Abstract
The transesterification of phytosterol and soybean oil was performed using Novozym 435 in supercritical carbon dioxide (SC-CO2). The transesterification reaction was conducted in soybean oil containing 5–25 % phytosterol at 55–95 °C and free-water solvent. The effects of temperature, reaction time, phytosterol concentration, lipase dosage and reaction pressure on the conversion rate of transesterification were investigated. The optimal reaction conditions were the reaction temperature (85 °C), reaction time (1 h), phytosterol concentration (5 %), reaction pressure (8 Mpa) and lipase dosage (1 %). The highest conversion rate of 92 % could be achieved under the optimum conditions. Compared with the method of lipase-catalyzed transesterification of phytosterol and soybean oil at normal pressure, the transesterification in SC-CO2 reduced significantly the reaction temperature and reaction time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytosterols are known to have a hypocholesterolemic effect by lowering plasma total and low-density lipoprotein (LDL) cholesterol levels without affecting plasma high-density lipoprotein (HDL) cholesterol concentration [1, 2]. It is generally believed that plasma total and LDL cholesterol levels can be reduced up to 10–20 % with a dose of 1.5–3.0 g phytosterols/day in humans [3, 4].
Because of such health attributes of phytosterols, they have been recently applied as a dietary supplement in various types of food products such as margarine, spread, salad dressing, edible oil and milk. However, phytosterols have limited usage as a dietary supplement since they possess very low solubility in edible oil and have very high melting point. Therefore, to overcome such problems of free forms of phytosterols, phytosteryl esters are preferred in food formulations, since phytosteryl esters have much greater solubility in oils and much lower melting point compared to the corresponding phytosterols. They can be easily incorporated into a wide variety of diets and fat-based food products. They provide easily the daily intake of phytosterol needed for sufficient reduction of cholesterol absorption. Moreover, many studies in recent years have shown that phytosteryl esters can also effectively reduce plasma total and LDL cholesterol levels in a similar manner as phytosterols [5–8].
Various attempts have been made to develop phytosterol-containing edible oils. Special margarines fortified with steryl esters are now commercially available as functional foods with the ability to reduce both total and low-density lipoprotein (LDL) cholesterol levels [9]. Phytosteryl esters of FAs are presently synthesized by chemical esterification and transesterification, but the chemical method involves some problems such as the formation of side products, excessive energy cost, need for removal of catalyst from the product and so on (e.g., dehydrated or oxysterols).
Enzymatic methods can overcome these problems which allow mild reaction conditions and no chemical waste is produced [10–13]. Hence, in recent years, several enzymatic procedures using lipases (triacylglycerol acylhydrolases, E.C. 3.1.1.3) obtained from diverse kinds of microbial sources for the preparation of phytosteryl esters of FAs have been developed to overcome such shortcomings of chemical methods [14–16]. The lipase-catalyzed esterification and transesterification were carried out successfully in organic solvents or oil/water two phase and in oil itself [17].
In combination with an environmentally benign and safe medium, such as supercritical carbon dioxide (SC-CO2), enzymatic catalysis makes supercritical fluids extremely attractive to the food industry [18]. In particular, it has demonstrated that lipases can be used in the presence of SC-CO2 to conduct several reactions valuable to the food industry [17] such as hydrolysis, esterifications, transesterifications, acylations, glycerolysis and randomization of fats, among others.
The main reason for the frequent use of lipase in supercritical fluids (SF) is the increased solubility of hydrophobic lipid substrates in nonpolar SF and the reversal of hydrolysis reactions in favor of synthesis, such as esterification and transesterification. The separation and recovery of the enzyme catalysts after the reaction are possible due to their insolubility in nonpolar SF. Nonpolar substrates such as lipids are more soluble in dense gases than in aqueous solution. Moreover, the advantages of carbon dioxide as a supercritical fluid include its low toxicity, low cost, lack of flammability, lack of reactivity, wide range of solvent properties at different pressures and temperatures and improved properties of separated components in certain cases [19].
The present study focuses on synthesizing phytosteryl esters of soybean oil by the lipase-catalyzed transesterification reaction between phytosterols and soybean oil. The effects of reaction parameters on the conversion rate of transesterification in SC-CO2 were evaluated, such as temperature, reaction time, phytosterol concentration, reaction pressure, lipase dosage and stirring speed, and then the optimal reaction conditions were determined.
Materials and methods
Materials
Novozym 435 (immobilized Candida antarctica lipase, 10,000 PLU/g) was obtained from Novozymes (China) Investment Co. Ltd. Refined soybean oil was obtained from the local supermarket. Phytosterol was supplied by the Sky Bioengineering Co., Ltd. (Xian, China). The phytosterol was analyzed and found to contain three major phytosterol analogs (β-sitosterol 48.1 %, campesterol 24.7 %, dihydrobrassicasterol 22.2 %) by GC in our laboratory. All other chemicals were commercially available and of analytical grade.
Methods
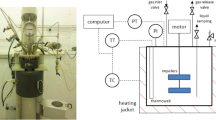
A certain amount of phytosterol, soybean oil and Novozym 435 lipase was taken in a high-pressure reactor. Then the high-pressure reaction kettle was sealed and CO2 introduced for tightness test. The experiment was conducted by introducing CO2 and adjusting the pressure, temperature, stirring speed and reaction time. After the reaction, the high-pressure reactor was cooled to room temperature, the air was discharged from the reactor and then the products from the reactor were separated. Transesterified samples were collected and analyzed. The range for each factor in this research was preliminarily investigated for optimization.
Analysis
The composition of the reaction products was analyzed with a GC using a DB-1ht (0.32 mm × 0.1 μm × 5 m) column (Agilent Technologies, Palo Alto, CA). The column temperature was raised from 150 to 370 °C at a rate of 15 °C/min and the split ratio was 80:1. The temperature of the injector and detector was both set at 370 °C. The carrier gas was helium at a flow rate of 7.0 mL/min.
Degree of conversion
The sample from the reaction was subjected to GC to calculate the conversion rate (%) using the following equation:
The peaks of β-sitosterol and β-sitosteryl ester were identified by GC using standard reagents. The peak areas were confirmed to be proportional to the concentrations.
Statistical and chemometric methods
All the measurements were carried out in triplicate and mean values and standard deviations were calculated. Differences were considered statistically significant at p < 0.05. The variety values for each compound were compared to the mean of all varieties by calculating a confidence interval. One-way ANOVA was performed for mean differences. Statistical analyses were conducted using the XLSTAT software (http://www.xlstat.com).
Results and discussion
Effect of lipase dosage on the conversion rate
The effect of a different amount of lipase was examined in the range of 0.5–2.5 %, reaction time of 3 h, reaction temperature of 85 °C, reaction pressure of 8 Mpa and phytosterol dosage of 5 %. As shown in Fig. 1, the results show that the conversion rate increased rapidly when the mass amount of lipase was increased up to 1 %, but it did not significantly increase the reaction rate above that amount. The optimal lipase dosage was achieved at 1 % with a conversion at 91.7 %. Obviously, a great amount of lipase provides abundant active sites and sufficient mass contact and consequently leads to a higher conversion rate. When the lipase dosage was more than 1 %, the conversion rate remained unchanged. It may be due to the lack of substrates corresponding to the active sites of enzyme, and the difficulties in maintaining uniform suspension of the biocatalysts. Hence, lipase dosage (1 %) was used in the subsequent experiment. Weber et al. [20] also observed that the enzyme amount increased from 50 to 100 mg can not be obvious increasing the degree of esterification and transesterification.
Effect of reaction temperature on conversion rate
Figure 2 presents the effect of reaction temperature on the conversion rate of transesterification reaction. The enzymatic transesterification was carried out under the following conditions: reaction temperature in the range of 55–95 °C, reaction time of 3 h, phytosterol concentration of 5 %, reaction pressure of 8 Mpa and lipase dosage of 1 %. The results indicate that temperature had a significant effect on the conversion rate. Figure 2 shows that the conversion rate increased by increasing reaction temperature between 55 and 85 °C significantly (p < 0.05). In fact, an increase in temperature reduces the mixture viscosity of substrates and thus reduces mass transfer limitations which favor the interactions between enzyme particles and substrates. Furthermore, the conversion rate decreased with the further increase of temperature at temperature ranges higher than 85 °C significantly (p < 0.05), which could be due to partial denaturation of the enzyme and loss of its hydrolytic activity at higher temperature. Therefore, the highest conversion rate was observed at a temperature of 85 °C.
These observations were in accordance with what was previously shown by Weber et al. [21] in which authors reported that the highest conversion by esterification (89 %) was achieved at 80 °C in vacuo using Novozym 435 as catalyst, whereas conversions at 40 and 60 °C were markedly decreased.
Effect of phytosterol concentration on the conversion rate
The effect of phytosterol concentration on the conversion rate of transesterification reaction is shown in Fig. 3. The effect of phytosterol concentration was examined in the range of 5–25 %, reaction time of 3 h, reaction temperature of 85 °C, reaction pressure of 8 Mpa and lipase dosage of 1 %. The results show that the conversion rate decreased with an increase in phytosterol concentration significantly (p < 0.05). This result can be explained by considering that the phytosterol could not be completely dissolved in soybean oil at phytosterol concentration greater than 5 %; therefore, the transesterification reaction was not carried out perfectly. The results suggest that the optimum phytosterol concentration was 5 %.
Effect of reaction time on the conversion rate
Figure 4 illustrates the effect of reaction time on the conversion rate of transesterification reaction. The effect of reaction time was evaluated between 30 min and 4 h, phytosterol concentration of 5 %, reaction temperature of 85 °C, reaction pressure of 8 Mpa and lipase dosage of 1 %. As presented in Fig. 4, the conversion rate increased with increasing reaction time from 0.5 to 1 h significantly (p < 0.05). On the other hand, the conversion rate increased slowly with further increase of reaction time from 1.5 to 4 h. Therefore, the optimum reaction time was determined at 1 h.
Effect of reaction pressure on the conversion rate
Due to the high solubility in SC-CO2 as the reaction medium, it can greatly reduce the viscosity and improve the reaction speed. Therefore, the reaction pressure is the main factor affecting the conversion rate of the transesterification in SC-CO2. The effect of reaction pressure on the conversion rate of the transesterification was investigated between 6 and 10 Mpa, phytosterol concentration of 5 %, reaction temperature of 85 °C, reaction time of 1 h and lipase dosage of 1 %. As shown in Fig. 5, the conversion rate increased more remarkably with the increase of reaction pressure from 6 to 8 Mpa, and then the conversion rate increased slowly with the increase of reaction pressure. Therefore, the optimum reaction pressure was 8 Mpa. In a previous study, Venskutonis et al. (2008) also reported that in all cases, the yield of the extract increased more than twice after increasing the extraction pressure from 15 to 25 Mpa. However, the increase slows down after further compression of CO2 to 35 Mpa [ 22 ].
Conclusion
Due to the high solubility in SC-CO2, the viscosity of the reaction medium was greatly reduced and the reaction speed was improved. Compared with lipase-catalyzed transesterification of phytosterol and soybean oil at normal pressure, the transesterification in SC-CO2 reduced the reaction temperature and reaction time significantly. The optimal reaction conditions for transesterification of phytosterol and soybean oil in SC-CO2 were determined as follows: reaction temperature 85 °C, reaction time 1 h, phytosterol concentration 5 %, reaction pressure 8 Mpa and lipase dosage 1 %. The highest conversion rate of 92 % could be achieved under the optimum conditions. Therefore, it was concluded that lipase-catalyzed transesterification of phytosterol and soybean oil in SC-CO2 was suitable for producing phytosterol-containing edible oils.
Change history
27 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00449-021-02575-x
References
Lees AM, Mok HY, Lees RS, McCluskey MA, Grundy SM (1977) Plant sterols as cholesterol-lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis 28:325–338
Wester I (2000) Cholesterol-lowering effect of plant sterols. Eur J Lipid Sci Technol 102:37–44
Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R (2003) Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 78:965–978
Miettinen TA, Gylling H (2004) Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann Med 36:126–134
Jones PJH, MacDougall DE, Ntanios F, Vanstone CA (1997) Dietary phytosterols as cholesterol-lowering agents in humans. Can J Physiol Pharmacol 75:217–227
Law M (2000) Plant sterol and stanol margarines and health. Br Med J 320:861–864
Neil HAS, Meijer GW, Roe LS (2001) Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterolenriched fat spread. Atherosclerosis 156:329–337
Weststrate JA, Meijer GW (1998) Plant sterol-enriched margarines and reduction of plasma total and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 52:334–343
Ling WH, Jones PJH (1995) Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci 57:195–206
Lilin L, Wei D, Dehua L, Li W, Zebo L (2006) Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J Mol Catal B Enzym 43:58–62
Zeng CX, Qi SJ, Li ZG, Luo RM (2015) Enzymatic synthesis of phytosterol esters catalyzed by Candida rugosa lipase in water-in-[Bmim]PF6 microemulsion. Bioprocess Biosyst Eng 38:939–946
Li Z, Yang DP, Jiang L, Ji JF (2007) Lipase-catalyzed esterification of conjugated linoleic acid with l-carnitine in solvent-free system and acetonitrile. Bioprocess Biosyst Eng 30:331–336
Negishi S, Hidaka I, Takahashi I, Kunita S (2003) Transesterification of phytosterol and edible oil by lipase powder at high temperature. J Am Oil Chem Soc 80:905–907
Villeneuve P, Turon F, Caro Y, Escoffier R, Barea B, Barouh B (2005) Lipase-catalyzed synthesis of canola phytosterols oleate esters as cholesterol lowering agents. Enzyme Microb Technol 37:150–155
Vu PL, Shin JA, Lim CH, Lee KT (2004) Lipase-catalyzed production of phytosteryl esters and their crystallization behavior in corn oil. Food Res Int 37:175–180
Weber N, Weitkamp P, Mukherjee KD (2002) Cholesterollowering food additives: lipase-catalysed preparation of phytosterol and phytostanol esters. Food Res Int 235:177–181
Jackson MA, King JW, List GR, Neff WE (1997) Lipase-catalyzed randomization of fats and oils in flowing supercritical carbon dioxide. J Am Oil Chem Soc 74:635–639
Gunnlaugsdottir H, Järemo M, Sivik B (1998) Process parameters influencing ethanolysis of cod liver oil in supercritical carbon dioxide. J Supercrit Fluids 12:85–93
King JW (2000) Advances in critical fluid technology for food processing. Food Sci Technol Today 14(4):186–191
Weber N, Weitkamp P, Mukherjee KD (2002) Cholesterol-lowering food additives: lipase-catalysed preparation of phytosterol and phytostanol esters. Food Res Int 35(2–3):177–181
Weber N, Weitkamp P, Mukherjee KD (2001) Steryl and stanyl esters of fatty acids by solvent-free esterification and transesterification in vacuo using lipases from Rhizomucor miehei, Candida antarctica, and Carica papaya. J Agric Food Chem 49:5210–5216
Venskutonis PR, Daukšas E, Sivik B (2008) Use of immobilised lipase from Candida antarctica in supercritical fluid extraction of borage seed Oil. Food Technol Biotechnol 46(2):157–163
Acknowledgments
This work was supported by a grant from the Heilongjiang Provincial Basic Scientific Research Business Foundation: Synthesis of phytosteryl esters by esterification and transesterification in vacuo and without solvent using lipases (No: DADOU2014-2). This work was supported by a grant from the National Natural Science Foundation of China (NSFC): Study on the method of controlling TFAs in oil by orientated hydrogenation and mechanism of molecular reaction under CO2 supercritical state (No: 31271886). This work was also supported by a grant from the Key Laboratory of Soybean Biology in Chinese Ministry of Education Northeast Agricultural University, Harbin, China, 150030. The authors are also grateful to the anonymous referees and the editor for helpful comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, L., Llibin, S., Li, J. et al. Lipase-catalyzed transesterification of soybean oil and phytosterol in supercritical CO2 . Bioprocess Biosyst Eng 38, 2343–2347 (2015). https://doi.org/10.1007/s00449-015-1469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1469-5