Abstract
A pilot-scale reactor treating domestic sewage was operated to promote anaerobic digestion and denitrification using endogenous electron donors. While 55 % of organic matter was removed, nitrogen and sulfur showed a different dynamics during the operation. Pyrosequencing analysis clarified this behavior revealing that specific microbial communities inhabited the anaerobic (47.05 % of OTUs) and anoxic (31.39 % of OTUs) chambers. Analysis of 16S rRNA gene partial sequences obtained through pyrosequencing revealed a total of 1727 OTUs clustered at a 3 % distance cutoff. In the anaerobic chamber, microbial community was comprised of fermentative, syntrophic and sulfate-reducing bacteria. The majority of sequences were related to Aminobacterium and Syntrophorhabdus. In the anoxic chamber, the majority of sequences were related to mixotrophic and strictly autotrophic denitrifiers Arcobacter and Sulfuricurvum, respectively, both involved in sulfur-driven denitrification. These results show that pyrosequencing was a powerful tool to investigate the microbial panorama of a complex system, providing new insights to the improvement of the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic technology is being increasingly used for cost-effective domestic sewage treatment in warm-climate countries. The removal of nutrients is still an issue, though, and the development of novel technologies and strategies is essential for viability and expansion of treatment systems that comprise biological nutrients removal [1, 2].
Alternatives to the so-called conventional denitrification (i.e., using organic electron donors) must be searched for, due to the lack of readily biodegradable organic matter in anaerobic effluents. Interestingly, potential sources for alternative electron donors may be found within the own anaerobic process. Anaerobic reactors produce sulfides in both dissolved and gaseous forms, as well as methane that, together with the residual organics, could all be used as sources of endogenous electron donors for denitrification [1], reducing costs in the post-treatment of effluents produced by such reactors.
Autotrophic denitrification using reduced sulfur compounds as electron donors and methane-utilizing denitrification as well as anaerobic oxidation of ammonium have been studied in the past years, showing the potentials of such alternative processes for nitrogen removal of different types of wastewater [3, 4]. System configurations designed to take into account the occurrence of alternative denitrification processes have also been recently reported [3, 4].
It is well known that microbial communities play an essential function in biological treatment systems. The knowledge of structure, composition and role of microorganisms associated with physicochemical monitoring tools have provided comprehensive information with respect to the overall performance of treatment facilities and have helped to improve design efficiency and applicability of anaerobic sewage treatment [4, 5]. Although a physiological complex microbiota is known to be involved in the anaerobic treatment of organic waste streams, such as domestic sewage, the true nature and function of several bacteria and Archaea populations involved in the process is still unknown. There is also a lack of information about the microorganisms and their role in sulfur-driven denitrification in bioreactors treating domestic sewage.
Molecular approaches based on 16S rRNA genes, such as fluorescent in situ hybridization (FISH), polymerase chain reaction coupled to denaturing gel gradient electrophoresis (PCR-DGGE) and clone library are able to characterize the so-called “uncultivable community” and thus have greatly expanded the field of microbial ecology, including the communities involved in wastewater treatment processes [5]. Nevertheless, these techniques are sensitive to detect only the most abundant populations, restricting the analysis of microbial communities in a broader and complete scale. The recently developed sequencing approaches, also called next generation or high-throughput sequencing (e.g., 454 pyrosequencing), has emerged as a powerful tool for analyzing complex microbial communities, since it can generate more than 400,000 reads with average read length up to several hundred base pairs and average quality of more than 99.5 % [6].
Pyrosequencing is a powerful tool to access the microbial community structure of wastewater treatment bioreactors and it has been successfully applied in wastewater treatment systems [4, 7–9]. Nevertheless, utilization of pyrosequencing for identification of microbial community driven sulfur-denitrification is scarce [9] were pioneers in the use of pyrosequencing to access the microbial community of a lab-scale bioreactors operated for sulfur-driven denitrification.
In this study, the pyrosequencing technique was used to obtain a deeper insight into microbial communities and consequently a better understanding of the phenomena occurring inside the chambers of a novel system configuration treating domestic sewage. The system was designed for anaerobic digestion, nitrification and denitrification using endogenous electron donors such as sulfides and methane, and so was based in complex interactions among different microbial communities. Pyrosequencing was employed in two different operational phases (consisting of different recycling ratios and described below) with two different purposes: (1) to characterize the microbial community in the anoxic chamber in phase I; (2) to corroborate the hypothesis of denitrification occurrence in the anaerobic chamber in phase II. In this way, the technique was able to assess aspects of the treatment system not easily identified by physicochemical analyses.
Materials and methods
Pilot-scale system
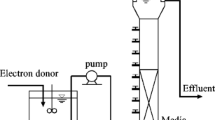
The pilot-scale system was built in glass fiber and consisted of two continuous upflow reactors (RA and RB, Fig. 1), containing immobilized biomass. The tubes and connections were made of PVC, and the system was subject to ambient temperature, varying from 10 to 30 °C along the year. RA had a diameter of 0.80, 4.00 m of height, an empty-bed volume of 1.58 m3, and contained two separate chambers, one for anaerobic digestion and one for denitrification. The hydraulic retention time (HRT) was kept at 8 h for both chambers. RB was an auxiliary reactor used for nitrification, and had a diameter of 0.40 m, a height of 4.50 m and an empty-bed volume of 0.39 m3. This second reactor was injected with a constant airflow and subject to different HRT (8–24 h), depending on the experimental condition. Chambers were filled with commercial support material consisting of polyurethane foam caged in plastic cylinders (Biobob®, BIO PROJ, Brazil).
Pilot-scale system scheme: 1 RA feed pump, 2 RA lower (anaerobic) chamber, 3 RA intermediary chamber, 4 RA upper (anoxic) chamber, 5 biogas separation, 6 RB feed pump, 7 air diffuser, 8 air compressor, 9 RB nitrifying (aerobic) chamber, 10 anaerobic chamber outlet sampling point, 11 anoxic chamber inlet sampling point
The system was designed to promote anaerobic digestion, nitrification and denitrification, and thus secondary treatment and nitrogen removal were aimed. The chambers for each process were arranged so that no intermediary tanks were necessary, to avoid losing sulfides by volatilization or oxidation. According to Fig. 1, influent was pumped through the base of RA, entering the lower chamber designed for anaerobic digestion. A part of the effluent from the lower chamber was then pumped to RB for nitrification, and the nitrified effluent was collected at the top of this unit and sent back to RA by gravity, being introduced at the bottom of the upper chamber of RA. At this point, the mixture between both parcels of the effluent from the lower chamber—the one that was pumped to RB and the one that remained in RA—could be achieved, before entering the upper chamber, designed for denitrification.
The advantage of this novel configuration is that the only fraction of the effluent transferred between reactors is the one subject to nitrification. The remaining part, containing the sulfides and that should not be exposed to the atmosphere or to pumping turbulence, is kept undisturbed inside RA, and thus sulfide loss is avoided. This favors the occurrence of autotrophic denitrification when both parcels of the effluent—nitrified (containing nitrates) and non-nitrified (containing sulfides)—are mixed together before entering the upper chamber. Another positive characteristic of the system is the fact that the denitrifying chamber is located on top of the anaerobic digestion chamber, and so the biogas produced in the lower chamber obligatorily pass through the upper chamber. In this way, CH4 and H2S present in the biogas could be used for denitrification, while CO2 could be a source of alkalinity and inorganic carbon for denitrifiers. As a result, the system is able to use more efficiently the by-products of anaerobic digestion when it comes to nitrogen removal.
Wastewater, inoculation and operation
The pilot-scale system was fed with domestic sewage collected from the sewer system of the city of São Carlos, São Paulo, Brazil. The wastewater was pumped out of an equalizing tank connected to the sewage municipal emissary that was located close to the building, collecting sewage from the neighborhood. Entrance valves were protected with stainless steel grids to prevent solids entering the pump and the reactor. Inoculation of the support material was performed naturally, by feeding the system only with wastewater, for a period of approximately 75 days. This procedure was initially applied only to RA, for the development of anaerobic digestion in this unit, and later full operation was established to allow nitrifiers to grow in RB, by sending 20 % of the total flow rate to RB. The system was operated with a total flow rate of 55 L h−1, and subject to two different experimental conditions, based on the proportion of the total flow rate that was pumped to RB for nitrification: 20–30 % (phase I) and 40–60 % (phase II), maintained during approximately 100 and 60 days, respectively.
COD, pH, sulfide, sulfate, ammonium, nitrite and nitrate were measured in the inlet and outlet of both anaerobic and anoxic chambers during operation. All physical chemical analyses were done according to the Standard Methods for the Examination of Water and Wastewater [10]. Sulfide was measured by the methylene blue method. Sulfate was determined by the turbidimetric barium chloride method. COD, ammonium, nitrite and nitrate were determined by spectrophotometry.
Sampling, DNA extraction and pyrosequencing
Biobobs® samples were collected from the upper chamber of RA at the end of phase I and from the top of the lower chamber of RA at the end of phase II (Fig. 1). In phase I pyrosequencing was used to characterize the microbial community involved in denitrification, while in phase II this technique was used to test the hypothesis of denitrification occurring in the anaerobic chamber. It is important to note that samples were taken from different chambers in phases I and II, and thus it was not the scope of this work to compare microbial communities in the same chamber between different phases. Microbial biomass was removed manually from the plastic cylinders by successive washing in PBS-buffer (PBS: 0.13 M NaCl, 7 mM Na2HPO4, 3 mM Na2H2PO4, pH 7.2) and subsequent centrifugation for 10 min at 6000 rpm, at 4 °C, after which the pellet (1 g) was collected and kept at −20 °C until DNA extraction. Genomic DNA was extracted using a phenol:chloroform protocol adapted from Griffiths et al. [11]. Extractions were performed by the addition of 0.5 g of glass beads (150–212 um), 1 mL of phenol buffered with Tris (pH 8.0), 1 mL of chloroform and 1 ml of PBS buffer (PBS: 0.13 M NaCl, 7 mM Na2HPO4, 3 mM Na2H2PO4, pH 7.2). Samples were lysed via vortex for 1 min and the aqueous phase containing nucleic acids were separated by centrifugation (6000 rpm) for 10 min at 4 °C. The aqueous phase (0.8 mL) was then transferred to a microtube and nucleic acids were extracted, via vortex, with an equal volume of phenol, followed by centrifugation (10,000 rpm) for 10 min at 4 °C. Aqueous phase (0.6 mL) was then transferred to another microtube and nucleic acids were extracted, via vortex, with an equal volume of chloroform, followed by centrifugation (10,000 rpm) for 10 min at 4 °C. Extraction with phenol–chloroform was repeated twice. Total nucleic acids were subsequently purified using illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare) following the instructions given by the manufacturer.
Pyrosequencing was performed on both phases I and II samples using barcoded primers that amplify the V4 hypervariable region of the 16S rRNA gene [12]. The primer and barcode sequences were used as follows: forward primer F563 5′-AYTGGGYDTAAAGNG-3′, reverse primer R802 5′-TACNVGGGTATCTAATCC-3′ (RDP website http://pyro.cme.msu.edu/pyro/help.jsp), barcode for anaerobic chamber 5′-CACGCTACGT-3′, and barcode for anoxic chamber 5′-CAGTAGACGT-3′. PCR reactions and pyrosequencing were performed in a 454 GS FLX Titanium (Roche) at the Instituto de Agrobiotecnologia Rosario (INDEAR Facility, Argentina). PCR was performed according to the procedures described in [13]. Pyrosequencing data from aerobic and anoxic chambers were deposited in the NCBI Short Read Archive (SRA) under the accession numbers SRX1336504 and SRX1336505, respectively.
Sequences were processed on mothur 1.24.0 [14]. Chimeric sequences were detected by the Chimera Slayer algorithm [15] using the Silva “Gold” aligned database as reference. Putative chimeras and sequences shorter than 160 bp were excluded. The remaining sequences were normalized (randomly subsampled) taking into account the sample with the fewest number of sequences. The resulting sequences were aligned against the SILVA 102 reference database [16] and further clustered into operational taxonomic units (OTU) at 3 % distance cutoff, which was considered as species taxonomic level [17]. In order to estimate the complexity of the microbial communities, alpha diversity indices were calculated on mothur for each sample using richness (Chao1) and diversity (Shannon) estimators, as well as by Good’s diversity coverage. A Venn diagram was constructed to calculate the number of specific and shared OTU between each phase. Finally, all sequences from both chambers (i.e., without subsampling) were assigned to taxonomic level of Genera with the Classifier tool in the Ribosomal Database Project (RDP) release v10.18 (http://rdp.cme.msu.edu/index.jsp) using a 50 % confidence threshold [18].
Results and discussion
Physicochemical analyses
The pilot-scale system was operated for approximately 180 days, and was considered stable after a 75 day period (no inoculation sludge). A neutral pH (6.8–7.0) was kept during the entire operation period. COD removal efficiencies ranged from 50 to 55 % in both phases. However, nitrogen and sulfur compounds had different dynamics in the chambers depending on the operational phase (Table 1). After the acclimation period, nitrates were continuously observed in the outlet of RB at concentrations of up to 20 mg N L−1.
The most evident changes between the two phases were observed in the anaerobic chamber. In phase I, sulfide was produced in this chamber, while in phase II no evident production was observed. On the other hand, sulfate was completely consumed in phase I, and not particularly affected in phase II. A small consumption of ammonium was observed in phase I, and a more consistent consumption in phase II. Although expected in the measurements, nitrate was not detected significantly in the sample points of RA, and neither was nitrite in none of the phases.
Few effects were observed, though, regarding the anoxic chamber. Most parameters remained practically unchanged between the inlet and outlet, with the exception of sulfide, which was consistently produced in phase II, according to Table 1.
The results show a rather complex environment in which several phenomena may have occurred simultaneously, including denitrification using residual organics, sulfides or methane. This complexity was increased by the fact that the sampling at the outlet of the anaerobic chamber and inlet of the anoxic chamber may not have been completely representative. Sampling in pilot-scale systems usually suffers hydrodynamic effects, and this may have been the case for those sample points, especially because the interchange of effluents between RA and RB was occurring in these regions. Therefore, the dynamics occurring in the intermediary chamber located between anaerobic and anoxic chambers could not be completely assessed.
Analyzing the inlet and outlet data of the entire RA reactor, it was evident that denitrification activity was occurring inside the reactor. Approximately 20 and 40 % of the total inorganic nitrogen were removed in phases I and II, respectively. This fraction corresponds roughly to the proportion of the total flow rate that was recirculated to RB in each phase, and thus was subject to nitrification–denitrification processes. The physicochemical data obtained for inlets and outlets of each chamber were not able to explain, though, which processes were responsible for the nitrogen removal.
The shift in the behavior of the anaerobic chamber regarding sulfur compounds in phase II, the non-detection of nitrates in RA (which were measured in the outlet of RB) and the apparent lack of denitrification activity in the anoxic chamber could be explained by the following hypotheses: (1) denitrification activity in the pipes connecting RB to RA, containing the flow of nitrified effluent; (2) denitrification activity in the intermediary chamber due to suspended biomass activity; (3) denitrification activity on the upper part of the anaerobic chamber in phase II, because of unexpected mixing in the intermediary chamber, which could explain the presence of sulfate at the outlet of this chamber due to autotrophic denitrification; (4) intense mixing of nitrified and anaerobic effluent in the intermediary chamber of RA and in the inlet of the anoxic chamber in such a way that the nitrates in the sample points were already denitrified due to the contact with the biomass of the chamber.
Since physicochemical analyses alone were not enough to clarify these phenomena, auxiliary techniques were needed for a better understanding of the microbial community acting on the controversial sampling points. In this way, pyrosequencing technique was as a suitable tool to increase the data pool in order to adequately evaluate the biological processes and improve the design of the system. Pyrosequencing analyses were applied in two situations, to evaluate two main hypotheses: (1) samples collected from the lower region of the anoxic chamber in phase I, to evaluate if a denitrifying community was present in this chamber; (2) samples collected from the upper region of the anaerobic chamber in phase II, to evaluate if the increase in the recirculation rate between RA and RB could be causing nitrates to be denitrified at this region.
Pyrosequencing
Massive parallel sequencing resulted in a total of 13,665 sequences with an average read length of 286 ± 13.6 bp. From this total, the anaerobic chamber had 6288 sequences and the anoxic chamber resulted in 7377 sequences. Therefore, for the OTU-based analysis, the number of sequences from the anoxic chamber was set to 6288 randomly chosen sequences. Alpha diversity indices were calculated for both chambers at a 3 % distance cutoff (Table 2). Good’s coverage rates showed values higher than 90 %, which is considered the minimum coverage necessary to analyze OTU with confidence [19].
The total observed OTU in the anaerobic chamber (815 OTU) was higher when compared to the anoxic chamber (629 OTU). Similarly, the richness (Chao1) and diversity (Shannon) indices were higher in the anaerobic chamber. These results indicate that the anaerobic chamber contained a greater number of different microorganisms and also a more evenly distributed number of phylotypes among the OTUs. Therefore, the anoxic chamber seemed to be a more selective environment than the anaerobic. In order to evaluate how much of this diversity is shared between the anaerobic and anoxic chambers, a Venn diagram was constructed with the observed OTU (Fig. 2). The majority of the OTU’s were specific for the anaerobic (47.05 % of total OTU) or the anoxic chamber (31.39 %). The remaining 21.55 % of the OTU were shared between the two samples, which may represent populations surviving in both chambers, for example the metabolically versatile mixotrophic bacteria that take advantage of different environmental conditions, or inactive/decaying cells that entered the anoxic chamber coming from the anaerobic chamber.
Microbial community in anoxic chamber
Pyrosequencing analysis in the anoxic chamber yielded a total of 7377 sequences, of which 6486 were related to the domain bacteria and 831 were unclassified bacteria.
At the phylum level, sequences belonging to the Proteobacteria were the most abundant, with 71 % of frequency (Fig. 3). All five classes within Proteobacteria were represented, being Epsilonproteobacteria the most numerous, with 62 % of all bacterial sequences (Fig. 4). Members of the class Epsilonproteobacteria are increasingly recognized as predominant auto or mixotrophic organisms globally ubiquitous in marine and terrestrial ecosystems, being of major importance in biogeochemical processes at oxic-anoxic interfaces, particularly those that are sulfur-dependent [20]. These results show that the operational and environmental conditions imposed in the anoxic chamber of RA promoted the selection of a microbiota related to sulfur metabolism. Within this group, the most abundant sequences were related to Arcobacter (Campylobacteraceae) and Sulfuricurvum (Helicobacteriaceae) genera (Fig. 5). Cultivable representatives of both genera are facultative anaerobic, growing in microaerophilic conditions, with the ability to oxidize sulfur reduced compounds (sulfide, thiosulfate, elemental sulfur) and hydrogen, while reducing oxygen or nitrate to nitrite. They do not use nitrite as electron acceptor, though [21, 22]. Representatives of Arcobacter and Sulfuricurvum genera are mixotrophic and strictly autotrophic, respectively. Sequences related to Arcobacter were more abundant than those related to Sulfuricurvum, showing that mixotrophic metabolism was favored in the anoxic chamber. [23] reported the dominance of sequences related to Arcobacter sp in a continuously stirred tank reactor fed with synthetic sewage containing 100 mg/L of S−2–S and 70 mg/L of NO−3–N. In a recent study using the pyrosequencing technique, [24] detected a great number of sequences classified as Arcobacter in the inflow sewage of wastewater treatment plants, and [21] isolated Arcobacter defluvii sp. nov. from raw sewage samples. Although the microbiota of the raw sewage was not analyzed, these microorganisms most probably were present in the raw domestic sewage and were selected by the operational and environmental conditions applied to the bioreactor. Regarding the occurrence of Sulfuricurvum, this is the first report of sequences related to Sulfuricurvum in a domestic sewage bioreactor. Sulfuricurvum kujiense strain YK-1 [22], the only strain cultivated so far, is strictly autotrophic and can grow on crude oil by coupling oxidation of reduced sulfur compounds to reduction of nitrate. The predominance of Epsilonproteobacteria with presence of sequences related to Arcobacter and Sulfuricurvum is an important evidence that this system configuration was adequate to develop a microbial consortium of nitrate-reducing bacteria able to denitrify under autotrophic and/or heterotrophic conditions.
The classes Gammaproteobacteria, Deltaproteobacteria were detected in minor frequency (Fig. 4). Within the class Gammaproteobacteria, comprising 715 sequences (9.8 % of frequency), the family Pseudomonadaceae was dominant, with all 556 sequences related to the genus Pseudomonas. In minor proportion (0.8 %), there was the family Aeromonadaceae with 91 sequences, of which 62 were related to the genus Tolumonas (Fig. 5) and 23 sequences to the genus Aeromonas. It is well known that members of the genus Pseudomonas are widespread and metabolically versatile, and are frequently detected in environments where denitrification is achieved, including domestic sewage treatments systems. Cultivable representatives are facultative chemolithotrophs, facultative anaerobes with respiratory metabolism and in anoxic or aerobic condition can use nitrate or nitrite as electron acceptor. For example, Pseudomonas stutzeri strains SU2 and QZ1 have been reported to be associated with aerobic denitrification and denitrification coupled to oxidation of sulfide, respectively, in wastewater treatment systems [25, 26]. The Aeromonadaceae family is a group of microorganisms widespread in aquatic ecosystems. Members of this family are chemo-organotrophs and facultative anaerobes capable of respiratory and fermentative metabolism. Oxygen is a universal electron acceptor and some groups can reduce nitrate [27]. Sequences related to Aeromonas were detected in the anoxic chamber and may have been associated with nitrate reduction. The type genus Aeromonas was isolated from domestic sewage treatment system and was related with reduction of nitrate [28]. Interestingly, sequences related to Tolumonas were more abundant than those related to Aeromonas, even though for Tolumonas the reduction of nitrate has not been reported. Although sequences related to Tolumonas are not directly involved in denitrification they may have had and indirect influence by producing electrons donors for denitrifiers. For example, the type strain Tolumonas auensis strain TA 4T produces acetate, ethanol and formate as products of glucose fermentation [29]. These substrates can be used for denitrification.
The class Deltaproteobacteria comprised 665 sequences or 9 % of all bacteria sequences. In this class, it was observed a dominance of sequences related to incompletely sulfate-reducing bacteria belonging to the genus Desulfobulbus, with 394 sequences, followed by Desulfovibrio, with 178 sequences (Fig. 5). Cultivable representatives, such as Desulfobulbus propionicus and Desulfovibrio desulfurican, besides reduction of sulfate, can also couple reduction of nitrate or nitrite to ammonium with completely oxidation of sulfide, as well as oxidize inorganic sulfur compounds and organic substrates incompletely to acetate under microaerophilic conditions [30]. The sequences related to Desulfobulbus and Desulfovibrio may indicate the role of these genera in carbon, sulfur and nitrogen cycles by oxidation of sulfur under microaerophilic condition as well as by reduction of nitrate linked to sulfur oxidation and by production of acetate for heterotrophic denitrification. The class Betaproteobacteria comprised 2.8 % of all bacteria sequences with 206 sequences. In this class, sequences related to the genus Janthinobacterium (Oxalobacteraceae) were dominant, with 72 sequences (Fig. 5). It is well known that microorganisms of this genus are chemo-organotrophs with strictly aerobic metabolism and with the ability to reduce nitrate or nitrite to nitrogen gas. In overall, due to their versatility, the classes and genera presented here may have found in the anoxic chamber a suitable environment for growth, because of the simultaneous presence of nitrates, sulfides, sulfate, residual organic matter and residual dissolved oxygen.
In minor frequency, sequences related to the phylum Verrucomicrobia were detected, comprising 209 sequences or 2.8 % of all bacterial sequences (Fig. 3), with the majority of sequences (145 sequences) belonging to Subdivision3_genera_incertae_sedis (class Subdivision 3) (Fig. 5). Verrucomicrobia is a recently described phylum that are ubiquitous in soils, and most of isolates are free-living organisms, mesophilic, facultative or obligate anaerobic, saccharolytic and oligotrophic [31]. Detection of this group has been recently reported in activated sludge systems, anaerobic sludge digester and in a bioreactor operated for aerobic/anoxic deammonification [8, 32, 33]. Sequences related to this group suggest their role in heterotrophic metabolism in the anoxic chamber as well as in the anaerobic chamber, where sequences related to Verrucomicrobia were also detected.
The phyla Acidobacteria and Synergistetes were also detected in minor frequency. The phylum Acidobacteria comprised 190 sequences, of which 166 belonged to the family Holophagaceae, being represented in high number by sequences (123 sequences) related to Holophaga, also detected in the anaerobic chamber. The type strain Holophaga foetida [34] is obligate anaerobic, homoacetogenic and the presence of sequences related to Holophaga suggests anaerobic zone in the anoxic chamber. The presence of sequences related to this organism may indicate its role in denitrification, by supplying nitrate reducers with organic electron donors for heterotrophic denitrification. The phylum Synergistetes comprised 224 sequences, being the majority related to Aminobacterium (84 sequences), Aminomonas (47 sequences), Synergistes (25 sequences) and Cloacibacillus (16 sequences). These sequences were also observed in the anaerobic chamber and all cultivable taxa share the ability to ferment amino acids and/or peptides to hydrogen and fatty volatile acids, which are consumed by mixed microbial populations [35]. Sequences related to these genera indicate the presence of fermentative metabolism inside the anoxic chamber using organic residuals provided by the anaerobic chamber. This fraction of the microbial community may also have contributed for denitrification by supplying nitrate-reducing microorganisms with fermentation by-products that could be used as electron donors.
In summary, the operational and environmental conditions promoted the selection of a complex microbial community in the anoxic chamber with microorganisms directly and indirectly involved in sulfur-driven denitrification as well as in heterotrophic denitrification. The majority of sequences related to Proteobacteria within Epsilon class such as Arcobacter and Sulfuricurvum, as well as the detection of sequences related to Desulfobulbus and Desulfovibrio (Deltaproteobacteria), shows that this system configuration was capable of developing a microbial community of nitrate-reducing sulfur-oxidizing bacteria in the anoxic chamber as well as mixotrophic and heterotrophic denitrifiers (Pseudomonas, Aeromonas, Janthinobacterium), and in minor proportion those with homoacetogenic (Holophaga) and fermentative metabolism (Tolumonas, Aeromonas and sequences related to the phylum Synergistes).
Microbial community in anaerobic chamber
A total of 6288 sequences were analyzed for the anaerobic chamber, of which 4834 were classified to different bacterial Phyla and 1442 were unclassified bacteria. Sequences related to the phylum Synergistetes dominated in the anaerobic chamber, with 1898 sequences or 30 % of frequency (Fig. 3). In this phylum it was observed a dominance of sequences related to the genera Aminobacterium and Aminomonas, followed by Synergistes, and in lower number Cloacibacillus (Fig. 5). Synergistetes is a candidate phylum recently proposed that includes anaerobic, gram negative, rod-shaped bacteria that are ubiquitous in humans but with few isolates so far from anaerobic treatment systems and all cultivable taxa share the ability to ferment amino acids and/or peptides to hydrogen and fatty volatile acids which are consumed by mixed microbial populations, such as methanogens, homoacetogenic and sulfate-reducing bacteria [35]. As sequences related to Synergistetes were dominant (Fig. 3) and protein component of domestic sewage is reportedly greater than 40 % [36], the microorganisms of this phylum most probably played an important role in the acidogenic phase during the anaerobic degradation of amino acids and/or peptides presented in the sewage, under the operational and environmental conditions applied.
The phylum Proteobacteria was the second largest phylum comprising a total of 1169 sequences (Fig. 3). All five classes within Proteobacteria were represented, but in contrast with the anoxic chamber, Deltaproteobacteria was the most numerically represented, with 881 sequences corresponding to 14 % of all bacterial sequences (Fig. 4). Deltaproteobacteria contains the major lineages of sulfate reducers and also syntrophic substrate-degrading anaerobes, and this may explain the microbial activity in the anaerobic chamber, which generated sulfides to be consumed in the anoxic chamber. The more abundant sequences were related to the genus Syntrophorhabdus (333 sequences), followed by Desulfobulbus (199 sequences) and in less number by Smithella (89 sequences), Desulfovibrio (74 sequences), and Desulfonema (45 sequences) (Fig. 5). Syntrophorhabdus is largely represented by abundant bacteria within anaerobic ecosystems mainly decomposing aromatic compounds. The type species, Syntrophorhabdus aromaticivorans, isolated from an UASB reactor treating wastewater from a manufacturing terephthalic acid, degrades phenol and other aromatic compounds in syntrophic association with hydrogen-scavenging methanogens [37]. In addition, this organism is also able to degrade isophthalate in syntrophic association with Desulfovibrio sp. Although phenol and other aromatic compounds are characteristic of some industrial effluents, they can also be present in sewage due, for example, to the use of detergents. Aromatic compounds can also be formed during anaerobic degradation of aromatic amines present in sewage [38]. The high number of sequences related to Syntrophorhabdus may indicate the role of this group in syntrophic degradation of phenol and other aromatic compounds possibly present in the sewage. A total of 89 sequences were related to another syntrophic bacterium related to the genus Smithella (Fig. 5). Smithella is known to participate in the syntrophic oxidation of propionate to acetate for use, for example, by acetoclastic methanogens [39]. As propionate is an important fermentation product in the acidogenic phase of anaerobic digestion, it is not surprising that sequences related to syntrophic propionate consumer were detected. Regarding sulfate-reducing bacteria (SRB), high number of sequences were related to the incompletely oxidizing Desulfobulbus and Desulfovibrio, as well as the completely oxidizer Desulfonema (Fig. 5). The presence of OTUs related to these genera strongly indicates the role of them in sulfate reduction using hydrogen and/or organic acids from fermentative metabolism. In addition to sulfate, SRB have been found to use many other electron acceptors and moreover can promote dissimilatory reduction of nitrate to ammonium as well as respiration under microaerophilic conditions [30].
The third largest phylum was the Firmicutes comprising 814 sequences or 12 % of all bacteria (Fig. 3). A majority of sequences (674 sequences), nearly 11 % of the total, were related to the class Clostridia (Fig. 4), while the class Bacilli was represented by only five sequences. Two major representative genera were Clostridium sensustricto (family Clostridiaceae) and Clostridium XI (family Peptostreptococcaceae) with 45 and 34 sequences, respectively (Fig. 5). Members of these genera are ubiquitous chemoorganotrophic microorganisms, important participants of anaerobic degradation of fermentative metabolism of organic compounds. The metabolites produced, such as H2, CO2 and organic acids can be used by other members of the anaerobic microbial community, such homoacetogens, sulfate-reducing bacteria, denitrifiers and methanogens. So the presence of these sequences pointed out the occurrence of fermentative metabolism in the anaerobic chamber, as expected.
In minor proportion (3–5 %), there were the phyla Chloroflexi, Verrucomicrobia and Acidobacteria (Fig. 3). Chloroflexi comprised 321 sequences, with all of them belonging to the family Anaerolineaceae. Sequences related to Anaerolineaceae have been found in anaerobic environments, such as UASB reactors treating high-strength organic wastewaters, and their presence suggest their contribution to the degradation of carbohydrates and other cellular components, such as amino acids [40]. Among the few cultivable strains, the most representative genera identified in this study were sequences related to Longilinea followed by Leptolinea, with 108 and 71 sequences, respectively (Fig. 5). Members of both genera are obligate anaerobes, non-spore-forming, gram negative, multicellular filamentous microorganisms that ferment carbohydrates and peptides, and can grow in co-culture with hydrogenotrophic methanogens [41, 42]. In the anaerobic chamber, sequences related to these microorganisms may have contributed to fermentation of carbohydrates and peptides present in the sewage, releasing metabolites used by other members of microbial community.
The phylum Verrucomicrobia comprised 301 sequences with the majority (269 sequences) belonging to Subdivision3_genera_incertae_sedis (class Subdivision 3), as also observed in the anoxic chamber. The phylum Acidobacteria comprised 221 sequences with the majority (80 sequences) related to the genus Holophaga. Sequences related to this phylum have been recovered from diverse environments including wastewater treatment plants, and it was also observed in the anoxic chamber. The type strain of the genus Holophaga, Holophaga foetida, is obligate anaerobic, homoacetogenic bacterium that uses, for example, pyruvate and trihydroxybenzenes as organic sources [34]. The presence of sequences related to Holophaga may indicate the role of this organism in pyruvate metabolism, a common intermediate of anaerobic metabolism, as well as in anaerobic degradation of aromatic compounds that could be present in the sewage.
In summary, the selection of sequences belonging to the phyla Synergistetes followed by Deltaproteobacteria and Firmicutes reveals the predominance of fermentative metabolism of organic compounds by fermenting peptide, amino acid and carbohydrate bacteria, mainly related to Aminobacterium as well as Aminomonas, Synergistes, Subdivision 3 genera incertae sedis and, in minor proportion, Longilinea, Leptolinea and Clostridium, with production of intermediate metabolites which could have been used by other members of the anaerobic microbial community, such as Syntrophorhabdus, syntrophic propionate consumer Smithella, syntrophic pyruvate degrader Holophaga and sulfate-reducing bacteria Desulfobulbus and Desulfovibrio.
Overall assessment of the phenomena occurring in the system
The presence of a consistent denitrifying microbiota involved with sulfur and mixotrophic metabolism in the anoxic chamber in phase I, and the lack of evidence of denitrifying community in the anaerobic chamber in phase II are very important data to better assess the phenomena occurring inside the system. The presence of sulfide-oxidizing as well as mixotrophic denitrifiers in the anoxic chamber showed that the system’s configuration was successful in promoting denitrification at this region, even though physicochemical analyses did not detect this activity. Based on these results, it is reasonable to assume that the hypothesis (4) presented in the “Results and discussion” section was most probably the one occurring inside reactor RA. Unknown hydrodynamics of the system may have caused an intense mixing in the intermediary chamber of RA, in such a way that sampling points in this region were subject to samples already denitrified by the anoxic chamber. The fast consumption of nitrates by the biomass in the anoxic chamber may also have contributed to the phenomenon. The contact of nitrates with the anaerobic chamber was not related to the observed denitrification, though. These results indicate that pyrosequencing is indeed a powerful tool to assess the microbial panorama of a complex system, in order to elucidate aspects that could not be determined otherwise, reinforcing the importance of the coupling between physicochemical and molecular biology techniques and providing new insights in the improvement of the system. In this way, pyrosequencing analyses showed that hydrodynamics of the intermediate chamber of RA is one of the drawbacks of the proposed system, requiring measures for better performance in this regard.
Conclusions
This study demonstrated the usefulness of the pyrosequencing technique to evaluate a bioreactor in which only physicochemical results were not enough to describe the observed phenomena. In the anoxic chamber, results showed a microbial community directly and indirectly involved in sulfur-driven denitrification, but due to fast use of nitrates or hydrodynamics, denitrification was not clearly distinguished by physicochemical analyses. In the anaerobic chamber, pyrosequencing showed a typical anaerobic microbial community, and so the hypothesis of denitrification occurrence was not corroborated. These results are important, since they elucidated aspects of a complex pilot-scale bioreactor, contributing to its future improvement.
References
Foresti E, Zaiat M, Vallero M (2006) Anaerobic processes as the core technology for sustainable domestic wastewater treatment: consolidated applications, new trends, perspectives, and challenges. Rev Environ Sci Biotechnol 5:3–19
von Sperling M (2015) Comparison of simple, small, full-scale sewage treatment systems in Brazil: UASB–maturation ponds–coarse filter; UASB–horizontal subsurface-flow wetland; vertical-flow wetland (first stage of French system). Water Sci Technol 71(3):329–337
Moraes BS, Orrú JG, Foresti E (2013) Nitrogen and sulfide removal from effluent of UASB reactor in a sequencing fed-batch biofilm reactor under intermittent aeration. J Biotechnol 164(3):378–385
Gonzalez-Martinez A, Osorio F, Rodriguez-Sanchez A, Martinez-Toledo MV, Gonzalez-Lopez J, Lotti T, van Loosdrecht MC (2015) Bacterial community structure of a lab-scale anammox membrane bioreactor. Biotechnol Prog 1:186–193
Sanz JL, Kochling T (2007) Molecular biology techniques used in wastewater treatment: an overview. Process Biochem 42:119–133
Glenn TC (2011) Field guide to next generation DNA sequencers. Mol Ecol Resour 15:759–769
Hu M, Wang X, Wen X, Xia Y (2012) Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour Technol 117:72–79
Zhang T, Shao MF, Ye L (2012) 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6:1137–1147
Park S, Yu J, Byun I, Cho S, Park T, Lee T (2011) Microbial community structure and dynamics in a mixotrophic nitrogen removal process using recycled spent caustic under different loading conditions. Bioresour Technol 102:7265–7271
American Public Health Association/American Water Works Association/Water Environment Federation (APHA/AWWA/WEF) (2005) Standard methods for the examination of water and wastewater, 21st edn. Washington DC, USA
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of dna and rna from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66(12):5488–5491
Sul WJ, Cole JR, Jesus EC, Wang Q, Farris RJ, Fish JA, Tiedje JM (2011) Bacterial community comparisons by taxonomy-supervised analysis independent of sequence alignment and clustering. Proc Natl Acad Sci USA 35:14637–14642
Cardenas E, Wei-Min WU, Leigh ML, Carley J, Carroll S, Gentry T, Luo J, Watson D, Gu B, Ginder-Vogel M, Kitanidis PK, Jardine PM, Zhou J, Criddle CS, Marsh TL, Tiedje JM (2010) Significant association between sulfate-reducing bacteria and uranium-reducing microbial communities as revealed by a combined massively parallel sequencing-indicator species approach. Appl Environ Microbiol 76(20):6778–6786
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methé B, DeSantis TZ, Human Microbiome Consortium, Petrosino JF, Knight R, Birren BW (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acid Res 35:7188–7196
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71(3):1501–1506
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) A naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Lemos LN, Fulthorpe RR, Triplett EW, Roesch LFW (2011) Rethinking microbial diversity analysis in the high throughput sequencing era. J Microbiol Meth 86:42–51
Campbell BJ, Engel AS, Porter ML, Takai K (2006) The versatile ε-proteobacteria: keyplayers in sulphidic habitats. Nat Rev Microbiol 4:458–468
Collado L, Levican A, Perez J, Figueras MJ (2011) Arcobacterdefluvii sp. nov., isolated from sewage samples. Int J Syst Evol Microbiol 61(9):2155–2161
Kodama Y, Watanabe K (2004) Sulfuricurvum kujiense gen. nov., sp. nov., a facultatively anaerobic, chemolithoautotrophic, sulfur-oxidizing bacterium isolated from an underground crude-oil storage cavity. Int J Syst Evol Microbiol 54(6):2297–2300
De Gusseme B, De Schryver P, De Cooman M, Verbeken K, Boeckx P, Verstraete W, Boon N (2009) Nitrate-reducing, sulfide-oxidizing bacteria as microbial oxidants for rapid biological sulfide removal. FEMS Microbiol Ecol 67(1):151–161
McLellan SL, Huse SM, Mueller-Spitz SR, Andreishcheva EN, Sogin ML (2010) Diversity and population structure of sewage derived microorganisms in wastewater treatment plant influent. Environ Microbiol 12(2):378–392
Mahmood Q, Zheng P, Hu B, Jilani G, Azim MR, Wu D, Liu D (2009) Isolation and characterization of Pseudomonas stutzeri QZ1 from an anoxic sulfide-oxidizing bioreactor. Anaerobe 15(4):108–115
Wen Y, Ren Y, Wei C-H, Li K-Y, Lin F-M, Chen X-Y (2010) A study on nitrogen removal efficiency of Pseudomonas stutzeri strains isolated from an anaerobic/anoxic/oxic wastewater treatment process. Afr J Biotechnol 9(6):869–873
Martin-Carnahan A, Joseph SW (2005) Genus I. Aeromonas Stanier 1943, 213AL. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey’s manual of systematic bacteriology, 2nd edn. vol 2 (The Proteobacteria), part B (The Gammaproteobacteria), Springer, New York, pp 557–558
Lim YW, Lee SA, Kim SB, Yong HY, Yeon SH, Park YK, Jeong DW, Park JS (2005) Diversity of denitrifying bacteria isolated from Daejeon sewage treatment plant. J Microbiol 43(5):383–390
Fischer-Romero C, Tindall BJ, Jüttner F (1996) Tolumonasauensis gen. nov., sp. nov., a toluene-producing bacterium from anoxic sediments of a freshwater lake. Int J Syst Bacteriol 46(1):183–188
Dannenberg S, Kroder M, Dilling W, Cypionka H (1992) Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch Microbiol 158(2):93–99
Bergmann GT, Bates ST, Eilers KG, Lauber CL, Caporaso JG, Walters WA, Knight R, Fierer N (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem 43(7):1450–1455
Chouari R, Le Paslier D, Dauga C, Daegelen P, Weissenbach J, Sghir A (2005) Novel major bacteria candidate division within a municipal anaerobic sludge digester. Appl Environ Microbiol 71(4):2145–2153
Gonzalez-Martinez A, Rodriguez-Sanchez A, Muñoz-Palazon B, Garcia-Ruiz MJ, Osorio F, van Loosdrecht MC, Gonzalez-Lopez J (2015) Microbial community analysis of a full-scale DEMON bioreactor. Bioprocess Biosyst Eng 38(3):499–508
Liesack W, Bak F, Kreft JU, Stackebrandt E (1994) Holophaga foetida gen. nov., sp. nov., a new, homoacetogenic bacterium degrading methoxylated aromatic compounds. Arch Microbiol 162(1–2):85–90
Vartoukian SR, Palmer RM, Wade WG (2007) The division “Synergistes”. Anaerobe 13(3–4):99–106
Ramsay IR, Pullammanappallil PC (2001) Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation 12(4):247–257
Qiu YL, Sekiguchi Imachi H, Kamagata Y, Tseng IC, Cheng SS, Ohashi A, Harada H (2004) Identification and isolation of anaerobic, syntrophic phthalate isomer-degrading microbes from methanogenic sludges treating wastewater from terephthalate manufacturing. Appl Environ Microbiol 70:1617–1626
Hofmann K, Hammer E (1999) Anaerobic formation and degradation of toxic aromatic compounds in agricultural and communal sewage deposits. Chemosphere 38(11):2561–2568
Ariesyady HD, Ito T, Yoshiguchi K, Okabe S (2007) Phylogenetic and functional diversity of propionate-oxidizing bacteria in an anaerobic digester sludge. Appl Microbiol Biotechnol 75(3):673–683
Yamada T, Imachi H, Ohashi A, Harada H, Hanada S, Kamagata Y, Sekiguchi Y (2007) Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia. Int J Syst Evol Microbiol 57(10):2299–2306
Yamada T, Sekiguchi Y, Hanada S, Imachi H, Ohashi A, Harada H, Kamagata Y (2006) Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new clases Anaerolineae classis nov. And Caldilineae classis nov. In the bacterial phylum Chloroflexi. Int J Syst Evol Microbiol 56(6):1331–1340
Yamada T, Sekiguchi Y, Imachi H, Kamagata Y, Ohashi A, Harada H (2005) Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl Environ Microbiol 71(11):7493–7503
Acknowledgments
This work was funded by FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo (Process numbers: 2007/58659-7, 2010/03286-4 and 2010/01735-6) and by CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saia, F.T., Souza, T.S.O., Duarte, R.T.D. et al. Microbial community in a pilot-scale bioreactor promoting anaerobic digestion and sulfur-driven denitrification for domestic sewage treatment. Bioprocess Biosyst Eng 39, 341–352 (2016). https://doi.org/10.1007/s00449-015-1520-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1520-6