Abstract
Volatile fatty acids (VFAs) that can be derived from food wastes were used for microbial lipid production by Chlorella protothecoides in heterotrophic cultures. The usage of VFAs as carbon sources for lipid accumulation was investigated in batch cultures. Culture medium, culture temperature, and nitrogen sources were explored for lipid production in the heterotrophic cultivation. The concentration and the ratio of VFAs exhibited significant influence on cell growth and lipid accumulation. The highest lipid yield coefficient and lipid content of C. protothecoides grown on VFAs were 0.187 g/g and 48.7 %, respectively. The lipid content and fatty acids produced using VFAs as carbon sources were similar to those seen on growth and production using glucose. The techno-economic analysis indicates that the biodiesel derived from the lipids produced by heterotrophic C. protothecoides with VFAs as carbon sources is very promising and competitive with other biofuels and fossil fuels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With depleting reserves of conventional petroleum resources and steadily rising oil prices caused by increasing demand from a growing world population and the rapid industrial development in many countries, there has been growing global interest in developing alternative sources of energy. Biofuels offer much promise on these frontiers. Biodiesel as a biofuel is being considered actively as a promising fossil fuel alternative or supplement, on the basis of its energy efficient proprieties [1–3]. In recent decades, microalgae have been mainly produced and sold as health foods, because of their high content of proteins, vitamins and other nutrient supplements. There has been a great upsurge in studies on microalgae as a source of a wide range of fine chemicals, oils, lipids and polysaccharides [4, 5].
Microbial lipids derived from fatty acids, one of the main cellular constituents produced by acetyl-CoA as precursors in many species of Chlorella, have been studied for 50 years [6–8]. Although microalgae can contain high quantities of microbial lipids [9], their photosynthetic capabilities require more acreage for cultivation compared to other oleaginous microorganisms. Therefore, heterotrophic microalgae have been receiving increased attention in recent years. Compared to the classical photosynthetic culture model, heterotrophic cultures that allow microalgae to accumulate a much higher proportion of fatty acids have offered a feasible process to produce microbial lipids for the biodiesel production in a large scale under optimal growth and production conditions controlled by a fermenter. Many research studies have demonstrated the possibility of growth and lipid accumulation under heterotrophic conditions for certain microalgae, including Chlorella [10–12]. Chlorella cells are capable of growing independent of light in ordinary stirred tank bioreactors, similar to the bioreactors used for most other microorganisms.
To date, most studies on the lipid production by heterotrophic Chlorella have been carried out using glucose as the sole carbon source due to its high efficiency for cell growth [13–15]. However, high cost of refined glucose is considered as an obstacle for the development of heterotrophic cultivation of microalgae, which precludes its use for the lipid production in industrial scales. Although the cost of aseptic operation is also a main problem concerning heterotrophic cultures of microalgae in the large-scale production of biodiesel [16], a clean-in-place (CIP) system which provides hot cleaning and sterilization chemicals using the energy recovered from the fermentation plant can reduce the cost of sterilization [17, 18].
The biofuel production from microalgae in heterotrophic cultures demands culture substrates to be utilized as efficiently and economically as possible [19]. Volatile fatty acids (VFAs), which can be produced from food wastes, sludge, and a variety of biodegradable organic wastes via a VFAs platform [20, 21], are a promising low-cost carbon source for lipid production. As a matter of fact, microalgae can directly convert those organic acids into acetyl-CoA by acetyl coenzyme-A synthetase, and this acetyl-CoA is then used for the biosynthesis of fatty acids and lipid accumulation [22, 23]. An important consideration for the choice of using VFAs for the lipid production is the economic potential of VFA production process through a food waste recovery platform [24]. A preliminary cost analysis in a previous report demonstrated that biodiesel production derived from VFAs-based microbial lipids accumulated by an oleaginous yeast is competitive with current agriculture-based biodiesels [25].
Till now, few results discuss the behavior of lipid accumulation by heterotrophic C. protothecoides cells under different culture conditions with various concentrations and ratios of VFAs in the culture medium. With the aim of producing biodiesels more economically and effectively, this study demonstrates the effects of VFAs as carbon sources on cell growth and lipid accumulation by Chlorella protothecoides. Two of the most widely used growth media (Basal and Bristol medium) for Chlorella were employed. The optimum culture temperature was examined for lipid accumulation using VFAs as carbon sources. Several nitrogen sources were also compared to obtain a higher lipid yield coefficient. Various concentrations and ratios of VFAs were used to study their effects on lipid accumulation. Furthermore, compositions of lipids produced using VFAs were analyzed and compared with the case of using glucose as the sole carbon source. A preliminary techno-economic analysis was finally carried out to compare four different scenarios for the biodiesel production.
Materials and methods
Microorganism and medium
Chlorella protothecoides (UTEX 25) was obtained from the Culture Collection of Alga at the University of Texas (Austin, Texas, USA) and maintained at 4 °C on nutrient agar slopes prepared from modified Bristol’s medium with 1 % proteose peptone and 1.5 % agar. The medium used in seed cultures (pH 6.3) consisted of 0.7 g/L KH2PO4, 0.3 g/L K2HPO4, 0.3 g/L MgSO4·7H2O, 0.1 g/L urea, 1 mL/L A5 trace. The A5 trace metal solution was prepared in 1,000 mL of distilled water containing 2.86 g H3BO3, 1.81 g MnCl2·H2O, 0.222 g ZnSO4·7H2O, 0.079 g CuSO4·5 H2O, 0.390 g Na2MoO4·2H2O and 0.049 g Co(NO3)2·6H2O [26]. The carbon source of seed cultures was 20 g/L glucose. A mixture of VFAs (acetic acid: propionic acid: butyric acid) was used as a sole carbon source in flask cultures, and the final concentrations of VFAs in the media were varied for different experiments. The purity of acetic acid, propionic acid, and butyric acid with ACS reagent grade used in all experiments was higher than 99.7 %. In all experiments, the ratio of VFAs (acetic acid: propionic acid: butyric acid) was 6:1:3, unless otherwise specified. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA).
Culture conditions
An inoculum (10 % of seed flask culture) of exponentially growing cells was used for inoculation. All microalgae were grown separately on glucose or VFAs in 250 mL baffled Erlenmeyer flasks that contained 50 mL medium in a batch mode in darkness for 168 h until cultures reached stationary phase. Flask culture experiments were continuously incubated in a rotary shaker at 150 rpm. The pH of the medium was adjusted by a pH meter (Mettler Toledo SevenEasy Model S20) using 2 mol/L HCl and 1 mol/L NaOH solutions.
The lipid accumulation by C. protothecoides in heterotrophic cultivation was investigated firstly by using two different media (namely, Basal medium and modified Bristol culture medium). The compositions of these two media are compared in Table 1. The optimal culture temperature was also tested in flask cultures. C. protothecoides was grown at 20, 25, or 30 °C with VFAs as carbon sources. The optimum culture medium and temperature were identified for the inoculum and flask cultures and employed in subsequent experiments.
Potassium nitrate, sodium nitrate, glycine, and urea were each used as separate nitrogen sources with an initial concentration of 20 mmol nitrogen/L. All nitrogen sources were ACS reagent grade with 99 % purity. Organic nitrogen sources (urea and glycine) were sterilized by membrane filtration (0.2 µm). Nitrogen sources were typically exhausted after 24 h of cultivation. The cells were harvested in the stationary phase.
The influence of VFAs on lipid accumulation was studied by comparing various ratios and concentrations. The ratio of individual VFAs could be modified based upon the products produced from food wastes through the VFAs platform [24]. Four different ratios (4:3:3, 8:1:1, 6:1:3, and 7:2:1) of VFAs (acetic acid: propionic acid: butyric acid) were tested for their effects on lipid accumulation. In these experiments, the concentration of VFAs was 2 g/L. The effects of VFA concentration on the lipid accumulation were investigated using VFA concentration of 1, 2, 4, and 8 g/L with a ratio of 6:1:3.
Cell growth analysis
Cell growth was monitored by the optical density (OD) measurement at 540 nm using a UV/Visible spectrophotometer (Evolution™ 60S Thermo Scientific, Waltham, MA, USA). Cell concentration was determined by measuring the cell dry weight (CDW). Samples (10 mL broth) were transferred to a pre-weighed centrifuge tube and centrifuged at 8,000 rpm for 10 min at 4 °C. After rinsing the pellet twice with distilled water, it was dried overnight in vacuo at 105 °C until no further decrease in weight was observed.
Measurement of nitrogen and carbon sources
Nitrogen source concentrations were analyzed by an ammonia assay kit (AA0100, Sigma-Aldrich Co. LLC). The concentrations of glucose and VFAs were determined by high-performance liquid chromatography (HPLC) (Varian, Inc. USA) fitted with a Bio-Rad Aminex HPX87H column (Bio-Rad Laboratories, Hercules, CA, USA). The column was eluted with 5 mmol/L H2SO4 (99.999°%, Sigma–Aldrich, Inc. USA) as mobile phase at 50 °C and a flow rate of 0.6 mL/min [25].
Lipid extraction
Culture broth of 20 mL was centrifuged at 8,000 rpm for 10 min, the supernatant was discarded, and the cell pellet was re-suspended in distilled water. A mixture of methanol and chloroform was added, and the mixture was shaken for several minutes and centrifuged at 8,000 rpm for 10 min. The chloroform layer (lower) with the lipids was then separated, and the alcoholic layer (upper), which contained the lipid residues, was re-extracted twice with the mixture of methanol and chloroform. The chloroform layers were combined and subjected to a “Folch wash” to remove all non-lipid contaminants [27]. The mixture was washed with 0.88 % (wt/vol) potassium chloride, followed by methanol/saline solution (1:1, vol/vol). The purified chloroform layer was carefully withdrawn and transferred to a glass vial, diluted with benzene (2 mL), and evaporated to dryness under a stream of nitrogen to avoid oxidation of unsaturated fatty acids. The residual material was immediately weighed to give total lipid content.
Fatty acid composition analysis
Sodium methoxide solution (1 mL) and 1 mL toluene were added to 10 mg microbial lipids, heated to 75 °C and held at that temperature for 20 min. After cooling, 1.5 mL toluene and 1.5 mL water were added and the mixture was shaken vigorously. After phase separation, the water was removed with a pipette and another 1.5 mL aliquot of water was added and the mixture was shaken again. After phase separation, an aliquot of the toluene phase was stored at 4 °C prior for further analysis.
The fatty acid components were analyzed by a HP 5,890 SERIES II plus Gas Chromatograph (GC) coupled to a HP 5973 Mass Spectrometer (MS) Detector (Hewlett-Packard, Palo Alto, CA, USA). The MS scanned a range of m/z from 50 to 550 using the SCAN mode. The column used to separate each compound was an equity-1 (Supelco), with dimensions of 30 m (length) × 0.25 cm (inner diameter) × 0.1 μm (thickness). A flame ionization detector (FID) operated at 280 °C was also employed and the sample entrance was 45 cm/s. Nitrogen was used as a carrier gas. The initial temperature was 120 °C for 5 min. The temperature was then raised at 3 °C/min to 180 °C, where it was maintained for 2 min. The temperature was then increased at 10 °C/min to 220 °C, where it was then sustained for 30 min. HP 5972 MS and data processing software (HP G1034C Chemstation Software) were used for measuring and analyzing the data. Peaks were identified by means of lipid standards fatty acid methyl ester mixture (C8:0–C24:0, Sigma-Aldrich Co.)
Results and discussion
Effects of culture medium and temperature on lipid accumulation with VFAs as carbon sources
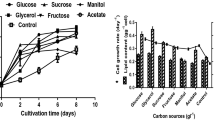
C. protothecoides was tested for the ability to produce lipid heterotrophically on both modified Bristol medium and Basal medium containing VFAs as carbon sources in flask cultures. Although both media supported cell growth as well as lipid accumulation in darkness (Fig. 1a, b), C. protothecoides grew faster in modified Bristol medium than in Basal medium (Fig. 1a). C. protothecoides produced higher biomass (0.56 g dry cells/L) and lipids (0.26 g/L) on modified Bristol medium as opposed to Basal medium. A higher lipid content of 47 % was also obtained from modified Bristol medium. As can be seen in Table 1, the Basal medium contains EDTA (500 mg/L), whereas the Bristol medium does not. Basal medium also contained higher concentrations of minerals than the modified Bristol medium. Although EDTA is the most commonly used chelating agent in microbial culture media, which plays important roles in stabilizing the sufficient supply of trace metal elements and the prevention of inhibitory effects of some metals [28], it is not ideal for C. protothecoides growth with VFAs as carbon sources at concentrations tested here. This phenomenon may be due to the inhibitory effect caused by the high concentration of EDTA present and the combination of EDTA with other trace elements in the Basal medium. Dou et al. [29] found the lipid production can be influenced significantly by trace elements of Cu2+, Fe3+, Zn2+, Mn2+, Mo6+, as well as EDTA and their relative concentrations in microalgae cultures. The effect of temperatures on cell growth of heterotrophic C. protothecoides is shown in Fig. 2a. The growth of C. protothecoides was inhibited significantly at 35 °C, which resulted in the lack of lipids at this culture temperature. The maximum biomass as well as the maximum total amount of lipids produced was observed at 25 °C (Fig. 2b). These results are in agreement with results from Pahl et al. [30], who concluded that the optimum temperature range for Cyclotella cryptica was 22.5–25 °C. However, Colla et al. [31] found that more biomass was obtained at 30 °C with Spirulina platensis. The discrepancy in these results may be due to different carbon sources and strains used for lipid accumulation. Since the modified Bristol medium and a cultivation temperature of 25 °C provided higher biomass and lipid contents, these two culture conditions were chosen for further experiments.
Effects of nitrogen sources on lipid accumulation with VFAs as carbon sources
The culture medium is commonly supplied with sufficient nutrients resulting in a lack of inhibition for high cell density growth of organisms. However, lipid accumulation is always triggered by the nitrogen limitation during the cultivation. Since potassium nitrate, sodium nitrate, glycine, and urea have been applied widely as common nitrogen sources for the cultivation of microalgae [26, 32–35], effects of these four nitrogen sources on cell growth and lipid accumulation with VFAs as carbon sources in the culture of C. protothecoides were compared. A lower limit nitrogen concentration of 20 mmol/L, therefore, was used in these heterotrophic cultures. Figure 3 shows the microalgae growth and lipid accumulation with four different nitrogen sources. Organic nitrogen sources provided higher cell growth with VFAs as carbon sources (Fig. 3a). The maximum biomass concentration of 0.605 g/L was obtained in cultures containing urea (Fig. 3b). The type of nitrogen source also affected lipid accumulation by C. protothecoides in heterotrophic cultures. Organic nitrogen sources like urea and glycine provided higher lipid weight and lipid content as compared to inorganic nitrogen sources. The maximum values of lipid production (0.287 g/L) and lipid content (47.5 %) were both found in cultures using urea, while the lowest values were found in cultures with sodium nitrate. The highest cell growth yield coefficient on VFAs (0.144 g/g) was also achieved in cultures with urea (Table 2). Similar conclusions were also reported by Pahl et al. [36] who found that the maximum specific growth rate and the lipid content were observed with urea as a nitrogen source in cultures of Cyclotella cryptica. Fidalgo et al. [37] reported that total fatty acid content in microalgae cells is influenced by the nitrogen source. However, results for lipid accumulation will differ with different strains. These results indicate that urea is generally superior to lipid accumulation by C. protothecoides in comparison with other commonly used nitrogen sources.
Effect of nitrogen sources on the cell growth (a) and lipid accumulation (b) in heterotrophic cultures of C. protothecoides (n = 3). Cultivation conditions: 25 °C, 150 rpm, pH 6.3, cultured for 168 h. Nitrogen source concentration was 20 mmol Nitrogen/L. VFAs concentration was 2 g/L with ratio of 6:1:3 in these cultures
Effects of VFAs ratio and concentration on lipid accumulation
Because the VFA ratio (acetic acid: propionic acid: butyric acid) could be modified through the VFAs platform [20, 21, 24], it is necessary to investigate the effects of different ratios on C. protothecoides growth and the lipid production. Table 3 shows the heterotrophic growth and lipid accumulation by C. protothecoides with four different VFA ratios. The highest biomass (0.65 g/L) and lipid production (0.317 g/L) were observed with a VFA ratio of 8:1:1. Similar results of biomass and lipid production were found when VFA ratio was 6:1:3 and 7:2:1. The highest lipid yield coefficient of 0.158 g/g and the highest biomass concentration (0.65 g/L) were achieved in cultures with a VFA ratio of 8:1:1 (Table 3). In general, higher acetic acid concentration in the culture provided more cell growth and lipid accumulation. As can be seen in Fig. 4, it is obvious that consumption of acetic acid predominated during the cell growth and lipid production. Prior to depletion of acetic acid in cultures, propionic acid and butyric acid were depleted by only 10–20 %. However, the consumption rate of propionic acid and butyric acid increased to 50 % after acetic acid exhaustion from the culture medium. These results indicate that acetic acid was more suitable for the lipid production by C. protothecoides than propionic acid and butyric acid in these heterotrophic cultures.
In heterotrophic cultures with dense Chlorella suspensions, the carbon substrate in the medium is depleted very rapidly, and thus a large quantity of carbon sources is needed for batch cultures to extend the growth period. It is necessary to determine what concentration of VFAs can be used without inhibiting the cell growth. Systematic investigations into the effect (either inhibition or promotion) of VFAs concentration on the cell growth are also needed. Therefore, the influence of initial concentration of VFAs on the heterotrophic growth of C. protothecoides was investigated in flask cultures. Figure 5 shows the effect of various concentrations of VFAs on the cell growth of C. protothecoides. It is obvious that lower the initial VFA concentration used in the culture medium, less is the inhibitory effect observed in the beginning of cultures. However, low concentrations of VFAs employed in this study resulted in low cell densities. There was no cell growth in the heterotrophic cultivation using 8 g/L VFAs, which may be due to an excess initial concentration of VFAs. As shown in Table 4, the biomass concentrations (0.58 g/L) and lipid content (48.2 %, w/w) in cultures containing 2 g/L initial VFAs were higher than those at lower or higher concentrations of carbon source. Theriault [38] found that glucose was the only carbon source, among many tested, which gave appreciable growth of Chlorella pyrenoidosa heterotrophically in flask cultures. However, as can be seen in Table 4, C. protothecoides is also able to use VFAs as carbon sources with a lipid yield of 0.14 g lipid/g VFAs. Although the VFAs provided lower lipid content (48 %) comparing with glucose did (55 %) [15], genetic modification work of microalgae can increase the total lipid content by improving metabolic fluxes from starch to TAG biosynthesis [39]. These results suggest that C. protothecoides could efficiently utilize those organic acids as carbon substrates for the cell growth and lipid accumulation. It is clear that inhibition effects of the VFA concentration on the cell growth and lipid accumulation were observed in cultures at higher VFAs concentrations.
Effect of carbon source (VFAs) concentrations on the cell growth in heterotrophic cultures of C. protothecoides (n = 3). No cell growth was observed in the culture with 8 g/L VFAs. Cultivation conditions: 25 °C, 150 rpm, pH 6.3, cultured for 168 h. Nitrogen source concentration was 20 mmol Nitrogen/L. VFAs ratio was of 6: 1: 3 in these cultures
The present study has indicated that the initial VFAs concentration of 2 g/L in the medium supported a relatively high growth rate and a high yield production of biomass and lipids. Acetic acid is the most favorable component of VFAs for the lipid accumulation by C. protothecoides. More inhibition effects were observed in cultivations containing higher VFAs concentrations. It has been demonstrated in this study that initial VFA concentrations affected cell density of Chlorella cultures, which is the most important parameter for the economical production of microalgal biomass and its products. Since microalgae can only tolerate relatively low concentrations of organic carbon substrates as compared with bacteria and yeasts, more systematic investigations into the development of high cell density heterotrophic processes are required. Based upon the optimum culture conditions explored in this work, high cell density and lipid production without inhibition effects of organic acids could potentially be achieved by using fed-batch culture, continuous culture or membrane cell recycling culture, in which the substrate concentration in the medium can be maintained at low levels [40, 41]. These culture modes may also provide a cost-effective, large-scale alternative method for culturing microalgae by utilizing low-cost carbon substances as their sole carbon and energy source.
Fatty acid composition of lipids accumulated by C. protothecoides with VFAs as carbon sources
Microbial lipids accumulated by C. protothecoides growing in flask cultures have been analyzed by gas chromatography mass spectrometry (GC–MS). As shown in Table 5, variations in the fatty acid composition from different carbon sources did not show distinct differences. The microbial lipids from heterotrophic cultures were characterized by the presence of C16 and C18 fatty acids, of which polyunsaturated fatty acids are considered as the major competent for biodiesel production [42, 43], predominated in lipids accumulated by C. protothecoides. Oleic acid (C18:1) content was particularly consistent with 50 %, compared to 10.8 % for linoleic acid (C18:2) and 21.5 % for palmitic acid (C16:0) using VFAs as carbon sources. The fatty acid composition will influence the cetane number (CN), which is one of the most significant properties to specify the quality of various biofuels used in a diesel engine. According to the Klopfenstein’s equation [44], microbial lipids produced with VFAs as carbon sources in this work had a CN value higher than 60 (Table 5). The minimal CN value by the biofuel standards of US and European Organizations has been set at around 50. Results from this study indicate that C. protothecoides can utilize both glucose and VFAs efficiently for the lipid accumulation and the fatty acids derived from microbial lipids are suitable for the production of premium biodiesel.
Preliminary techno-economic analysis of biodiesel production from heterotrophic cultivation using VFAs as a carbon source
The future of biodiesel production depends on several factors, and one of the most important factors is the cost of carbon source, accounting for up to 80 % of the total cost of raw materials [25]. A techno-economic analysis (TEA) is commonly used to guide investors and policy makers to select the most effective technology [45–47]. Therefore, a preliminary economic assessment was estimated to compare various scenarios of the biodiesel production. Using a TEA model developed in our previous study, the lipid and biodiesel cost could be evaluated based upon the data from this work. The economic consideration of a VFAs-based biotechnology for the biodiesel production could be compared with glucose- or sunlight-based biodiesel production.
Table 6 summarizes the biodiesel cost assessment based upon different carbon sources, microorganisms, and culture modes. Lipid cost was calculated using the following equation: Lipid cost ($/kg lipid) = raw material cost (including $30/ton carbon source and $150/ton NH4Cl) of $0.29/kg (based on the lipid yield on carbon source of 0.19 g/g) + utilities cost of $0.035/kg (0.1 $/kWh−1) [25] + labor cost of $0.056/kg [25] + general work cost of $0.029/kg [25]. The VFAs-based biodiesel appears to be competitive to completely displace fossil-based diesel. When VFAs-based lipids accumulated by C. protothecoides in heterotrophic cultivation were used for the biodiesel production, the cost of biodiesels could be as low as $2.3/gal. The microbial lipids-based biodiesel cost is much lower than other biodiesels produced from various feedstocks, such as soybean oil, castor oil, and autotrophic microalgae [25]. Furthermore, the cost of microbial lipid-based biodiesels could be reduced further since the production of VFAs is derived from food wastes, the price of which is in the range from ~$50 to 130/ton [48]. In the US, the federal government provides subsidies of $0.29 per energy equivalent liter (EEL) for biodiesels [49]. Comparing with the VFAs-based biodiesel from yeasts (Table 6), heterotrophic algae provide much higher lipid yield and content in this study. Although fixed capital investment has not been estimated in this preliminary techno-economic analysis, higher lipid content can reduce the total working volume of bioreactors, which can cut down the total fixed capital. A comprehensive techno-economic analysis will be developed in a future work to gain a better understanding of effects of various factors on the production of lipid and biodiesel.
Conclusions
The present work demonstrates the feasibility of using VFAs as solo carbon sources for the biodiesel production by microalga C. protothecoides during heterotrophic cultivations. The culture medium, culture temperature, and choice of nitrogen sources were investigated for the cell growth and lipid production. The highest lipid yield coefficient on VFAs and maximum lipid content was 0.187 g/g and 48.7 % (w/w), respectively. Present results indicate that VFAs are suitable carbon sources for the biodiesel production by C. protothecoides in heterotrophic cultures. Furthermore, the cost of the biodiesel production could also be reduced by using VFAs, which could be produced from food wastes. Results of a preliminary techno-economic analysis demonstrate that biodiesel production from C. protothecoides using VFAs in the heterotrophic cultivation is economically viable and technically feasible. Several driving forces such as lipid content, lipid yield, and carbon source cost predicted in the economic assessment will be improved in future experiments.
References
Schneider T, Graeff-Hönninger S, French W, Hernandez R, Merkt N, Claupein W, Hetrick M, Pham P (2013) Lipid and carotenoid production by oleaginous red yeas Rhodotorula glutinis cultivated on brewery effluents. Energy 61(1):34–43
Timilsina GR, Shrestha A (2011) How much hope should we have for biofuels? Energy 36(4):2055–2069
Nogueira LA (2011) Does biodiesel make sense? Energy 36(6):3659–3666
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131
Nichols B (1965) Light induced changes in the lipids of Chlorella vulgaris. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid. Metabolism 106(2):274–279
Piorreck M, Baasch K-H, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23(2):207–216
Vladimirova M, Klyachko-Gurvich G, Maslova I, Zholdakov I, Bartsevich E (2000) A comprehensive study of Chlorella sp. IPPAS C-48 and revision of its taxonomic position. Russ J Plant Physiol 47(5):644–654
Spoehr H, Milner HW (1949) The chemical composition of Chlorella; effect of environmental conditions. Plant Physiol 24(1):120
Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99(11):4717–4722
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35(5):281–285
Beal CM, Smith CH, Webber ME, Ruoff RS, Hebner RE (2011) A framework to report the production of renewable diesel from algae. BioEnergy Res 4(1):36–60
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97(6):841–846
Wen Q, Chen Z, Li P, Han Y, Feng Y, Ren N (2013) Lipid production for biofuels from effluent-based culture by heterotrophic Chlorella Protothecoides. BioEnergy Res 6(3):877–882
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126(4):499–507
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol 14(11):421–426
Pretreatment D-A (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. Contract 303:275–3000
Wooley R, Ruth M, Sheehan J, Ibsen K, Majdeski H, Galvez A (1999) Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis current and futuristic scenarios. DTIC Document
Chatzifragkou A, Makri A, Belka A, Bellou S, Mavrou M, Mastoridou M, Mystrioti P, Onjaro G, Aggelis G, Papanikolaou S (2011) Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36(2):1097–1108
Lim S-J, Kim BJ, Jeong C-M, Ahn YH, Chang HN (2008) Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour Technol 99(16):7866–7874
Lim S-J, Kim E-Y, Ahn Y-H, Chang H-N (2008) Biological nutrient removal with volatile fatty acids from food wastes in sequencing batch reactor. Korean J Chem Eng 25(1):129–133
Griffiths D, Thresher C, Street H (1960) The heterotrophic nutrition of Chlorella vulgaris (Brannon No. 1 strain): with two figures in the text. Ann Bot 24(1):1–11
Perez-Garcia O, Escalante FM, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36
Chang HN, Kim N-J, Kang J, Jeong CM (2010) Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol Bioprocess Eng 15(1):1–10
Fei Q, Chang HN, Shang L, Kim N, Kang J (2011) The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour Technol 102(3):2695–2701
Cerón-García M, Macías-Sánchez M, Sánchez-Mirón A, García-Camacho F, Molina-Grima E (2013) A process for biodiesel production involving the heterotrophic fermentation of < i > Chlorella protothecoides </i > with glycerol as the carbon source. Appl Energy 103:341–349
Papanikolaou S, Aggelis G (2002) Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour Technol 82(1):43–49
Oh-Hama T, Miyachi S (1988) Chlorella. In: Borowitzka MA, Borowitzka (eds) Micro-algal biotechnology. Cambridge UP, Cambridge, pp 3–6
Dou X, Lu X-H, Lu M-Z, Yu L-S, Xue R, Ji J-B (2013) The Effects of trace elements on the lipid productivity and fatty acid composition of nannochloropis oculata. J Renew Energy 2013:1–6. doi:10.1155/2013/671545
Pahl SL, Lewis DM, Chen F, King KD (2010) Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): effect of some environmental factors. J Biosci Bioeng 109(3):235–239
Colla LM, Oliveira Reinehr C, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98(7):1489–1493
Gao C, Zhai Y, Ding Y, Wu Q (2010) Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy 87(3):756–761
Prathima Devi M, Venkata Subhash G, Venkata Mohan S (2012) Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: effect of nutrient supplementation. Renewable Energy 43:276–283
Cheng Y, Lu Y, Gao C, Wu Q (2009) Alga-based biodiesel production and optimization using sugar cane as the feedstock. Energy Fuels 23(8):4166–4173
Zhang X, Yan S, Tyagi R, Surampalli R (2013) Biodiesel production from heterotrophic microalgae through transesterification and nanotechnology application in the production. Renew Sustain Energy Rev 26:216–223
Pahl SL, Lewis DM, King KD, Chen F (2012) Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): effect of nitrogen source and concentration. J Appl Phycol 24(2):301–307
Fidalgo J, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166(1):105–116
Theriault RJ (1965) Heterotrophic growth and production of xanthophylls by Chlorella pyrenoidosa. Appl Microbiol 13(3):402–416
Bellou S, Aggelis G (2012) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotechnol 164(2):318–329
Chang HN, Kim N-J, Kang J, Jeong CM, Fei Q, Kim BJ, Kwon S, Lee SY, Kim J (2011) Multi-stage high cell continuous fermentation for high productivity and titer. Bioprocess Biosyst Eng 34(4):419–431
Fei Q, Chang HN, Shang L (2011) Exploring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol Bioprocess Eng 16(3):482–487
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain Energy Rev 16(1):143–169
Liang Y, Cui Y, Trushenski J, Blackburn JW (2010) Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 101(19):7581–7586
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102(1):57–70
Sun A, Davis R, Starbuck M, Ben-Amotz A, Pate R, Pienkos PT (2011) Comparative cost analysis of algal oil production for biofuels. Energy 36(8):5169–5179
Fei Q, Guarnieri MT, Tao L, Laurens LML, Dowe N, Pienkos PT (2014) Bioconversion of natural gas to liquid fuel: opportunities and challenges. Biotechnol Adv 32(3):596–614. doi:10.1016/j.biotechadv.2014.03.011
Vlysidis A, Binns M, Webb C, Theodoropoulos C (2011) A techno-economic analysis of biodiesel biorefineries: assessment of integrated designs for the co-production of fuels and chemicals. Energy 36(8):4671–4683
Park GW, Fei Q, Jung K, Chang HN, Kim Y-C, Kim N-j, Choi J-d-r, Kim S, Cho J (2014) Volatile fatty acids derived from waste organics provide an economical carbon source for microbial lipids/biodiesel production. Biotechnol J. doi:10.1002/biot.201400266
Shapouri H, Gallagher P (2005) USDA’s 2002 ethanol cost-of-production survey. United States Department of Agriculture, Office of the Chief Economist, Office of Energy Policy and New Uses
Acknowledgments
Author Dr. Fei gratefully acknowledges Korea National Research Foundation (KRF Scholarship) for its financial support during his Ph.D study. The authors would like to thank Dr. Nagjong Kim and Dr. Jin-dal-rae Choi for their helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, Q., Fu, R., Shang, L. et al. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst Eng 38, 691–700 (2015). https://doi.org/10.1007/s00449-014-1308-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1308-0