Abstract
This study introduces activated carbon (AC) as an effective anode for microbial fuel cells (MFCs) using real industrial wastewater without treatment or addition of external microorganism mediators. Inexpensive activated carbon is introduced as a proper electrode alternative to carbon cloth and carbon paper materials, which are considered too expensive for the large-scale application of MFCs. AC has a porous interconnected structure with a high bio-available surface area. The large surface area, in addition to the high macro porosity, facilitates the high performance by reducing electron transfer resistance. Extensive characterization, including surface morphology, material chemistry, surface area, mechanical strength and biofilm adhesion, was conducted to confirm the effectiveness of the AC material as an anode in MFCs. The electrochemical performance of AC was also compared to other anodes, i.e., Teflon-treated carbon cloth (CCT), Teflon-treated carbon paper (CPT), untreated carbon cloth (CC) and untreated carbon paper (CP). Initial tests of a single air-cathode MFC display a current density of 1792 mAm−2, which is approximately four times greater than the maximum value of the other anode materials. COD analyses and Coulombic efficiency (CE) measurements for AC-MFC show the greatest removal of organic compounds and the highest CE efficiency (60 and 71%, respectively). Overall, this study shows a new economical technique for power generation from real industrial wastewater with no treatment and using inexpensive electrode materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scarceness of energy sources along with permanent energy requirements are the main constraints facing global progress and development. In addition, wastewater problems pose a significant risk for vital communities [1, 2]. Millions of tons of wastewater are produced daily, carrying bacteria, organic substrates, and other pollutants. These large amounts of wastewater require expensive treatment procedures for appropriate and acceptable use [3]. Therefore, many efforts have aimed to develop new techniques, which can save renewable energy resources based on exploiting waste sources [4,5,6]. Microbial fuel cells (MFCs) are a promising technique for recovering chemical energy stored in organic substrates and converting it into electricity [7,8,9,10,11]. The significance of this technology lies in decreasing wastewater treatment costs and creating advanced methods for power generation in both developing and industrialized countries. [12,13,14]. There are several advantages of MFCs, such as high conversion efficiency and low operating temperature and environmental footprint [15]. However, its application on a large scale is still limited due to low generated power and the high cost of its components [15, 16]. Therefore, the application of MFCs on large scale requires the use of inexpensive electrodes that exhibit good chemical stability, high mechanical strength and large surface area [17]. Anodic material is the main way to increase the power generation of MFCs due to its effect on bacteria attachment, the electron transfer process and substrate oxidation. Therefore, anodic materials must be conductive, biocompatible, and chemically stable. [15, 18]. Most of these properties are available in carbon materials; thus, the most common anode materials used in MFCs are based on carbon, such as carbon paper, cloth, felt, fibrous, foam, reticulated vitreous carbon (RVC), and graphite felt [15, 19, 20]. Carbon paper, cloth and foams have high costs for large industrial scale use (ca. $1000/m2) [21, 22]. However, graphite felt anodes are inexpensive and have the largest surface area; however, only 0.47 m2/g of their surface area is colonized by bacteria [15]. On the other hand, the use of granular activated carbon electrodes is restricted by their ultimate permeability in the packed bed direction [23]. High porosity of the bed is essential to avoid blockage and a decrease in the pressure; however, at the same time, electrical contact between specific granules is necessary to maintain appropriate conductivity [24]. Unsystematic carbon fibers have been considered as a substitute, but fiber clomping has an influence on the cell performance [21]. Overall, among all previous studies mentioned, no carbonaceous anode materials have displayed all of the required features of high porosity, superior interconnectivity, and high conductivity. Activated carbon materials have been investigated in several applications including supercapacitors, high-temperature filters and nano electronics [25]. It has been found that they are suitable for these applications due to their high surface area, low cost, excellent conductivity, low resistance and high micro porosity [26]. These impressive properties are believed to enhance bacteria cell adhesion, which can lead to increased power generation of MFC [27]. Hence, MFCs based on AC have been investigated using a sulfate-reducing bacterium (Desulfuricans) as the pure biocatalyst culture for sulfate reduction [28]. However, this pure culture is costly to grow and cultivate due to the stringent sterile special conditions.
In this study, the activated carbon performance is investigated as an effective anode material in a single-chamber air-cathode MFC using a novel electrolyte of real industrial wastewater obtained from a food manufacturing plant in Jeonju, South Korea; the wastewater was without treatment, filtration or addition of external microorganisms. In addition, the AC performance was compared to that of four carbonaceous anode materials.
Materials and methods
Electrodes
Carbon cloth (CC, EC–AC-cloth), carbon paper (CP, EC-TP1-120), and carbon cloth treated with Teflon (Poly Tetra Fluoro Ethylene (PTFE)) coating (CCT, EC–AC-Close) were purchased from Electro Chem. Inc., USA. Activated carbon (AC) sheets were obtained from the local market in Jeonju, South Korea. Pt-loaded carbon paper (0.5 mg/cm2) (EC-20-5, Electro Chem, Inc., USA) was used as the cathode. A commercial cation exchange membrane (CEM, CMI-7000) purchased from International Inc., NJ, USA, was used instead of the expensive Nafion117. All electrodes were cut into 2.5 × 2.5 cm sizes.
Microorganisms media
Real wastewater samples used in this study were collected from a local food factory in Jeonju, South Korea. The wastewater acted as a fuel and source of microorganisms without filtration in batch mode MFCs. The properties of the collected wastewater were characterized at the Water Environment Research Center, Jeonju, South Korea, as shown in Table S1. In addition, the mixed culture of microorganisms existing in the wastewater solution was examined by agar test. The culture dishes were examined under a microscope (U-25ND25 (BXSL) OLYMPUST2, JABAN) using 10× magnification (MPLOON10 × 0. 25) as shown in Figure S1A, and the bacteria number was counted as shown in Fig. S1B.
MFC design
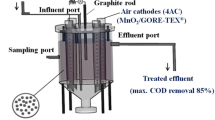
A single-chamber air-cathode MFC was used, in which the oxygen from the air was the oxidant, as shown in Fig. S2. The anode chamber had a volume of 84 cm3, and the space between the two electrodes was kept at 4 cm. CEM, fixed on the surface of the cathode, was used as the proton exchange membrane. In addition, 316 stainless steel plates with a thickness of 1 mm were used as the current collectors. The current density was calculated based on the anode surface area of 6.25 cm2.
Cell operation and the electrochemical measurements
Before starting the experiments, 80 ml of wastewater was purged with gaseous nitrogen for 20 min to remove all of the dissolved oxygen. Then, the wastewater was injected into the anode chamber at time zero, and the cell was operated in single cycle batch mode to immobilize the microorganisms onto the anode surface for more than 6 days until stabilization of the open circuit voltage (OCV). A potentiostat was used to measure the open circuit voltage (OCV). The potentials of the anode and the cathode were measured compared to a Ag/AgCl reference electrode, which was placed in the anode chamber. After OCV stabilization, when the two-cell electrode reactions reached equilibrium, the cell circuit was closed, and linear sweep voltammetry (LSV) was carried out at a scan rate of 1 mVs−1 to attain the polarization curve. Polarization curves are used for current characterization as a function of voltage by changing the external resistance. Therefore, the current–voltage relationship, I–V, was measured from the maximum OCV (i.e., minimum external resistance) to the zero voltage (i.e., maximum external resistance). The current density was measured for 2 h at a constant cell voltage of 0.2 V to check the cell stability. All measurements were carried out at room temperature (25 °C). All MFC experiments were repeated at least three times, and the intermediate values were used to portray accurate results.
Electrochemical analyses
A three-electrode cell consisting of the bio anode, Pt wire and Ag/AgCl electrode as the working, counter and reference electrodes, respectively, was used for the ex situ evaluation of the different electrodes in wastewater. Cyclic voltammetry (CV) was carried out using a potentiostat (Gamry Reference 600, US A) at different scan rates of 25 and 75 mV s−1 from +1 to −1 V. In situ evaluation of the anode electrode was carried out by fixing the electrodes in the MFC mentioned above. The cell voltage (V) and current (I) of the system were determined using linear sweep voltammetry (HA-151A POTENTIO STAT/GALVANOSTAT, Japan). All data were recorded using a GL220 instrument. The current density was calculated by dividing (I) by the anode surface area. The power (P) was calculated according to the following equation:
where I is the current (A), and V is the cell voltage (V). The power density (D) is normalized to the electrode surface area (A an) using the following equation:
where J, V and A an are the current density, voltage and anode surface area, respectively. The Coulombic efficiency (CE) is defined as the percentage of the total charge that is transferred to the anode from the substrate. The maximum possible charge can be obtained when the microorganisms producing the current can digest all substrates. It can be calculated from the equation (C p/C T) × 100%, where C p is the total coulombs calculated by integrating the current over time, and C T is the theoretical amount of coulombs that can be produced from the substrate in the wastewater. Therefore, for a fed-batch system, CE can be calculated as:
where M is the molecular weight of oxygen (32), F is the Faraday’s constant, b = 4 (the number of electrons exchanged per mole of oxygen), V an is the volume of the liquid in the anode compartment, and COD is the change in the chemical oxygen demand (COD).
Characterization
The morphology of the different anodes was examined using a scanning electron microscope equipped with the EDX analysis tool (SEM, Hitachi S-7400, Japan). After the experiments, the electrodes were dried at 50 °C for approximately 1 h before being used for SEM analysis. The crystal structures of the anode materials were examined by XRD using a Rigaku X-ray diffractometer (XRD, Rigaku, Japan) with Cu Kα (λ = 1.540 Å). The diffraction pattern was recorded over a 2θ range from 10° to 100° at a step size of 0.02°. Moreover, the hydrophilicity of the different anode materials was measured. The sessile drop method was used to measure the water contact angle of the anodes using DROP image Standard Device (Version 2.4), where approximately 3 μL of deionized water was dropped onto the anode surface, and the value of the water contact angle was recorded at room temperature.
Results and discussion
Characterization of the anode materials
Chemical structure of the anode materials (XRD)
The crystal structures of the different anode materials were examined by XRD as shown in Fig. S3. As shown, all of the investigated anode materials have the main carbon peak at 2θ of ~26° [d (002), JCPDS; 41-1487]. However, the carbon paper (CP) had the highest intensity, followed by CC and AC. These results confirm that the activated carbon is more amorphous than CC and CP. The amorphous surface of the material enhances the adhesion of microorganism, due to the large space for microorganism attachment [29]. Therefore, it is estimated that this amorphous morphology enhances the electron transfer efficiency and improves current generation [30, 31].
Elemental composition of the investigated anode materials (EDX)
Figure. S4 shows the surface morphology of the different anode materials investigated. All of the investigated anode materials are composed of carbon. However, it can be observed that the fluorine element for two peaks has different percentages, i.e., 18.26 and 7.55% for treated carbon paper and treated carbon cloth, respectively. The fluorine element is attributed to the Teflon material coating, which is used to enhance the surface properties of CPT and CCT anodes.
On the other hand, a simple avometer device was used to measure the electric resistances of the investigated carbon sheets; the results show that activated carbon has the highest electrical conductivity. Numerically, the observed specific electrical resistances are 0.8, 1.4 and 4.6 Ω cm−1 for the activated carbon, carbon cloth and carbon paper, respectively.
Surface wettability of the investigated anode materials
The wettability of the anode is a very important factor since hydrophobicity resists the wetting of the surface with water and thus decreases microorganism adhesion. Moreover, it is expected that a hydrophobic surface will increase the interface resistance between the biofilm and the anode surface, which inhibits the electron transfer process. Figure 1 displays the water contact angles for the different electrodes. As shown, the AC electrode possesses the lowest hydrophobicity. The water contact angles are 115.4°, 120.5°, 129.3°, 130.6° and 141.6° for AC, CCT, CC, CPT and CP electrodes, respectively. The lowest hydrophobicity leads to the highest wetted surface area of the anode by wastewater (electrolyte). Accordingly, very good attachment of the cells is expected with the AC electrodes, which minimizes the electron transfer resistance and increases the current generation.
The electrochemical properties of the examined anode materials
Ex situ cyclic voltammetry of the examined anode materials
Cyclic voltammetry of the different anode materials in 20 ml of real wastewater is shown in Fig. 2. The results reveal that the activated carbon anode achieves the highest current densities at different investigated scan rates (25 and 75 mVs−1). The generated current densities for MFC based on the different carbonaceous anode materials are 9, 8, 6, 4 and 3 mAcm−2 for AC, CCT, CC, CPT and CP, respectively. This finding indicates that the electron transfer resistance from the microorganism cell to the anode surface in the case of activated carbon is the smallest. In other words, the activated carbon depicts the greatest improvement in the performance of the electrochemically active biofilm and thus leads to a great enhancement of current generation at different scan rates. The most remarkable feature of the activated carbon electrode is the process ability due to its good surface characteristics and flexibility.
In situ evaluation of the investigated anode materials
Biofilm layers on different anode materials investigated in microbial fuel cells
Figure 3 shows the SEM investigation of microorganism cell adhesion on the different utilized anode surfaces before and after MFC operation. The first column represents the image of the anode before use. The second column displays the SEM magnification of the anodes after utilization in the MFC. The adhesion of microorganism cells can be observed more clearly on the surface and between the fibers of the different anode materials. Nevertheless, it can be concluded that the maximum adhesion is achieved on the surface of the AC anode; the microorganism cells cover the AC surface completely. This maximum adhesion can be attributed to the impressive characteristics of the AC anode, such as amorphous morphology, surface biocompatibility and hydrophilicity natural of AC, which increase microorganism attachment and enhance the electron transfer process. Furthermore, it can be observed that the attachment of microbial cells to the carbon cloth material surface is greater than that to the carbon paper material surface; this finding is related to the higher porosity of CC compared to CP. The porosity gives a larger available surface area for bacteria to adhere and enhances EET between the microorganism and the electrode surface. Moreover, the comparison of CCT and CPT to pristine CC and CP anodic materials confirms that more adhesion of microorganism cells can be achieved in the case of CCT and CPT electrode materials due to the Teflon coating. Teflon is widely used as a binder material for coating electrodes in fuel cells because it has high chemical stability. Moreover, Teflon coating leads to the formation of a porous microstructure network on the electrode surfaces, which enhances and increases the active area on the anode surface and increases the efficiency of electron transfer [32]. Based on the aforementioned investigations, it can be confidently stated that the collected food wastewater has active exogenous microorganisms, which are considered as the actual anode catalyst in the MFC. Moreover, these naturally existing microorganisms have the ability to transfer electrons and generate power in the microbial fuel cells.
Electrode potential and open cell voltage
Figure 4a, b) shows the relation among OCV, anode potential (E a) and cathode potential (E c) versus time for the assembled MFCs using different investigated anode materials. As shown in Fig. 4a, the anode potential in all cases decreases with time, which in turn leads to an increase in the OCV with time in the same trend as the equation, OCV = E c − E a. The decrease in the anode potential is due to reactions happening throughout the fermentation process. In this process, microbes produce most of their energy content in the form of ATP and NADH, which convert back to NAD+ for completing the metabolic reaction. Therefore, during the OCV, the concentrations of the reduced species, such as NADH, build up within the growing bacteria until steady state is reached. This stage is called the accumulation period. In other words, the decrease in the anode potential (E a) during the MFC operation can be attributed to the adhesion of the exogenous microorganisms. Once the MFC circuit is closed, the electrons move from the anode to the cathode through the external circuit [33]. However, little change is observed for the cathode potential versus time for all investigated anode materials as shown in Fig. 4b, in which the initial value of the cathode potential is 0.73, and after MFC operation, it reaches 0.68 V. These results confirm that the increase in OCV versus time is attributed to the decrease in the anode potential.
During the accumulation stage, E a decreases until minimum values of approximately −0.268, −0.242, −0.2, −0.112 and −0.105 V are reached; at the same time, the OCV increases to maximum values of 0.84, 0.815, 0.789, 0.762 and 0.743 for AC, CCT, CC, CPT and CP, respectively. The variation in the time and values from one electrode to another is related to the difference in the structure, electrical resistivity and wettability among the different electrodes.
Experimentally, as shown in Fig. 4a, b, the AC anode attains the minimum anode potential, which leads to the maximum OCV over time compared to the other investigated anode materials. On the other hand, CPT and CCT attain higher OCV than untreated CP and CC. The high performance of the treated CP and CC compared to the untreated ones can be attributed to the addition of the polymer coating (Teflon), which modifies and enhances the surface properties to facilitate the continuous growth of microorganisms. Moreover, Table S2 summarizes the electrochemical measurement results.
Current generation and stability
Figure 5a shows the stability of the produced current density from the MFCs at 0.2 V. Cell voltage was fixed by the linear sweep voltammetry system, which adjusts the specific external resistance against the MFC to keep the voltage value of the cell at 0.2 V. It is observed from Fig. 5a that in the case of MFCs with an AC anode, the maximum current density is achieved, followed by CCT, CC, CPT and CP with values of 1792, 482, 300, 280 and 196 mAm−2, respectively. Moreover, it can be seen that the current density starts at a high value and then decreases with time until a stable state is reached. The decrease in the initial current density is normal due to the fast depletion of the accumulated electrons on the anode surface during the MFC operation. Later on, the produced current matches the instantly produced electrons from the microorganisms, which leads to the observed current stability in all formulations.
Overall, MFCs with different carbonaceous anode materials achieve an average current density of approximately 1376, 328, 193, 223 and 155 for AC, CCT, CPT, CC and CP, respectively. These results show that the AC anode generates a current density 8, 6, 7 and 4 times higher than CP, CC, CPT, and CCT, respectively.
Power generation
Figure 5b shows the generated power from the assembled MFCs using different carbonaceous anode materials. It can be observed from the figure that the power generation is 338, 123.6, 78, 73 and 56 mWm−2 for AC, CCT, CC, CPT and CP, respectively. The observed high power in the case of the AC anode can be attributed to its promising biological and electrochemical properties. The AC anode has high bio-catalytic activities that lead to high adhesion of exogenous bacteria to its surface and improve the electron transfer process between bacteria and the surface. Accordingly, this desirable feature translates into a large decrease in the anode potential as well as a considerable increase in the OCV during the accumulation stage, which leads to an increase in the efficiency of the electron transfer process and enhances the current and power generation. Moreover, the porous surface of the AC anode is highly susceptible for continuous bacterial activity, leading to a complete growth process and more electron production. Most of the porous anode materials generate more power per geometric surface area compared to their smooth counterparts. Therefore, the generated power in the MFC with CC is higher than that with CP. This is mainly due to the larger surface area available to bacteria per unit volume of the anode chamber in the case of the porous anode materials. Moreover, Table S3 displays the determined maximum current and power densities for all of the investigated anode materials. Moreover, the produced power is compared with values reported based on our literature review; the generated power of the AC anode in this study is higher than many previous recent studies as shown in Table 1.
Internal resistance of the system
MFC internal resistance indicates the resistance of electrons to flow through the membrane, electrolytes, electrodes and interconnections [39, 40]. The internal resistance (R int) of the MFC can be determined from the slope of the linear polarization curve, which equals (∆E/∆I), as shown in Fig. 5b.
Overall, the only difference between the internal resistances of the different cells investigated in this study is in the anode materials properties. Figure 5c shows a comparison of the overall internal resistances for MFCs based on the investigated anode materials. As shown, the internal resistance in the case of MFCs with AC is the least, followed by CCT, CPT, CC and CP, respectively. These results confirm that AC as an anode material decreases the electron charge resistance and enhances the electron transfer, which leads to an increase in the generated power and current.
Evaluation of the MFC in wastewater treatment (COD and Coulombic efficiency)
MFC performance in wastewater treatment was investigated by measuring the chemical oxygen demand (COD) before and after the MFC test. The initial value of COD before the MFC test is 961; this value decreases after 6 days of MFC operation, reaching 379, 412, 463, 578 and 675 for AC, CCT, CPT, CC, and CP, respectively. This result confirms that MFC is a promising technology to simultaneously generate electricity and treat wastewater from organic materials. As shown in Fig. 6a, AC-based MFCs have the highest COD removal among all of the used MFCs. The corresponding COD removal for the MFC with an AC anode is over 60%, while it is 56, 39, 51 and 29% for CCT, CC, CPT and CP anode materials, respectively. The highest removal of organic materials in the case of the AC anode MFC supports the previous results, as it can be translated to more consumption of the organic matter. Moreover, the aforementioned descending order of COD removal with respect to the used anode materials also matches the corresponding results of current density and power generation. For estimating the relationship between the generated current from MFC and COD removal percentage, the Coulombic efficiency (CE) of the fabricated MFCs is calculated and presented in Fig. 6b. CE is affected by different factors such as microorganism’s cultures and adhesion, internal resistance of the system, and electrode material properties. Therefore, the effect of different anode materials on the CE is investigated according to Eq. 3, and the results reveal that the AC electrode has the highest Coulombic efficiency (71%) compared to the other anode materials. Typically, CCT, CC, CP and CPT anode-based MFCs have a CE efficiency of 28, 20, 13 and 12.5%, respectively. This finding can be exploited to explain the highest current production with the AC anode compared to the other anode materials. This achieved current is attributed to the production of electrons due to the consumption of organic substrate by microbes for growth. Therefore, due to the low resistance and high conductivity of the AC anode compared to the other anode materials, the consumption of organic compounds and thus the released electrons are high.
Conclusion
Real industrial wastewaters can be exploited as a fuel without treatment for generating electricity using the MFC technique. Activated carbon was presented as a promising anode material for MFC wastewater treatment. The large surface area, high hydrophilicity and porous structure of AC enhanced microorganism growth and adhesion. Its practicality as a MFC anode was shown by preliminary tests in a single air cathode microbial fuel cell in which its bio-electrochemical performance was higher compared to the other anodes. The highest power generation, current density, COD removal and Coulombic efficiency can be obtained using the AC-MFC. Overall, this study opens a new avenue for using activated carbon-based microbial fuel cells as an effective technique for generating power and wastewater treatment simultaneously.
References
Blanco J, Malato S, Fernández-Ibañez P, Alarcón D, Gernjak W, Maldonado M (2009) Review of feasible solar energy applications to water processes. Renew Sustain Energy Rev 13:1437–1445
Wetser K, Sudirjo E, Buisman CJ, Strik DP (2015) Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl Energy 137:151–157
Pandey P, Shinde VN, Deopurkar RL, Kale SP, Patil SA, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723
Pham T, Rabaey K, Aelterman P, Clauwaert P, De Schamphelaire L, Boon N, Verstraete W (2006) Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6:285–292
Kim JR, Min B, Logan BE (2005) Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl Microbiol Biotechnol 68:23–30
Poddar S, Khurana S (2011) Geobacter: the electric microbe! Efficient microbial fuel cells to generate clean, cheap electricity. Indian J Microbiol 51:240
Moon H, Chang IS, Kim BH (2006) Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Biores Technol 97:621–627
Gil G-C, Chang I-S, Kim BH, Kim M, Jang J-K, Park HS, Kim HJ (2003) Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens Bioelectron 18:327–334
Li Y, Williams I, Xu Z, Li B, Li B (2016) Energy-positive nitrogen removal using the integrated short-cut nitrification and autotrophic denitrification microbial fuel cells (MFCs). Appl Energy 163:352–360
He H, Zhou M, Yang J, Hu Y, Zhao Y (2014) Simultaneous wastewater treatment, electricity generation and biomass production by an immobilized photosynthetic algal microbial fuel cell. Bioprocess Biosyst Eng 37:873–880
Baranitharan E, Khan MR, Prasad DMR, Teo WFA, Tan GYA, Jose R (2015) Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioprocess Biosyst Eng 38:15–24
Qiao Y, Bao S-J, Li CM (2010) Electrocatalysis in microbial fuel cells—from electrode material to direct electrochemistry. Energy Environ Sci 3:544–553
Mohamed HO, Obaid M, Khalil KA, Barakat NA (2016) Power generation from unconditioned industrial wastewaters using commercial membranes-based microbial fuel cells. Int J Hydrogen Energy 41:4251–4263
Kim M, Cha J, Yu J, Kim C (2016) Stackable and submergible microbial fuel cell modules for wastewater treatment. Bioprocess Biosyst Eng 39:1191–1199
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Logan BE, Regan JM (2006) Microbial fuel cells-challenges and applications. Environ Sci Technol 40:5172–5180
Zhu N, Chen X, Zhang T, Wu P, Li P, Wu J (2011) Improved performance of membrane free single-chamber air–cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes. Bioresour Technol 102:422–426
Adeniran J, Huberts R, De-Koker J, Arotiba O, Olorundare O, Van-Zyl E, Du-Plessis S (2016) Energy generation from domestic wastewater using sandwich dual-chamber microbial fuel cell with mesh current collector cathode. Int J Environ Sci Technol 13:2209–2218
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102:9335–9344
Xiao L, Damien J, Luo J, Jang HD, Huang J, He Z (2012) Crumpled graphene particles for microbial fuel cell electrodes. J Power Sources 208:187–192
Logan B, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air–cathode microbial fuel cells. Environ Sci Technol 41:3341–3346
Zhang F, Cheng S, Pant D, Van Bogaert G, Logan BE (2009) Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem Commun 11:2177–2179
Jiang D, Li B (2009) Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): a design suitable for large-scale wastewater treatment processes. Biochem Eng J 47:31–37
Logan BE (2007) Microbial fuel cells. Wiley, New Jersey
Liu CK, Lai K, Liu W, Yao M, Sun RJ (2009) Preparation of carbon nanofibres through electrospinning and thermal treatment. Polym Int 58:1341–1349
Paul P (2009) Value Added Products from Gasification Activated Carbon. Combustion Gasification and Propulsion Laboratory Department of Aerospace Engineering Indian Institute of Science, Bangalore
Smith L, Ma P (2004) Nano-fibrous scaffolds for tissue engineering. Colloids Surf B 39:125–131
Zhao F, Rahunen N, Varcoe JR, Chandra A, Avignone-Rossa C, Thumser AE, Slade RC (2008) Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ Sci Technol 42:4971–4976
Chambrion P, Suzuki T, Zhang Z-G, Kyotani T, Tomita A (1997) XPS of nitrogen-containing functional groups formed during the C–NO reaction. Energy Fuels 11:681–685
Jansen R, Van Bekkum H (1995) XPS of nitrogen-containing functional groups on activated carbon. Carbon 33:1021–1027
Li B, Zhou J, Zhou X, Wang X, Li B, Santoro C, Grattieri M, Babanova S, Artyushkova K, Atanassov P (2014) Surface modification of microbial fuel cells anodes: approaches to practical design. Electrochim Acta 134:116–126
Liu H, Logan BE (2004) Electricity generation using an air–cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Kim JR, Zuo Y, Regan JM, Logan BE (2008) Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol Bioeng 99:1120–1127
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662
Sayed ET, Tsujiguchi T, Nakagawa N (2012) Catalytic activity of baker’s yeast in a mediatorless microbial fuel cell. Bioelectrochemistry 86:97–101
Zuo Y, Cheng S, Logan BE (2008) Ion exchange membrane cathodes for scalable microbial fuel cells. Environ Sci Technol 42:6967–6972
Kasem ET, Tsujiguchi T, Nakagawa N (2013) Effect of metal modification to carbon paper anodes on the performance of yeast-based microbial fuel cells part ΙΙ: in the case with exogenous mediator, methylene blue. Key Eng Mater 534:82–87. doi:10.4028/www.scientific.net/KEM.534.82
Hoogers G (2002) Fuel cell technology handbook. CRC Press
Larminie J, Dicks A, McDonald MS (2003) Fuel cell systems explained. Wiley, Chichester, UK
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MISP) (Grant Number 2014R1A4A1008140).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamed, H.O., Obaid, M., Sayed, E.T. et al. Electricity generation from real industrial wastewater using a single-chamber air cathode microbial fuel cell with an activated carbon anode. Bioprocess Biosyst Eng 40, 1151–1161 (2017). https://doi.org/10.1007/s00449-017-1776-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1776-0