Abstract
High cell density is an important factor in achieving high bioreactor productivity. To meet the oxygen demand with density at >100 × 106 cells/mL, a frit sparger is often used. In this study, the impact of Pluronic® F68 on a perfusion process using a frit sparger was studied. The perfusion process was developed using an alternating tangential flow device with a 0.2 µm PES hollow fiber filter. Pluronic® F68 at 2 g/L was sufficient in preventing cell damage at gas flow rate of ~0.20 vvm from a drilled hole sparger (0.5 mm) but inadequate at ~0.025 vvm from a frit sparger (20 µm). Increase of Pluronic® F68 concentration to 5 g/L prevented cell death at up to ~0.10 vvm from the frit sparger and was able to maintain high cell density at high viability in the range of 60–80 × 106 cells/mL. Such positive effect was demonstrated in both 3- and 200-L bioreactors. Supplementing additional Pluronic® F68 was also effective in restoring cell growth/viability from low viability cultures. Increased Pluronic® F68 concentration had no adverse impact on target antibody, HCP, and Pluronic® F68 transmissions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-density perfusion cultures using filtration-based cell retention devices have attracted a great deal of interest for continuous biomanufacturing [1,2,3]. Perfusion enables continuous fresh media replenishment and waste product removal, which supports high cell densities with high viability. One of the challenges in maintaining high density at 50 to >100 × 106 cells/mL is the high oxygen demand. A frit sparger is frequently used to ensure sufficient oxygen supply due to its superior oxygen k L a (volumetric mass transfer coefficient) [4, 5]. However, using a frit sparger is often associated with more cell damage from hydrodynamic stress, especially bubble-bursting-associated cell death due to the smaller bubbles generated [6,7,8]. Cell culture medium additives have been widely used to protect animal cells from agitation- and aeration-associated cell damage [8,9,10,11]. Among a pool of options, Pluronic® F68 is the most widely used (here referred to as PF68). PF68 is a poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) triblock copolymer with an average molecular weight of 8400 Da. It is commonly used as a nonionic surfactant, which is also known under other brand names such as Lutrol® F68 or Kolliphor® P188. With the increase in cell density and high gas flow rates through the frit sparger, the typical PF68 concentration at 1–2 g/L used in cell culture media may become insufficient [12]. For example, we have shown that gas flow rate at as low as 0.016 vvm could cause cell damage when a 20 µm frit sparger was used in a fed-batch culture [13]. Recently, PF68 concentration at up to 5 g/L has been investigated in high-density fed-batch cultures, and no impact on cell growth and product quality was found for such concentration, though the sparger type was not specified [14]. Increasing PF68 concentration in the cell culture media has also been discussed as a mitigation approach for the observed PF68 lot-to-lot variation [15].
Increasing PF68 concentration could negatively impact filtration performance [16, 17]. In perfusion cultures using hollow fiber filters as the cell retention device, the adsorption of media components, proteins, antifoam, and cell debris on the filter surface could cause filter fouling over time. As a result, product (e.g., proteins, antibodies) transmission across the filter decreases [18, 19]. This leads to product accumulation in the bioreactor and compromises process yield since the product is collected from the permeate side of the filter. The extended product retention in the bioreactor due to fouling could also potentially impact product quality via proteolytic degradation. Replacement of filters in the process is often required due to filter fouling [20]. Though the use of high PF68 concentration has been recently reported for hollow fiber-based perfusion cultures [21], little understanding is available regarding the impact of the increased PF68 concentration on filter performance and product transmission.
In this study, we evaluate a range of PF68 concentrations (2–5 g/L) on hollow fiber filter-based perfusion culture performance at both 3- and 200-L scales. The impact of PF68 concentration on target monoclonal antibody (MAb) product and impurities (HCPs, host cell proteins) transmission across a 0.2 µm polyether sulfone (PES) filter is investigated. PF68 transmission across the filter is also discussed.
Materials and methods
Cell line and inoculum expansion
A recombinant Chinese hamster ovary (CHO) cell line was used. It was a glutamine synthetase (GS) cell line designed to produce a monoclonal antibody. The inoculum train started from vial thaw and expanded in shake flasks. Shake flasks were maintained in an incubation shaker with a 25 mm throw (Multitron, Infors AG, Bottmingen, Switzerland) operated at 5% CO2, 36.5 °C, and 100 rpm shaking speed.
Perfusion bioreactors
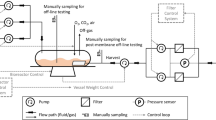
Both 3-L glass bioreactors (Sartorius Stedim, Göttingen, Germany) and 200-L single-use bioreactors (SUB) (XDR200, GE Healthcare, Marlborough, MA, USA) were used in the study. Their engineering information has been reported previously [22]. For both 3- and 200-L bioreactors, temperature was controlled at 36.5 °C; dissolved oxygen (DO) was controlled at 30% of air saturation using pure O2, and pH was controlled at >6.75. Bioreactors were inoculated at a target cell density of 1 × 106 cells/mL. For 3-L bioreactors, a marine impeller and a drilled hole sparger (DHS; 14 × 0.5 mm holes) or a frit sparger (20 µm pore size) were used with a working volume of 1.3 L. Agitation speed was controlled at 260 rpm (note no increase of agitation speed as previously reported was required [23]). For 200-L SUB, a single pitched-blade impeller and a sparger disk with different hole/pore sizes (10 × 1 mm holes and 20 µm pores) were used with a working volume of 100 L. Constant air (0.05–0.06 vvm) was supplied through the 1 mm holes for CO2 removal, and O2 was supplied through the 20 µm pores as needed for DO control. Agitation speed was controlled in the range of 90–140 rpm. EX-CELL® antifoam (1% Simethicone Emulsion, Sigma-Aldrich, St. Louis, MO, USA) was added continuously using a syringe pump (KDS-200, KD Scientific, Holliston, MA, USA) at 0.2–0.3 mL/h for foam control for all the 3-L bioreactor studies from day 6. Antifoam was added as needed for the 200-L SUBs.
An alternating tangential flow-2 (ATF-2) or alternating tangential flow-6 (ATF-6) filtration system (Repligen Corporation, Waltham, MA, USA) was used for the 3- or 200-L bioreactors, respectively. A 0.2 µm PES hollow fiber filter was used, with different surface areas at different scales (ATF-2 0.13 m3; ATF-6 2.5 m3). ATF-2 flow rate used was 0.8 LPM, and ATF-6 flow rate used was 15 LPM. Proprietary media were used for both batch growth and perfusion. Medium exchange was typically started on day 2 and maximum exchange rate of 1 vvd (medium exchange volume per bioreactor volume per day) was used. Medium was continuously supplied to the bioreactor and liquid stream containing antibody was continuously collected from the permeate side of the filter. Cell bleed was started on day 8 at 0.06 vvd.
Cell culture sample analysis
Samples were taken daily for offline analysis. Bioreactor supernatant samples were collected from the supernatant layer after centrifuging the cell culture samples at 2000 g for 10 min, and permeate samples were collected directly after the 0.2 µm PES filter. Viable cell density (VCD) and viability were measured using the trypan blue exclusion method on a Cedex Hi-Res cell counter (Roche Diagnostics GmbH, Mannheim, Germany). Offline pH, pO2, and pCO2 were measured using an ABL80 blood gas analyzer (Radiometer, Denmark). Glucose, lactate, glutamine, and glutamate were measured using a YSI Model 2700 analyzer (YSI Life Sciences, Yellow Springs, OH, USA). Lactate dehydrogenase (LDH) was measured using a RX Imola analyzer (Randox Laboratories, Ltd., Crumlin, UK). Bioreactor supernatant and permeate antibody concentrations were analyzed using an Agilent 1100 high-performance liquid chromatography (HPLC, Agilent Technologies, Santa Clara, CA, USA) with a reversed phase column [24].
Host cell protein measurement
Host cell proteins from both bioreactor supernatant and permeate samples were determined using an enzyme-linked immunosorbent assay (ELISA). Test samples were serially diluted in microtiter well plates for HCP quantification, with HCP standards provided from the 3rd generation Cygnus kit (Cygnus Technologies, Inc., Southport, NC, USA). Briefly, 100 µL of anti-CHO-HRP (Cygnus #F551) and 50 µL of HCP standards, samples, and controls were added to the anti-HCP antibody pre-coated assay plate and incubated at room temperature for 2 h. After incubation, the plate was washed four times with washing buffer before adding 100 µL TMB (3,3′,5,5′-Tetramethylbenzidine) substrate. The colorimetric reaction was stopped after 30 min with the addition of stop solution provided in the kit and then the plate was read at 450/650 nm using a SpectroMax® M5e microplate reader (Molecular Devices, LLC., Sunnyvale, CA, USA) and results were analyzed by using non-linear four-parameter regression analysis.
PF68 measurement
Both Pluronic® F68 (10% solution, Part no: 24040-032, Life Technologies, Grand Island, NY, USA) and Kolliphor® P188 (Part no: K4894, Sigma-Aldrich, St. Louis, MO, USA) were used to develop the PF68 assay with comparable results. The P188 powder was dissolved in purified water to prepare the 10% (100 g/L) solution. The stock solution was 0.2 µm filtered (KLEENPAK™ Capsules with Fluorodyne® II DFL membrane, Pall Corporation, Westborough, MA, USA) prior to usage.
For PF68 concentration determination, a high-throughput method was adapted from the colorimetric method described by Ghebeh et al. [25]. Briefly, 50 µL of PF68 standards at a concentration range from 0.2 to 4 g/L or samples were mixed with 25 µL of cobalt thiocyanate reagent, 50 µL of ethyl acetate, and 20 µL of absolute ethanol in the wells of 96-well protein precipitation plate to form blue precipitates. The liquids were then drained by applying vacuum using a Vacuum Manifold Filtration System for SPE Sample Filtration (Analytical Sales & Services, Inc., Pompton Plains, NJ, USA). The precipitates were washed three times with ethyl acetate before eluting into a collection plate with acetone. The absorbance from each was then read at 328 nm using a SpectraMax M2 plate reader (Molecular Devices, LLC., Sunnyvale, CA, USA). PF68 concentrations in the samples were calculated based on a linear curve fitting of absorbance at 328 nm versus the known standard concentration.
Determination of daily transmission and total retention
Daily MAb, HCP, and PF68 transmission was calculated using the following equation:
The level of adsorption of analytes such as PF68 on the filter is unknown in the study, and the transmission calculation did not take the potential adsorption on the filter into consideration.
The total MAb retention was calculated using the following equation:
Results and discussion
Cell culture performance with different spargers
The same perfusion process was evaluated in the 3-L bioreactors with a drilled hole sparger or a frit sparger (20 µm) using media containing 2 g/L PF68 (Fig. 1). Significant distinctions between the two spargers were observed. When a DHS was used, oxygen flow at ~0.20 vvm (gas volume flow per unit of liquid volume per minute) was required to support cell density at 60–70 × 106 cells/mL due to the low oxygen k L a through the DHS (k L a data in Ref. [22]). Cell viability was still maintained at >95% on day 15 even at such high vvm (Fig. 1c). On the other hand, cell viability declined from day 7 in the frit sparger condition, when oxygen demand was increased and pulsed oxygen supply at maximum of ~0.05 vvm was needed. Additionally, peak cell density reached was only ~65% of that in the DHS condition. Since sparger is the only difference between the two conditions, it was hypothesized that such cell culture performance difference was caused by cell damage from using the frit sparger. Much smaller bubbles are generated from the 20 µm sparger compared with those generated by a DHS. Bubble bursting at the air–liquid interface has been demonstrated to be the primary cause of cell damage in sparged bioreactors, where cell death increases with the decrease in bubble radii due to the greatly increased energy dissipation rate with smaller bubbles [6,7,8]. Considerable amount of literature is available for the effectiveness and necessity of PF68 in preventing cell damage in sparged bioreactors [7, 8, 10, 26,27,28,29,30,31,32,33,34,35]. In our study, the level of oxygen flow through the frit sparger was similar to the lethal level reported in high-density CHO and BHK (Baby Hamster Kidney) perfusion cultures at 20 × 106 cells/mL, where sparge rates above 0.054 vvm using a 15 µm sparger, or above 0.025 vvm using a 0.5 µm sparger were found to be detrimental to cells, although the exact PF68 concentration used was not specified [36].
To avoid cell damage, large bubbles from a DHS are preferred. However, we anticipate that the oxygen k L a of the DHS could be insufficient to support high cell density in large-scale bioreactors. Additionally, high gas flow rates may cause undesired bag pressure increase for SUBs. To manage the total gas throughput while maintaining desired oxygen k L a, a frit sparger is to be used. Based on the frit sparger oxygen k L a characteristics [22] and cell specific oxygen uptake rates (3–5 pmol/cell/day) in our perfusion processes, it is estimated that ~0.05 vvm O2 could be required to support cell density at 100 × 106 cells/mL in a 200-L SUB with power input per volume (P/V) at ~50 W/m3. Further increase of impeller speed could improve oxygen k L a and reduce the O2 vvm required. However, it will be at the expense of increased local energy dissipation rate, which could also cause cell damage.
Increasing PF68 concentration may be required to maintain cell viability when scaling up perfusion cultures using a frit sparger for O2 supply. Although it has been widely reported that 1–2 g/L PF68 in general provides sufficient protection from shear damage, such concentration is inadequate with small bubbles at high gas flow rates. Earlier study showed that 5 g/L PF68 was required in an airlift bioreactor with only ~0.003 vvm sparge rate for Spodoptera frugiperda (SF-9) insect cell cultures for cell density at <4 × 106 cells/mL when a 20 µm frit sparger was used, while 2 g/L PF68 provided little protection [37]. Similarly, the protective effect of PF68 was found to increase with its concentration in the range of 0–3 g/L for SF-9 cells at late exponential growth phase under laminar shear stress [10]. Increasing PF68 concentration was found to be necessary to reduce cell attachment to bubbles with the increase in cell concentration [33]. Though no direct cell culture data were shown, it provided critical information on the expected PF68 concentration requirement with the increase in cell density. Additionally, the uptake of PF68 by the cells may also play a bigger role in high-density cultures. Average uptake of PF68 for CHO cells was measured to be 11.7 ± 6.7 µg/106 cells [38], which translates to 0.25–0.92 g/L PF68 uptake at a cell density of 50 × 106 cells/mL. Such level of PF68 uptake can severely affect its concentration in the culture if only 1–2 g/L PF68 was used. For the CHO cell line studied here, PF68 concentration at 2 g/L appears to be insufficient in preventing cell damage at low sparge rates via the 20 µm frit sparger.
Impact of PF68 concentration on perfusion culture and MAb transmission
Cell growth and viability at different PF68 concentrations (2, 3, 5, and 10 g/L) were first evaluated in shake flasks. No negative impact on cell doubling time and viability was observed for PF68 concentration at up to 5 g/L (Supplementary Materials Figure S1). This is similar to previous reports where no adverse impact was noted from PF68 concentration at 5 g/L or higher for industrial CHO cell lines [14, 15]. A control with 0 g/L F68 was not pursued since a large number of prior studies already showed that most cells do not grow well in sparged bioreactors without PF68 or other similar shear protectants [26,27,28,29, 37, 39], and the results in the previous section showed that cell growth and viability was suboptimal at 2 g/L PF68.
To understand the level of PF68 required to prevent cell damage when a 20 µm frit sparger was used at the target density of 60–80 × 106 cells/mL, the same perfusion medium with PF68 at 2, 3, 5 g/L was used (Fig. 2). Cell growth profiles were similar for all conditions up to day 6 with cell density reached 25.4–30.6 × 106 cells/mL. Rapid increase in pCO2 to ~100 mmHg was observed, largely due to the poor CO2 stripping with a frit sparger. A constant air flow at 0.025 vvm was started on day 6 and maintained until end of the culture to reduce CO2 accumulation and test the impact of continuous gas sparge rate on cell damage (Fig. 3). The continuous air flow caused immediate divergence in cell culture performance. In the control condition with 2 g/L PF68, cell growth was inhibited and cell viability declined rapidly (18.5% drop in 24 h). Similar decline in cell viability was observed in the 3 g/L PF68 condition (15.8% drop). Correlating to the viability decline, significant LDH increase was also observed in both conditions from day 6 to day 7. The 5 g/L PF68 condition prevented cell damage considerably, with only 3% viability decline and modest LDH increase. Due to the continuous air sparging, the pCO2 level decreased to ~45 mmHg for all conditions. Cell viability kept declining in the 2 g/L PF68 condition to 45.7%, with VCD slowly increased to 43.7 × 106 cells/mL on day 14. In the same duration, cell viability declined to 66.7% with a VCD of 61.4 × 106 cells/mL in the 3 g/L PF68 condition. In contrast, viability was maintained at 97.1% with a VCD of 74.3 × 106 cells/mL in the 5 g/L condition. It is clear that PF68 concentration at 2 g/L was not sufficient in preventing cell damage at as low as 0.025 vvm continuous gas flow from a frit sparger, and 3 g/L PF68 only marginally improved that. The additional oxygen sparging required for maintaining DO posed additional stress on the system. On the other hand, PF68 concentration at 5 g/L was able to prevent cell damage at the maximum total gas flow of ~0.10 vvm (Fig. 3). Increasing PF68 concentration was found to be necessary to reduce cell damage, and the protection effectiveness was concentration dependent in the range of 2–5 g/L [10, 33, 37].
Culture performance with different PF68 concentrations using a frit sparger in the 3-L bioreactors. a VCD, b viability, c LDH–bioreactor, d pCO2, e MAb titer–bioreactor, f MAb titer–permeate, g MAb transmission, h total MAb retention. Perfusion started from day 2 and maintained at 1 vvd throughout the culture duration from day 4. To avoid cell bleed variation, the same bleed rate at 0.06 vvd was applied for all the conditions starting from day 8. For the 2 g/L PF68 condition, additional 2 g/L PF68 was supplemented to the perfusion media on day 14; thus, day 14–29 media contain 4 g/L PF68
Gas flow rate profiles in the 3-L bioreactors. Continuous air flow at 0.025 vvm was started on day 6 and maintained until day 29. O2 was supplied as needed for DO control. Both air and O2 were supplied through the frit sparger. The oxygen flow rates corresponded to the cell growth profiles shown in Fig. 2. For the 2 g/L PF68 condition, supplementing additional 2 g/L PF68 from day 14 led to immediate increase in O2 flow
To further confirm the protection effectiveness of PF68, additional 2 g/L PF68 was supplemented to the 2 g/L PF68 condition from day 14, making the final PF68 concentration at 4 g/L. Cell viability started recovering within the next day, together with the increase in cell growth/O2 demand and fast decrease in LDH (Figs. 2, 3). Such immediate protection from PF68 addition and restoration of cell growth is in agreement with previous reports [39, 40]. Cell viability recovered to an average of 83.3% with peak cell density reached >80 × 106 cells/mL. The recovery of cell growth/viability with the additional PF68 supplementation supports the hypothesis that insufficient PF68 led to the viability decline shown in Fig. 1. The effect of PF68 on restoring cell viability was further evaluated in other perfusion processes using the same frit sparger, and the same viability recovery was achieved (Supplementary Materials Figure S2), which shows that such viability restoration is highly reproducible. One notes that the negative impact of low viability on MAb transmission across the 0.2 µm PES filter was not reversed, even though cell culture performance was recovered through PF68 supplementation (Fig. 2e–h). Daily MAb transmission declined rapidly in the 2 g/L PF68 condition, and kept declining while cell growth and viability recovered after day 14 with the additional 2 g/L PF68 supplementation. Such decline is understandable since cell debris and particles in the bioreactor are difficult to remove if their sizes are >0.2 µm. Additionally, adsorption of host cell residuals (HCP, DNA) and antifoam to membrane could also contribute to filter fouling and reduction in transmission efficiency. As a result, the compromised filter performance was difficult to reverse, which was different than the cell culture performance.
Though 5 g/L PF68 was found to be beneficial in maintaining high cell viability when a 20 µm frit sparger was used, it is desired to understand whether this increase had any real impact on product transmission. The poor antibody transmission in the 2–3 g/L PF68 conditions appears to largely relate to early viability decline. A good baseline for comparison is to have similar cell density and viability profiles maintained. To create such baseline, the same process was performed with a DHS using perfusion media containing either 2 or 5 g/L PF68. As shown in Fig. 1, PF68 concentration at 2 g/L was sufficient in preventing cell damage at high gas flow rates through the DHS, and similar cell density and viability could be achieved in both 2 and 5 g/L PF68 conditions. For all the conditions tested, MAb transmission within the same culture duration has no statistical difference (one-way analysis of variance (ANOVA), P = 0.8693) (Table 1). This confirms that there was no negative impact of increased PF68 concentration at up to 5 g/L on filter performance, regardless of the sparger used.
HCP and PF68 transmissions
Though the molecular weight of HCPs varies, they are still able to easily pass through a 0.2 µm filter. Similar to the MAb retention profiles, higher HCP retention was observed for the conditions with low viabilities (Fig. 4). Overall HCP transmission was the highest in the 5 g/L PF68 condition. Different than MAb transmission, there was an initial HCP transmission increase, correlating to the advent of significant cell damage caused by continuous air sparging from day 6 (especially in the 2 and 3 g/L PF68 conditions). It was speculated that HCPs were rapidly generated, thus creating high HCP levels in the bioreactor and a low apparent transmission value since it would take 3 days for 95% of the bioreactor volume to be replaced at 1 vvd medium exchange rate. With the HCPs being exchanged out over time, the transmission values gradually increased. On the other hand, there was no sudden impact of sparging on antibody production, and MAb transmission followed a typical filter fouling characteristic with gradual transmission decline. It is to note that the HCP transmission trends observed here were due to the specific experiments performed in which large amount of HCPs were generated from cell damage. Overall, the HCP transmission profiles confirmed that increasing PF68 concentration actually improved protein transmission due to better-sustained cell viability. This implies that cell viability could be the critical parameter impacting protein transmission across the PES filter, especially with the accumulated loading on the filter over time (e.g., >200 L/m2 after 30 days in perfusion).
PF68 was also transmitted through the 0.2 µm filter freely at the concentration of 5 g/L (Fig. 5). The molecular weight of PF68 is 8400 Da, which theoretically should be able to pass through the 0.2 µm filter unless it is adsorbed to the filter surface. In contrast, PF68 transmission was lower when its concentration was maintained at 2 g/L and viability was low. The membrane fouling due to low cell viability may have also caused the lower PF68 transmission in the 2 g/L condition.
Pilot scale comparison
The effectiveness of different PF68 concentrations (2 vs. 5 g/L) in perfusion cultures was also evaluated at pilot scale (200 L SUB) with an ATF-6 (Fig. 6). The cell culture performance was similar to those in Figs. 1 and 2, where viability declined quickly with the 2 g/L PF68 condition when O2 flow increased to ~0.02 vvm and high viability was maintained in the 5 g/L PF68 condition at a similar vvm. For the 2 g/L PF68 condition, one noticeable difference is that MAb transmission through the hollow fiber filter declined rapidly in the 200-L SUB, at a rate much faster than that in the 3-L bioreactors. The membrane flux was 0.42 LMH (L/m2/h) for ATF-2, and was 1.67 LMH for ATF-6. This difference might potentially cause filter performance difference as the total filter capacity used was four times higher in ATF-6 within the same duration. In a mock study, HCCFs (harvested cell culture fluids) containing 5 g/L PF68 were used to challenge the PES filter at different fluxes in ATF-2. The HCCFs used were from previous perfusion experiments, thus containing comparable levels of MAb and HCPs as shown in Figs. 2 and 4, though the antifoam level in the HCCFs was unknown. Two different media exchange rates, 1 and 4 vvd, were used to mimic the flux of 0.42 and 1.67 LMH, respectively. Little to no protein retention was observed in 12 days at both exchange rates (Fig. 7). Additionally, PF68 was not retained in the bioreactor. This serves as an indirect evidence that PF68 likely has very little adsorption to the filter surface, if any. It also confirms that PF68 at up to 5 g/L should have little impact on filter performance in large-scale perfusion cultures when high viability is maintained. The faster decline of MAb transmission with the decrease in cell viability in the 200-L SUB is yet to be understood better, such as the impact of flux difference. Still, superior MAb transmission at 5 g/L PF68 concentration was achieved at 200-L scale, similar to that at 3-L scale.
Cell culture performance in the 200-L single-use bioreactors with ATF-6 using the same media containing 2 or 5 g/L PF68. a VCD, b viability, c MAb transmission, d oxygen flow rates through the 20 µm sparger and agitation speeds used. Slightly higher O2 vvm was observed in the 2 g/L PF68 condition due to the lower agitation speed used
Mock run with HCCF in the 3-L bioreactors at perfusion rate of 1 or 4 vvd with 5 g/L PF68. Transmissions of a MAb, b HCP, c PF68 were comparable between the two conditions. The HCCF (MAb titer 2.1 g/L; HCP level 1.1 mg/mL; LDH level 4250 U/L) was collected from the permeate end of a previous perfusion run and spiked with 100 g/L PF68 stock solution to reach 5 g/L final concentration
Even the higher flux tested here is still far lower than the typical flux experienced in media filtration and downstream processing. The maximum filter capacity utilized in the study was also modest, at <600 L/m2. Though no negative impact of 5 g/L PF68 on the PES hollow fiber filter was observed, the impact of such PF68 increase (compared to the typical 1–2 g/L PF68 used) on high flux filtration operations deserves further studies [16, 17].
Maintaining appropriate PF68 levels to prevent cell death
The appropriate PF68 concentration should be determined by a few factors including cell density, gas sparge rate, and bubble size. With the advancement of cell line and media, current perfusion cultures cell density routinely reaches 50–100 × 106 cells/mL, which is accompanied by increased O2 demand. Increased cell damage is expected with the increase in cell density, as cell attachment per bubble increases with the increase in cell density [33]. Additionally, the protective effectiveness of PF68 is directly linked to bubble size and gas sparge rate, when the same PF68 concentration was used (Fig. 1). Significant decrease of cell-bubble interaction with the increase in PF68 concentration has been noted for large bubbles, while such decrease was less for microbubbles [11]. Based on the results shown here, it is desired to adjust the PF68 concentration based on the target cell density and the expected gas flow rates via a particular sparger design. For example, the same PF68 concentration works well for a DHS, but not sufficient for a frit sparger as shown in this study. Assuming the number of bubbles increase proportionally with the increase in vvm and the mean bubble diameter does not change significantly, and cellular PF68 uptake is similar on a per cell basis, one could estimate the available PF68/bubble at a particular vvm (Supplementary Materials Figure S3). Maintaining similar PF68/bubble is expected to ensure sufficient protection at different vvm and cell densities. Such approach could benefit process design and media optimization when developing high-density perfusion cultures with target operational attributes.
Conclusion
Little is known about the impact of PF68 concentration on the hollow fiber filter performance in perfusion cultures, though the protective effect of PF68 on cells has been well characterized. In this study, we showed that increased PF68 concentration (4–5 g/L) was necessary to avoid cell damage when using a 20 µm frit sparger for oxygen supply in high-density perfusion cultures at both 3- and 200-L scales. PF68 concentration at 2–3 g/L was insufficient in preventing cell damage at as low as 0.025 vvm gas sparge rate through the frit sparger for the cell line studied here. Increasing PF68 concentration to 5 g/L had no adverse effect on protein (both MAb and HCPs) transmission across the 0.2 µm PES hollow fiber filter in the 3-L bioreactors. The transmissions actually were the highest with 5 g/L PF68, due to the minimized cell damage from gas sparging leading to filter fouling. Such positive impact of high PF68 concentration on MAb transmission was also shown in the 200-L bioreactors. The results here confirmed that cell viability and cell lysis played a dominating role in filter fouling and protein retention. Preventing cell damage and maintaining a highly viable culture are critical in maintaining high product transmission in hollow fiber filter-based perfusion cultures.
References
Warikoo V, Godawat R, Brower K, Jain S, Cummings D, Simons E, Johnson T, Walther J, Yu M, Wright B, McLarty J, Karey KP, Hwang C, Zhou W, Riske F, Konstantinov K (2012) Integrated continuous production of recombinant therapeutic proteins. Biotechnol Bioeng 109:3018–3029
Clincke M-F, Mölleryd C, Zhang Y, Lindskog E, Walsh K, Chotteau V (2013) Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor™. Part I. Effect of the cell density on the process. Biotechnol Prog 29:754–767
Xu S, Gavin J, Jiang R, Chen H (2017) Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnol Prog. doi:10.1002/btpr.2415
Marks DM (2003) Equipment design considerations for large scale cell culture. Cytotechnology 42:21–33
Zhang S, Handa-Corrigan A, Spier RE (1993) A comparison of oxygenation methods for high-density perfusion culture of animal cells. Biotechnol Bioeng 41:685–692
Wu J, Goosen MFA (1995) Evaluation of the killing volume of gas bubbles in sparged animal cell culture bioreactors. Enzyme Microb Technol 17:241–247
Meier SJ, Hatton TA, Wang DIC (1999) Cell death from bursting bubbles: role of cell attachment to rising bubbles in sparged reactors. Biotechnol Bioeng 62:468–478
Chisti Y (2000) Animal-cell damage in sparged bioreactors. Trends Biotechnol 18:420–432
Papoutsakis ET (1991) Media additives for protecting freely suspended animal cells against agitation and aeration damage. Trends Biotechnol 9:316–324
Goldblum S, Bae Y-K, Hink WF, Chalmers J (1990) Protective effect of methylcellulose and other polymers on insect cells subjected to laminar shear stress. Biotechnol Prog 6:383–390
Hu W, Rathman JJ, Chalmers JJ (2008) An investigation of small-molecule surfactants to potentially replace pluronic F-68 for reducing bubble-associated cell damage. Biotechnol Bioeng 101:119–127
Heath C, Kiss R (2007) Cell culture process development: advances in process engineering. Biotechnol Prog 23:46–51
Xu S, Hoshan L, Jiang R, Gupta B, Brodean E, O’Neill K, Seamans CT, Bowers J, Chen H (2017) A practical approach in bioreactor scale-up and process transfer using a combination of constant P/V and vvm as the criterion. Biotechnol Prog. doi:10.1002/btpr.2489
Tharmalingam T, Goudar CT (2015) Evaluating the impact of high Pluronic® F68 concentrations on antibody producing CHO cell lines. Biotechnol Bioeng 112:832–837
Peng H, Hall KM, Clayton B, Wiltberger K, Hu W, Hughes E, Kane J, Ney R, Ryll T (2014) Development of small scale cell culture models for screening poloxamer 188 lot-to-lot variation. Biotechnol Prog 30:1411–1418
Roush DJ (2008) Primary recovery options for MAb purification: evolution and scale-up of a flexible platform process. Cell Cult Eng XI, Queensland
Schulz C, Vogel JH, Scharfenberg K (1997) Influence of Pluronic F-68 on the ultrafiltration of cell culture supernatants. In: Carrondo MJT et al (eds) Animal cell technology. From vaccines to genetic medicine. Springer, Netherlands, pp 373–378
Wang S, Godfrey S, Ravikrishnan J, Lin H, Vogel J, Coffman J (2017) Shear contributions to cell culture performance and product recovery in ATF and TFF perfusion systems. J Biotechnol 246:52–60
Karst DJ, Serra E, Villiger TK, Soos M, Morbidelli M (2016) Characterization and comparison of ATF and TFF in stirred bioreactors for continuous mammalian cell culture processes. Biochem Eng J 110:17–26
Woodside SM, Bowen BD, Piret JM (1998) Mammalian cell retention devices for stirred perfusion bioreactors. Cytotechnology 28:163–175
Walther J (2016) Overcoming process intensification challenges to deliver a manufacturable and competitive integrated continuous biomanufacturing platform. Cell Cult Eng XV, La Quinta
Xu S, Chen H (2016) High-density mammalian cell cultures in stirred-tank bioreactor without external pH control. J Biotechnol 231:149–159
Xu S, Hoshan L, Chen H (2016) Improving lactate metabolism in an intensified CHO culture process: productivity and product quality considerations. Bioprocess Biosyst Eng 39:1689–1702
McLaughlin K, Shah D, Wu E, Schussler S, Kilgore B, Li H, Linden T (2015) Reverse phase high performance liquid chromatography (RP-HPLC) as an in-process analytics core competency for upstream and downstream process development. In: 249th ACS National Meeting, Denver, CO
Ghebeh H, Handa-Corrigan A, Butler M (1998) Development of an assay for the measurement of the surfactant Pluronic F-68 in mammalian cell culture medium. Anal Biochem 262:39–44
Trinh K, Garcia-Briones M, Chalmers JJ, Hink F (1994) Quantification of damage to suspended insect cells as a result of bubble rupture. Biotechnol Bioeng 43:37–45
Murhammer DW, Goochee CF (1988) Scaleup of insect cell cultures: protective effects of Pluronic F-68. Nat Biotech 6:1411–1418
Oh SKW, Nienow AW, Al-Rubeai M, Emery AN (1992) Further studies of the culture of mouse hybridomas in an agitated bioreactor with and without continuous sparging. J Biotechnol 22:245–270
Jöbses I, Martens D, Tramper J (1991) Lethal events during gas sparging in animal cell culture. Biotechnol Bioeng 37:484–490
Michaels JD, Nowak JE, Mallik AK, Koczo K, Wasan DT, Papoutsakis ET (1995) Interfacial properties of cell culture media with cell-protecting additives. Biotechnol Bioeng 47:420–430
Xie L, Metallo C, Warren J, Pilbrough W, Peltier J, Zhong T, Pikus L, Yancy A, Leung J, Aunins JG, Zhou W (2003) Large-scale propagation of a replication-defective adenovirus vector in stirred-tank bioreactor PER.C6™ cell culture under sparging conditions. Biotechnol Bioeng 83:45–52
Handa-Corrigan A, Emery AN, Spier RE (1989) Effect of gas-liquid interfaces on the growth of suspended mammalian cells: mechanisms of cell damage by bubbles. Enzyme Microb Technol 11:230–235
Ma N, Chalmers JJ, Aunins JG, Zhou W, Xie L (2004) Quantitative studies of cell-bubble interactions and cell damage at different Pluronic F-68 and cell concentrations. Biotechnol Prog 20:1183–1191
Ramírez OT, Mutharasan R (1990) The role of the plasma membrane fluidity on the shear sensitivity of hybridomas grown under hydrodynamic stress. Biotechnol Bioeng 36:911–920
Apostolidis PA, Tseng A, Koziol M-E, Betenbaugh MJ, Chiang B (2015) Investigation of low viability in sparged bioreactor CHO cell cultures points to variability in the Pluronic F-68 shear protecting component of cell culture media. Biochem Eng J 98:10–17
Qi H, Jovanoic G, Michaels J, Konstantinov K (2001) The art & science of micro-sparging in high-density perfusion cultures of animal cells. Animal Cell Technology: From Target to Market: Proceedings of the 17th ESACT Meeting, pp 412–415
Murhammer DW, Goochee CF (1990) Sparged animal cell bioreactors: mechanism of cell damage and Pluronic F-68 protection. Biotechnol Prog 6:391–397
Gigout A, Buschmann MD, Jolicoeur M (2008) The fate of Pluronic F-68 in chondrocytes and CHO cells. Biotechnol Bioeng 100:975–987
Michaels JD, Petersen JF, McLntire LV, Papoutsakis ET (1991) Protection mechanisms of freely suspended animal cells (CRL 8018) from fluid-mechanical injury. Viscometric and bioreactor studies using serum, pluronic F68 and polyethylene glycol. Biotechnol Bioeng 38:169–180
Palomares LA, Gonzalez M, Ramirez OT (2000) Evidence of Pluronic F-68 direct interaction with insect cells: impact on shear protection, recombinant protein, and baculovirus production. Enzyme Microb Technol 26:324–331
Acknowledgement
We would like to thank Mike Caruso, Jack Cardoso, James Jimenez, Angel Rodriguez, Debbie Lutz, Billy Alcaide, and Cornelia Amoah for media preparation and bioreactor operations, the In-Process Analytics and Purification group for titer analysis, Brandon Yu for assisting PF68 measurement, and John Troisi for HCP measurement. We would also like to thank Nihal Tugcu, David Roush, and Balrina Gupta for their careful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, S., Jiang, R., Chen, Y. et al. Impact of Pluronic® F68 on hollow fiber filter-based perfusion culture performance. Bioprocess Biosyst Eng 40, 1317–1326 (2017). https://doi.org/10.1007/s00449-017-1790-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1790-2