Abstract
Based on the screening of biocatalysts and reaction conditions including solvent, water content, temperature, enzyme loading, and reaction time, lipase from porcine pancreas (PPL) showed the prominent promiscuity for the Knoevenagel condensation between 1,3-dihydroindol-2-one heterocycle and aromatic aldehydes. Under the optimized procedure, both electron-withdrawing and electron-donating substituent of aldehydes substrates could react efficiently, and benzylidene-indolin-2-ones were obtained in excellent yields (75.0–96.6 %).
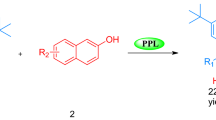
Graphical abstract
Benzylidene-indolin-2-ones derivatives were efficiently synthesized by the Knoevenagel condensation between various aromatic aldehydes and 1,3-dihydroindol-2-one catalyzed by lipase from porcine pancreas with excellent yields obtained.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzylidene-indolin-2-one derivatives as a kind of alkaloids are found to possess significant pharmacological and chemical activity in medicinal and organic chemistry [1–3]. For example, they are important intermediates for the synthesis of sunitinib which is currently widely used in the clinics as a multi-targeting tyrosine kinase inhibitor with anti-angiogenic activity [4–6]. These derivatives were usually obtained by the Knoevenagel condensation of aromatic aldehydes with 1,3-dihydroindol-2-one catalyzed by organic base such as pyridine and piperazine [6–9], or facilitated by MW irradiation [9, 10], phase transfer catalysts [11] and functional ionic liquids [12]. Considering the importance of benzylidene-indolin-2-ones, we think it is still significant to develop more efficient and environmentally sound technologies to prepare these compounds.
Enzyme as a kind of biocatalysts has attracted remarkable attention due to their high selectivity and environmental friendliness [13, 14]. Recently, it was found that enzyme had the promiscuity ability to catalyze C–C bond forming reactions including Aldol condensation, Knoevenagel condensation, Mannich reaction, Michael addition, Henry reaction and so on [15–17]. Recently, we also reported the lipoprotein lipase from Aspergillus niger could efficiently catalyze the Knoevenagel condensation [18]. As far as we know the active methylene substrates of lipase-catalyzed Knoevenagel reactions are limited to chain compounds, and there is no literature reported systematically about the reaction of heterocyclic active methylene substrates with carbonyl compounds yet [19–21]. In continuation of our interest in lipase-catalyzed organic synthesis [22–24], herein, we reported the first discovery that lipase from porcine pancreas (PPL) had the efficient biocatalytic promiscuity for the Knoevenagel condensation between aromatic aldehydes and 1,3-dihydroindol-2-one heterocycle, and a series of benzylidene-indolin-2-ones products were synthesized in excellent yields.
Materials and methods
Materials
Porcine pancreas lipase (PPL) and Candida rugosa lipase (CRL) were purchased from Sigma. Novozym 435 (lipase B from Candida antarctica, immobilized on a macroporous acrylic resin) was purchased from Novo Nordisk. Pseudomonas cepacia lipase (PCL) was kindly donated from Amano Pharmaceuticals. Lipase lipoprotein from Aspergillus niger (LPL) was purchased from Ningxia Sunson group corporation. Other reagents were commercially available and were used without further purification.
Characterization
Melting points were determined on WRS-1B digital melting point apparatus and were not corrected. The NMR spectra were measured on a Bruker Advance 2B 400 MHz instrument with CDCl3 or (CD3)2SO as the solvent and TMS as internal standard (shown in supporting information). Chemical shifts (δ) were reported in ppm and coupling constants (J) in Hz.
General procedure for lipase-catalyzed Knoevenagel reaction
A mixture of aromatic aldehyde (2 mmol), lipase (125 mg) and 1,3-dihydroindol-2-one (2 mmol) in DMSO (4 mL) and deionized water (1 mL) was stirred at 45 °C. The reaction was monitored by thin layer chromatography (TLC) and terminated by filtering off the catalyst. The filtrate was diluted with ethyl acetate (10 mL) and washed with water (5 mL × 2). The aqueous phase was back-extracted with ethyl acetate (10 mL × 2). Combined organic phase was washed with water and dried over anhydrous Na2SO4, then concentrated to give the crude product which was further purified by flash column chromatography (ethyl acetate/petroleum ether = 1:9–1:2, v/v).
Results and discussion
Catalytic activities of different lipases for Knoevenagel condensation
In initial research, the reaction of 4-nitrobenzaldehyde and 1,3-dihydroindol-2-one as model reaction was conducted according to the procedure developed in our previous research [18] (Scheme 1) , while only 30.0 % yield was obtained. As confirmed the heterocyclic structure of the substrate played crucial effect for the lipase-catalyzed Knoevenagel reaction compared with acyclic active methylene compounds. Five lipases from different sources were screened to catalyze this model reaction and the results were shown in Table 1. Poor yield was reached in the absence of lipase (Table 1, entry 1), and PPL (Table 1, entry 2) showed the best activity with 65.6 % yield obtained. Meanwhile, CRL, PCL, LPL, Novozym435 (Table 1, entries 3–6) also showed the ability to catalyze the reaction, while their catalytic activities were less efficient. Therefore, PPL was consequently used throughout the further studies (Scheme 1).
Effect of solvents on the Knoevenagel condensation catalyzed by PPL
Based on the concept that reaction media is one of the key factors influencing the enzyme catalytic performance [25–27], seven commonly used organic solvents were investigated (Table 2). The results indicated that DMSO was the best reaction medium for the model reaction, while no clear correlation between solvent polarity and the enzyme activity was observed, as is consistent with the literature [19, 28] and our previous report [18].
Effect of water content on the Knoevenagel condensation catalyzed by PPL
The role of water content in the reaction medium is very important, as it may dramatically influence both the stability and activity of the enzymes and, probably, their conformational flexibility [29, 30]. Previous reports also demonstrated that water was essential in the enzymatic reactions of carbon–carbon bond formation [19, 28, 31, 32]. We found that the yield could be raised up to 65.6 % when the water content increased to 20 % (v/v). However, once the water content surpasses 20 %, the yield decreased evidently and this is probably due to the insolubility of the substrates. This result is different from the Knoevenagel condensation of aromatic aldehydes with acyclic active methylene compounds catalyzed by LPL, where the highest yields were obtained without the addition of water [18] (Fig. 1).
Effect of temperature on the Knoevenagel condensation catalyzed by PPL
Temperature may significantly influence the activity and stability of a biocatalyst [18]. To optimize the experimental conditions, we then examined the effect of temperature on this reaction. The yield was increased from 37.0 to 94.2 % and declined after 45 °C, which may be due to the conformational change of the active site of PPL caused by excessively high temperature. Thus, 45 °C was selected as the optimum temperature for further experiments (Fig. 2).
Effect of enzyme loading and reaction time on the Knoevenagel condensation catalyzed by PPL
To improve the reaction yields, enzyme loading was further investigated (Table 3). The yield of product was improved greatly by increasing the enzyme amount from 25 to 125 mg and reached a plateau after 125 mg. The influence of reaction time was also considered. As showed in Table 3, the yield was increased from 5 to 10 h. After 10 h, the yield increased negligibly, which indicated the reaction nearly reached reaction equilibrium.
PPL-catalyzed Knoevenagel condensation of various aromatic aldehydes and 1,3-dihydroindol-2-one (Scheme 2).
Based on the above results, we extended the reaction with a series of aromatic aldehydes to investigate the generality for the scope of this new biocatalytic procedure, as was summarized in Table 4. All the products were characterized by melting points and NMR spectra that were consistent with the literature data. It was found that both electron-withdrawing and electron-donating substituents of aromatic aldehydes (Table 4, entries 1–9) could effectively participate in PPL catalyzed Knoevenagel reactions to give benzylidene-indolin-2-one derivatives in excellent yields (89.8–94.2 %). While in reported enzymatic Knoevenagel condensation research, usually only low to moderate yields were obtained for substrates bearing electron-donating groups, and some procedures were even limited to aldehydes with electron-withdrawing groups [18, 20-21, 28]. Furthermore, the aromatic α,β-unsaturated aldehyde, Cinnamic aldehyde (Table 4, entry 11) with 1,3-dihydroindol-2-one also could be completed efficiently with high yield obtained (96.6 %). Compared with the common chemistry method, our procedure was performed in a lower reaction temperature, a relatively longer reaction time was needed, while comparable yields could be obtained. Generally, the mixture of Z- and E- benzylidene-indolin-2-ones could be formed when using traditional chemical catalysts [2, 6, 7, 9, 12]. It is also consistent with the common enzymatic Knoevenagel condensation that Z- and E- configuration products are obtained, and the ratio are different for various substrates [19, 28]. Noteworthily, satisfactory yield was also obtained with high selectivity of E-configuration for 2,6-dichlorobenzaldehyde (Table 4, entry 10), as could be attributed to the enhanced steric hindrance with the number of substituent adjacent to the –CHO increased [28, 33]. Moreover, the developed method can be applied to a gram-scale synthesis with high yield obtained (Table 4, entry 12).
Spectroscopic date for representative products
(E)-3-(2,6-Dichlorobenzylidene)indolin-2-one (10): mp 180–181 °C, 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 7.63 (s, 1H), 7.44 (d, J = 8.1 Hz, 2H), 7.35–7.30 (m, 1H), 7.22 (t, J = 7.7 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H).
(E)-1,3-dihydro-3-(3-phenyl-2-propen-1-ylidene)2H-Indol-2-one (11): mp 208–209 °C, 1H NMR (400 MHz, DMSO) δ 10.5 (s, 1H), 8.48 (dd, J = 15.6, 11.6 Hz, 1H), 7.63–7.55 (m, 4H), 7.45 (t, J = 7.4 Hz, 2H), 7.41–7.35 (m, 1H), 7.22–7.16 (m, 2H), 6.96 (td, J = 7.6, 0.8 Hz, 1H), 6.82 (d, J = 7.6 Hz, 1H).
(Z)-1,3-dihydro-3-(3-phenyl-2-propen-1-ylidene)2H-Indol-2-one (11): mp 205–206 °C, 1H NMR (400 MHz, DMSO) δ 10.5 (s, 1 H), 7.99 (d, J = 7.6 Hz, 1H), 7.83–7.78 (m, 2H), 7.75 (dd, J = 15.2, 12.4 Hz, 1H), 7.48–7.35 (m, 4H), 7.30 (d, J = 12.0 Hz, 1H), 7.24 (td, J = 7.6, 0.8 Hz, 1H), 7.02 (td, J = 7.6, 1.2 Hz, 1H), 6.87 (d, J = 8.0 Hz, 1H).


Conclusions
In conclusion, we reported the first discovery that PPL had the effective promiscuity for the synthesis of benzylidene-indolin-2-ones via Knoevenagel condensations. Both electron-withdrawing and electron-donating substituent of aldehydes substrates could be proceeded smoothly and excellent yields could be obtained. This new procedure not only expanded the application of lipases to new chemical transformations, but also could be developed into a potentially valuable method for pharmaceutical & organic synthesis.
References
Mukherjee R, Jaggi M, Rajendran P, Siddiqui MJA, Srivastava SK, Vardhan A, Burman AC (2004) Betulinic acid and its derivatives as anti- angiogenic agent. Bioorg Med Chem Lett 14:2181–2184
Xu TT, Wang SY, Ji SJ (2006) Facile synthesis of Z/E-3-arylmethylidene-2,3-dihydro- indol-2-one under solvent-free conditions. Chin J Org Chem 26:1414–1417
Porcs-Makkay M, Volk B, Kapiller-Dezsofi R, Mezei T, Simig G (2004) New routes to oxindole derivatives. Monatsh Chem 135:697–711
Du TP, Zhu GG, Zhou J (2012) A facile method for the synthesis of 3-alkyloxindole. Lett Org Chem 9:225–232
Botta G, De Santis LP, Saladino R (2012) Current advances in the synthesis and antitumoral activity of SIRT1-2 inhibitors by modulation of p53 and pro-apoptotic proteins. Curr Med Chem 19:5871–5884
Zhang W, Go ML (2009) Functionalized 3-benzylidene-indolin-2-ones: inducers of NAD(P)H-quinone oxidoreductase 1 (NQO1) with antiproliferative activity. Bioorg Med Chem 17:2077–2090
Olgen S, Gotz C, Jose J (2007) Synthesis and biological evaluation of 3-(substituted-benzylidene)-1,3-dihydro-indolin derivatives as human protein kinase CK2 and p60(c-Src) tyrosine kinase inhibitors. Biol Pharm Bull 30:715–718
Kuchar M, Steinbach J, Wuest F (2009) Synthesis and radiopharmacological investigation of 3-[4’-[F-18] fluorobenzylidene] indolin-2-one as possible tyrosine kinase inhibitor. Bioorg Med Chem 17:7732–7742
Ding K, Wang GP, Deschamps JR, Parrish DA, Wang SM (2005) Synthesis of spirooxindoles via asymmetric 1,3-dipolar cycloaddition. Tetrahedron Lett 46:5949–5951
Villemin D, Martin B (1998) Potassium fluoride on alumina: dry synthesis of 3-arylidene-1,3-dihydro-indol-2-one under microwave irradiation. Synth Commun 28:3201–3208
Wang XS, Zeng ZS, Li YL, Shi DQ, Tu SJ, Wei XY, Zong ZM (2005) Synthesis of E-arylidene-2,3-dihydroindol-2-one derivatives in aqueous media catalyzed by triethylbenzylammonium chloride phase-transfer catalyst. Chin J Org Chem 25:1598–1601
Hu Y, Kang H, Zeng BW, Wei P, Huang H (2008) Facile synthesis of 3-arylidene-1,3-dihydroindol-2-ones catalysed by a Bronsted acidic ionic liquid. J Chem Res 11:642–643
Friedrich S, Hahn F (2015) Opportunities for enzyme catalysis in natural product chemistry. Tetrahedron 71:1473–1508
Reetz MT (2013) Biocatalysis in organic chemistry and biotechnology: past, present, and future. J Am Chem Soc 135:12480–12496
Miao YF, Rahimi M, Geertsema EM, Poelarends GJ (2015) Recent developments in enzyme promiscuity for carbon-carbon bond-forming reactions. Curr Opin Chem Biol 25:115–123
Kapoor M, Gupta MN (2012) Lipase promiscuity and its biochemical applications. Process Biochem 47:555–569
Ding Y, Huang H, Hu Y (2013) New progress on lipases catalyzed C–C bond formation reactions. Chin J Org Chem 33:905–914
Ding Y, Ni X, Gu MJ, Li S, Huang H, Hu Y (2015) Knoevenagel condensation of aromatic aldehydes with active methylene compounds catalyzed by lipoprotein lipase. Catal Commun 64:101–104
Wang Z, Wang CY, Wang HR, Zhang H, Sun YL, Ji TF, Wang L (2014) Lipase-catalyzed Knoevenagel condensation between α, β-unsaturated aldehydes and active methylene compounds. Chin Chem Lett 25:802–804
Jiang L, Yu HW (2014) Enzymatic promiscuity: Escherichia coli BioH esterase-catalysed Aldol reaction and Knoevenagel reaction. Chem Res Chin Univ 30:289–292
Lai YF, Zheng H, Chai SJ, Zhang PF, Chen XZ (2010) Lipase-catalysed tandem Knoevenagel condensation and esterification with alcohol cosolvents. Green Chem 12:1917–1918
Hu Y, Jiang XJ, Wu SW, Jiang L, Huang H (2013) Synthesis of vitamin E succinate by interfacial activated Candida rugosa lipase encapsulated in sol–gel materials. Chin J Catal 34:1608–1616
Liu WM, Hu Y, Jiang L, Zou B, Huang H (2012) Synthesis of methyl (R)-3-(4-fluorophenyl)glutarate via enzymatic desymmetrization of a prochiral diester. Process Biochem 47:1037–1041
Jiang XJ, Hu Y, Jiang L, Zou B, Huang H (2013) Optimization of enzymatic synthesis of l-ascorbyl palmitate by solvent engineering and statistical experimental designs. Biotechnol Bioproc Eng 18:350–357
Laane C, Boeren S, Vos K, Veeger C (2009) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 102:2–8
Salihu A, Alam MZ (2015) Solvent tolerant lipases: a review. Process Biochem 50:86–96
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Hu W, Guan Z, Deng X, He YH (2012) Enzyme catalytic promiscuity: the papain-catalyzed Knoevenagel reaction. Biochimie 94:656–661
Zaks A, Klibanov AM (1988) The effect of water on enzyme action in organic media. J Biol Chem 263:8017–8021
Narayan VS, Klibanov AM (1993) Are water-immiscibility and apolarity of the solvent relevant to enzyme efficiency? Biotechnol Bioeng 41:390–393
Li K, He T, Li C, Feng XW, Wang N, Yu XQ (2009) Lipase-catalysed direct Mannich reaction in water: utilization of biocatalytic promiscuity for C–C bond formation in a “one-pot” synthesis. Green Chem 11:777–779
Li C, Feng XW, Wang N, Zhou YJ, Yu XQ (2008) Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem 10:616–618
Sun FL, Xu G, Wu JP, Yang LR (2006) Efficient lipase-catalyzed kinetic resolution of 4-arylmethoxy-3-hydroxybutanenitriles: application to an expedient synthesis of a statin intermediate. Tetrahedron Asymmetry 17:2907–2913
Acknowledgments
This research was financially supported by the Hi-Tech Research and Development Program of China (Grant No. 2011AA02A209) and National Science Foundation for Distinguished Young Scholars of China (Grant No. 21225626).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, Y., Xiang, X., Gu, M. et al. Efficient lipase-catalyzed Knoevenagel condensation: utilization of biocatalytic promiscuity for synthesis of benzylidene-indolin-2-ones. Bioprocess Biosyst Eng 39, 125–131 (2016). https://doi.org/10.1007/s00449-015-1496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1496-2