. RSC Publishing, Cambridge, pp 201–227
Ramroop Singh N (2011) Biofuels. In: Speight JG (ed) The biofuels handbook. RSC Publishing, Cambridge, pp 160–198
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J. doi:10.1155/2014/298153
Câmara MM, Soares RM, Feital T, Naomi P, Oki S, Thevelein JM, Amaral M, Pinto JC (2017) On-line identification of fermentation processes for ethanol production. Bioprocess Biosyst Eng. doi:10.1007/s00449-017-1762-61
Murthy GS, Johnston DB, Rausch KD, Tumbleson ME, Singh V (2011) Starch hydrolysis modeling: application to fuel ethanol production. Bioprocess Biosyst Eng 34:879–890
Cárdona CA, Sanchez ÓJ (2007) Fuel ethanol production: process design trends and integration opportunities. Bioresour Technol 98:2415–2457
Brethauer S, Wyman CE (2010) Review: continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour Technol 101:4862–4874
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597
Bhatia L, Johri S, Ahmad R (2012) An economic and ecological perspective of ethanol production from renewable agro waste: a review. AMB Express 2:65
Goshima T, Tsuji M, Inoue H, Yano S, Hoshino T, Matsushika A (2013) Bioethanol production from lignocellulosic biomass by a novel Kluyveromyces marxianus strain. Biosci Biotechnol Biochem 77:1505–1510
Srivastava N, Rawat R, Oberoi HS, Ramteke PW (2015) A review on fuel ethanol production from lignocellulosic biomass. Int J Green Energy 12:949–960
Kossatz HL, Rose SH, Viljoen-Bloom M, van Zyl WH (2017) Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process Biochem 53:10–16
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem 51:1833–1842
Olofsson K, Bertilsson M, Lidén G (2008) A short review on SSF—an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1:7
Baeyens J, Kang Q, Appels L, Dewil R, Lv Y, Tan T (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88
Cardona CA, Quintero JA, Paz IC (2010) Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol 101:4754–4766
Quintero JA, Montoya MI, Sanchez OJ, Giraldo OH, Cardona CA (2008) Fuel ethanol production from sugar cane and corn: comparative analysis for Colombian case. Process Biochem 34:115–119
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Haq F, Ali H, Shuaib M, Badshah M, Hassan SW, Munis MFH, Chaudhary HJ (2016) Recent progress in bioethanol production from lignocellulosic materials: a review. Int J Green Energy 13:1413–1441
Yun M-S, Park J, Arakene M, Shiroma R, Ike M, Tamiya S, Takahashi H, Tokuyasu K (2011) An improved CARV process for bioethanol production from a mixture of sugar beet mash and potato mash. Biosci Biotechnol Biochem 75:62–64
Gupta A, Varma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567
van Thuijl E, Deurwaarder EP (2006) European biofuels policies in retrospect. Energy Research Centre of the Netherlands (ECN), Petten
Dodić S, Popov S, Dodić J, Ranković J, Zavargo Z, Jevtić Mučibabić R (2009) Bioethanol production from thick juice as intermediate of sugar beet processing. Biomass Bioenergy 33:822–827
Razmovski R, Vučurović V (2011) Ethanol production from sugar beet molasses by S. cerevisiae entrapped in an alginate-maize stem ground tissue matrix. Enzyme Microb Technol 48:378–385
Šantek B, Gwehenberger G, Ivančić Šantek M, Narodoslawsky M, Horvat P (2010) Evaluation of energy demand and the sustainability of different bioethanol production processes from sugar beet. Resour Conserv Recycl 54:872–877
Bankar Sandip B, Survasea Shrikan A, Singhal Rekha S, Granströma T (2012) Continuous two stage acetone-butanol-ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B 5313. Bioresour Technol 106:110–116
Ibrahim Nasser A, Phuong Lan Tran N, Lien Huong H, Suryadi I, Yi-Hsu J (2013) Bioethanol production from pretreated Melaleuca leucadendron shedding bark—simultaneous saccharification and fermentation at high solid loading. Bioresour Technol 136:213–221
Chi-Yang Y, Bo-Hong J, Kow-Jen D (2013) Production of bioethanol from carrot pomace using the thermotolerant yeast Kluyveromyces marxianus. Energies 6:1794–1801
Pinar K, Indre VR, Melek Ö, Irini A (2012) Alkaline peroxide pretreatment of rapeseed straw for enhancing bioethanol production by same vessel saccharification and co-fermentation. Bioresour Technol 104:349–357
Ichiro K, Yoshiyuki H, Sadatoshi M (2012) Integrated delignification and simultaneous saccharification and fermentation of hard wood by a white-rot fungus, Phlebia sp. MG-60. Bioresour Technol 126:137–141
Parameswaran B, Raveendran S, Reeta Rani S, Surender V, Lalitha D, Satya N, Noble K, Rajeev KS, Ashok P (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101:4767–4774
Zheng Y, Yu C, Shen Cheng Y, Lee C, Simmons WC, Dooley MT, Zhang R, Jenkins BM, van der Gheynst JS (2012) Integrating sugar beet pulp storage, hydrolysis and fermentation for fuel ethanol production. Appl Energy 93:168–175
Rodrıguez LA, Toro ME, Vazquez F, Correa-Daneri ML, Gouiric SC, Vallejo MD (2010) Bioethanol production from grape and sugar beet pomaces by solid-state fermentation. Int J Hydrog Energy 35:5914–5917
Šantek B, Ivančić M, Horvat P, Novak S, Marić V (2006) Horizontal tubular bioreactors in biotechnology. Chem Biochem Eng Q 20:389–399
Novak M, Trontel A, Slavica A, Horvat P, Šantek B (2017) Computational fluid dynamic (CFD) modeling of simultaneous extraction and fermentation process in a single sugar beet cossette. EuroBiotech J 1:19–27
Campbell LG, Storage Klotz KC (2006) In: Draycott AP (ed) Sugar beet. Blackwell Publishing, Oxford, pp 387–408
Richter H, Vlad D, Unden G (2001) Significance of pantothenate for glucose fermentation by Oenococcus oeni and for suppression of the erythritol and acetate production. Arch Microbiol 175:26–31
Richter H, Hamann I, Unden G (2003) Use of the mannitol pathway in fructose fermentation of Oenococcus oeni due to limiting redox regeneration capacity of the ethanol pathway. Arch Microbiol 179:227–233
Richter H, De Graaf AA, Hamann I, Unden G (2003) Significance of phosphoglucose isomerase for the shift between heterolactic and mannitol fermentation of fructose by Oenococcus oeni. Arch Microbiol 180:465–470
Wagner N, Hon Tran Q, Richter H, Selzer PM, Unden G (2005) Pyruvate fermentation by Oenococcus oeni and Leuconostoc mesenteroides and role of pyruvate dehydrogenase in anaerobic fermentation. Appl Environ Microbiol 71:4966–4971
Dejean L, Beauvoit B, Guerin B, Rigoulet M (2000) Growth of the yeast Saccharomyces cerevisiae on a non-fermentable substrate: control of energetic yield by the amount of mitochondria. Biochim Biophys Acta 1457:45–56
Terebiznik MR, Pilosof AMR (1999) Biomass estimation in solid state fermentation by modeling dry matter weight loss. Biotechnol Tech 13:215–219
Bhargav S, Panda BP, Ali M, Javed S (2008) Solid-state fermentation: an overview. Chem Biochem Eng Q 22:49–70
Steudler S, Böhmer U, Weber J, Bley T (2014) Biomass measurement by flow cytometry during solid-state fermentation of basidiomycetes. Cytom Part A. doi:10.1002/cyto.a.22592 (technical note)
Shell DJ, Dowe N, Ibsen KN, Riley CJ, Ruth MF, Lumpkin RE (2007) Contaminant occurrence, identification and control in a pilot-scale corn fiber to ethanol conversion process. Bioresour Technol 98:2942
van Dijken JP, Scheffers WA (1986) Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev 32:199–224
Weusthuis RA, Visser W, Pronk JT, Scheffers WA, van Dijken JP (1994) Effects of oxygen limitation on sugar metabolism in yeasts: a continuous-culture study of the Kluyver effect. Microbiology 140:703–715
Weinhandl K, Winkler M, Glieder A, Camattari A (2014) Carbon source dependent promoters in yeasts. Microb Cell Fact 13:5
Acknowledgements
The research was financially supported by Croatian Science Foundation under the project “Sustainable production of bioethanol and biochemicals from agricultural waste lignocelullosic raw materials (No. 9158)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pavlečić, M., Rezić, T., Šantek, M.I. et al. Bioethanol production from raw sugar beet cossettes in horizontal rotating tubular bioreactor. Bioprocess Biosyst Eng 40, 1679–1688 (2017). https://doi.org/10.1007/s00449-017-1823-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1823-x