Abstract
After an overview of the discussion about the existence of intra- and interspecific competition that illustrates the contradictory opinions I conclude that long-term field experiments are needed for firm conclusions. I discuss in some detail the role of two factors that limit population size of secondary cavity nesting birds e.g. territorial behavior and adequate cavities. This is followed by an overview of experimental long-term field studies in Belgium showing that intra- and interspecific competition in a great tit-blue tit system exists. By using nestbox configurations with high densities of nestboxes that differ in the diameter of their entrance hole in replicate study plots it is possible to manipulate the breeding densities of great tit Parus major and blue tit Cyanistes caeruleus independently, thereby varying the intensity of intra- and interspecific competition between these two coexisting species. When blue tit densities are experimentally increased local recruitment of great tits increases, and adult great tit post-breeding dispersal to other study plots decreases, implying that great tits use blue tit density to evaluate habitat quality and that high blue tit density results in heterospecific attraction. The reverse is not true. An experimental increase in great tit density leading to an increase in interspecific competition in a plot where blue tit density was already high leads to a decrease in blue tit nestling mass (illustrating interspecific competition for food), but to a gradual increase in blue tit body size. Both are primarily caused by an increase in the body size of immigrants (caused by intraspecific competition for protected roosting holes) in contrast to the control plot, where neither is observed. I also summarize behavioral, ecological and possible evolutionary effects of sparrowhawks on blue tits after sparrowhawks settled in an isolated study plot halfway through the study: adult survival substantially decreased for both sexes, but more for females that laid large clutches, leading to selection for females that laid a smaller clutch. This led to a change in the reproduction/survival life-history trade-off. Adult winter weights and nestling weights decreased, and the heaviest fledglings were selected against. Furthermore the frequency of polygyny increased. The long-term experiments also document the role of the use of public information and that species that compete can be attracted to sites in which competitor density is high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: does interspecific competition exist?
Biotic interactions exert selection pressures on individuals, which can translate into effects at population and community levels. In this overview, I will illustrate this using some examples from my long-term studies on tits, concentrating especially on competition and predation. I will place these in a brief historical perspective on discussions regarding intra- and interspecific competition. Most specific methods used are described in the papers cited.
In what follows, I will introduce the discussion as regards the existence of intra- and interspecific competition, provide experimental evidence for intra- and especially interspecific competition on demography, discuss factors that influence dispersal including the role of public information, explore possible evolutionary changes resulting from interspecific interactions, and finally summarize the multiple impacts of sparrowhawks on a blue tit population.
Competition is the negative effect which one organism has upon another by consuming, or controlling access to, a resource that is limited in availability (Keddy 1989). The word ‘consuming’ refers to exploitation competition, while the words ‘limiting access to’ refer to competition by interference. Intraspecific competition occurs between individuals of the same species, while interspecific competition involves individuals of different species. Competition impacts the fitness of individuals, which can lead to effects on population size or species distribution (Dhondt 2012). Directional changes in competition intensity could lead to evolutionary responses. The strongest evidence for the existence of competition results from carefully executed and replicated long-term field experiments which require a detailed understanding of what resources are limiting (Hairston 1989). If it is possible to manipulate resource availability or breeding density it should not only be possible to document the existence of interspecific competition but also to document its impact. While traditionally interactions between two or more species were defined either as competition or as facilitation (whereby both interacting individuals benefit from the interaction) it is now generally documented that interactions between individuals of different species can—depending on conditions—result either in competition or in facilitation (Forsman et al. 2008; Trautz et al. 2017), whereby inadvertent social information can play a major role (Jaakkonen et al. 2015; Mönkkönen and Forsman 2002; Parejo and Aviles 2016).
Because a necessary condition for the existence of interspecific competition is the existence of intraspecific competition (Reynoldson and Bellamy 1971) it is useful to give a brief overview of the long-term debate regarding its existence. Until about the mid-seventies there existed two opposing opinions. On the one hand, as summarized in two influential books, David Lack believed that intraspecific competition exists and was fundamental in regulating population size (Lack 1954, 1966), but that interspecific competition was at best transient and resulted in coexisting species using different resources (Lack 1971). In stark contrast Andrewartha and Birch (1954) simply rejected the existence of intraspecific competition, and thus by default the existence of interspecific competition. They wrote (p. 649): “The generalizations about “density-dependent factors” and competition in so far as they refer to natural populations are neither theory nor hypothesis but dogma”.
In a critical review of Lack’s 1966 book Chitty (1967), while not necessarily rejecting the existence of intraspecific competition, writes “A further consequence of this search for density-dependent factors is that an immense amount of time is spent in purely descriptive tasks, in the hope that if only we list enough causes of mortality we shall be that much closer to understanding how populations are regulated. This approach is a poor substitute for field experiments and has not been very productive, as indeed Lack admits (pp. 8, 288)”.
In the broader literature this debate was still very explicit at a 1970 meeting on “Dynamics of Numbers in Populations” in which Solomon placed the multiple general theories into two groups. One group were variants of the idea that populations cannot persist for long unless they are subject to density-dependent regulation (i.e. intraspecific competition), while the theories in the other group all contained the tenet that “the diversity of the habitat and of the population explains the persistence of many populations” (Solomon 1971). During the conference den Boer, one of several authors belonging to the second group, illustrated that density fluctuations are reduced as heterogeneity of the environment increases (den Boer 1971), and that therefore, density dependent factors are not needed for a population to persist. At the same meeting Charles Birch (1971) was even more explicit repeating that competition simply does not exist. On the other hand Kluyver (1971) (among others) illustrated experimentally that “intraspecific strife” was important in population regulation and dispersal of great tits (Kluyver 1971) and Dhondt (1971a) reported the importance of both density-dependent juvenile survival and territorial behavior in regulating great tit population size.
While, as discussed above, the existence and importance of intraspecific competition, although challenged, was supported by a fair number of scientists primarily under the impulse of David Lack, the ongoing existence of interspecific competition was much less generally accepted. Following Gause’s principle (Gause 1934) Lack (1966) concluded that different species can only coexist by avoiding interspecific competition altogether.
In the early seventies studies documenting ongoing interspecific competition in animal populations were quite rare (Connell 1971; Reynoldson and Bellamy 1971). The paradigm of “The Ghost of Competition Past” (Connell 1980) as described above by Lack drove thinking. The final sentence in Connell’s (1980) paper is:”. until some strong evidence is obtained from field experiments along the lines suggested above, I will no longer be persuaded by such invoking of “the Ghost of Competition Past”. In a review on interspecific competition in an ecological concepts symposium organized by the British Ecological Society Law and Watkinson (1989) wrote:” It is difficult to perceive a real sense of progress in our understanding of the role of competition in nature … rather we are left with an impression of a subject moving from one world view to another depending on the influence of the prevailing protagonists”. In this quote they emphasized that 35 years after the books by Lack and by Andrewartha and Birch we had not really gained much new understanding about the importance of interspecific competition.
I think that the remarks made by Chitty (1967) and Connell (1980) are crucial: to resolve a problem in ecology one must carry out field experiments that allow one to test contradictory predictions stemming from different theories or hypotheses. Of course, if one believes that interspecific competition does not occur, one would not design experiments to test its effects. It is interesting that in his review of experiments performed to study interspecific competition in birds John Wiens (1989) found that only very few had replicates and/or controls and very few had been performed over an extended period. Similarly, very few field experiments on birds survived Hairston’s critical evaluation in his important 1989 book. He concluded that “The whole book will irritate ecologists who adhere to the claim that direct observations of what are sometimes called “natural experiments” are sufficient to draw conclusions about ecological phenomena” (Hairston 1989).
The bottom line is experiments are essential in ecology, but they must be carried out correctly. Furthermore, in this entire debate as to the existence of interspecific (and even intraspecific) competition in birds, one potential interesting outcome of this biotic interaction, i.e. what selection pressures are the result of these interactions and to what possible evolutionary changes they can lead is overlooked. In this paper I will not only discuss short-term effects of intra- and/or interspecific competition, but also explore observations and experiments that provide some answers as to possible evolutionary responses of biotic interactions in birds.
Density dependence, intraspecific competition and population regulation
Intraspecific competition for a limiting resource is a process leading to density dependence and resulting in population regulation. Note that other factors, such as predation or parasitism, can also act in a density-dependent fashion and hence regulate population size. As explained in the introduction the mere existence of intraspecific competition leading to population regulation used to be widely not accepted. In what follows, I will discuss space and cavities as a limiting resource in cavity-nesting birds.
Does territorial behavior limit numbers? Or space as a limiting resource
Ever since Howard’s book “Territory in Bird Life” (Howard 1920) there has been an intense debate about the extent to which territorial behavior does limit breeding population size. The most explicit critic of this idea was David Lack. In 1933, he summarized his arguments why he believed that “there is not sufficient evidence that territory is an important factor in the prevention of overcrowding” (Lack and Lack 1933). In response Huxley (1934) wrote that it seems quite clear that when birds display territorial behavior it does play a part in determining the actual density of a breeding population. Based on some observations of territorial behavior of Eurasian coots Fulica atra Huxley introduced the elastic disk hypothesis that territories are compressible but not beyond a certain limit (Huxley 1934); hence breeding density of territorial species can vary between years. In her important summary on the topic Nice (1941) wrote that “pairs are spaced through the pugnacity of males towards others”, but also that birds which fail to obtain a territory form a reserve from which replacements come in case of the death a territory owner (Nice 1941). This led to the buffer hypothesis (Kluijver and Tinbergen 1953), further expanded by Brown (1969) and Fretwell and Lucas (1969) that basically claimed that territorial behavior causes birds to distribute themselves across habitats of diverse quality in such a way that densities vary in relation to habitat quality. Birds that cannot establish themselves form a surplus that may live in a different habitat, often flocking and queuing for access to a vacant territory (Baeyens 1979; Ens et al. 2014). This also led to Susan Smith’s “underworld” idea that explains social organization in territorial bird species in the tropics that breed year round (Smith 1978).
As an undergraduate I was interested in bird behavior and had the opportunity to work under the supervision of Prof. Dr. Jan Hublé. I participated in his great tit nestbox population study in and around Ghent, Belgium. As an undergrad one is inclined to believe that what has been published by senior scientists must be true. Nevertheless, one of the things which puzzled me was David Lack’s statement that although breeding density in a site could vary considerably territorial behavior did not limit the number of breeding pairs (Lack 1966). Various factors can influence territory size and hence breeding density. Male aggression could vary between years as found in red grouse Lagopus lagopus scotica (Watson and Miller 1971) or territory size could vary with male age (Dhondt 1971b) leading to a correlation between age composition and population size (Dhondt 1971a). Compelling experiments by John Krebs and collaborators showed that an experimental increase in settlement synchrony leads to an increase in the number of birds that can settle (Knapton and Krebs 1974), and that following the removal of territorial males in high quality territories these were rapidly replaced by birds from lower quality sites (Krebs 1971) or by birds from a non-breeding surplus (Krebs 1977). These, and many other studies, did show that intraspecific competition for space can indeed limit population size in many different species (Newton 1992).
Territorial behavior, however, is not limited to the breeding season. Some birds defend territories outside the breeding season, in their wintering grounds and even year round, which impacts the time of year when such behavior limits numbers. Piet Drent’s very detailed work underlined that we need to look further than territorial behavior in spring. What happens in the fall can also have major impacts and he concludes that many juvenile great tits present in the area in autumn fail to secure a territory, and emigrate confirming that territorial behavior in September is a density-limiting process (Drent 1984).
The impact of territorial behavior on limiting numbers is also linked to social organization during the non-breeding season as clearly illustrated by between species variation in social organization among temperate zone finch and tit species (Matthysen 1990; Newton 1972) and such behavior can lead to the exclusion from a study plot or mortality at different times of the year (Dhondt 1971a; Hogstad 1989; Watson and Jenkins 1968). During winter black-capped chickadees Poecile atricapillus, for example, live in groups that defend territories as a group. Within that group there exists a strict dominance hierarchy. Some individuals, however—called flock switchers by Smith (1987)—are technically floaters that move between flocks. These flock switchers rapidly replace a disappearing dominant flock member, but do not replace a subordinate one indicating that the floater strategy serves to increase their chance to obtain a territory later. She described that, as spring approached and flocking behavior changed into pair territorial behavior, the surplus birds that could not obtain a pair territory left the area being driven out (Smith 1967). Thus during winter intraspecific interactions for dominance limit population size, while in spring interactions for a territory do that. More studies of territorial behavior in tropical bird species with year round territory defense would help to better understand their population regulation possibly linked to their annual variation in testosterone level as, for example, done by Goymann and Landys (2011). Recent work suggests that the role of testosterone varies with life history strategy and that researchers should take advantage of the large diversity of life history strategies among tropical bird species to gain a better understatnding of the role of territorial behavior in tropical systems (Moore et al. 2019).
Are cavities limiting?
Since at least 1910 nestboxes have been used for the scientific study of secondary cavity nesting birds, especially tits (Kluijver 1951; Wolda 1913). Other than providing easy access to bird nests to the researchers the impact of providing artificial nest-holes varies. Tomasz Wesolowski and colleagues concluded that in Białowieża Forest in Poland, one of the few remaining undisturbed primaeval European forests, there is no shortage of natural cavities, and that therefore secondary cavity nesters are not limited through a lack of nest sites (Wesolowski 2007; Wesolowski et al. 2015). In most studies, though, the provision of nestboxes did result in an increase in the breeding density of at least some cavity nesting species illustrating that adequate nest sites are limiting, and that there exists a surplus of non-breeding birds (Newton 1994a, 1994b) even in largely natural forest (Robles et al. 2012). That was also true in our Belgian study sites: following the provision of nestboxes breeding densities increased; and surplus birds were present because when we removed territorial great tits in spring the vacancies were rapidly and completely replaced (Lambrechts and Dhondt 1988) as they were in a similar experiment by John Krebs in Oxford (Krebs 1977). I am not aware of any experiments in which blue tits were removed. Török’s “removal experiments” were actually exclusion experiments to test for competition for food during the breeding season between great and blue tits (Török and Tóth 1999), which they documented. The exclusions were performed by reducing the entrance holes of individual boxes, so that the intensity of competition was reduced because of fewer pairs breeding. Even in Wytham Woods near Oxford—a relatively unmanaged broadleaved woodland with a surplus of large-holed nestboxes—great tit breeding numbers decreased following a nestbox removal experiment. Note that blue tits, many of which used natural cavities, did not decline when the nestboxes were removed (East and Perrins 1988). Studies in South America confirm that even in natural tropical forest high quality nest cavities can be limiting (Cockle et al. 2010; Cornelius et al. 2008; Medina-Estrada et al. 2022; Tarazona-Tubens et al. 2022).
While I acknowledge that studies in manmade forest do not reflect natural conditions, they can nevertheless be used to test hypotheses.
In the nineteen sixties so-called selective nestboxes were being promoted to avoid the extirpation of the smaller tit species by the larger and more aggressive great tit in western European managed forests. In 1966, for example, Cees Stam (1968) and collaborators across The Netherlands and Belgium collected data from 17,638 nestboxes of different types in which all six W. European tit species and another 18 bird species were recorded. He illustrated how adding or replacing the traditional nestboxes (opening 32 mm or larger) with selective nestboxes that are smaller and/or have a smaller entrance hole changed the proportion of different species found in nestboxes from 87% great tit, 11% blue tit and 1% other tit species (n = 372) to 44% great tit, 36% blue tit and 20% other tit species (n = 367). One cavity, therefore is not the same as another cavity which opens an avenue to manipulate breeding densities of secondary cavity nesters as done, for example by Hans Löhrl (1977).
A method to manipulate breeding density of great and blue tit
Most European population studies of great and blue tit have used nestboxes with an opening large enough for great tits (30–32 mm diameter). These were also used by the smaller blue tits and some other species. In the Ghent study, we also used large-holed nestboxes (32 mm diameter), but for a reason unrelated to the study of competition we changed the large-holed boxes into small-holed nestboxes (26 mm) in one of the study sites. Not only did this lead to the intended reduction of great tit population size in the study plot, but an unexpected result of excluding great tits from the nestboxes was that large numbers of blue tits were found roosting in the small-holed nestboxes in winter, which than led to an increase in the size of their breeding population (Dhondt and Eyckerman 1980b). To test the idea that the increased use of nestboxes by blue tits was the result of interspecific competition for cavities as roosting sites and not caused by a preference of blue tits for small-holed nestboxes when available we performed aviary experiments. When in winter a single blue tit was kept in an aviary with both nestbox types it always used a large-holed nestbox for roosting (Dhondt and Eyckerman 1980b). If we then added a great tit to the aviary the blue tit shifted to a small-holed box for roosting without clear interactions with the great tit (Kempenaers and Dhondt 1991). This result is not really surprising. The larger great tit (18 g) can represent a mortal danger for the smaller blue tit (11 g) when together inside a nestbox, hence using a large-holed nestbox can be risky for blue tits (Löhrl 1977) as it can be for pied flycatchers Ficedula hypoleuca during the breeding season (Samplonius and Both 2019).
In 1974 I was appointed as a faculty member in the Department of Biology of the newly founded “Universitaire Instelling Antwerpen” (Belgium). Before starting a new research project I decided to first explore the extensive data on populations of great and blue tit that had been collected by Jan Hublé and collaborators at Ghent University in a suite of study sites since 1958. The data existed only on handwritten punch cards. To analyze the data and explore the extent to which competition could be documented, I first needed to computerize all data. Entering the data on IBM punch cards took about 18 months. After that the analyses rapidly provided correlational evidence for the existence of both intra- and interspecific competition in the great and blue tit system (Dhondt 1977). Based on that analysis I concluded that interspecific competition was caused by scramble competition for food during the breeding season because at higher density of either species nestling mass and fledging success of both species were reduced. The effect was asymmetric in that the impact of blue on great tits as measured by the competition coefficient (α = 1.2) was quite strong, while the reverse effect was much weaker (β = 0.13). This was attributed to the blue tits having a broader food niche than the great tit, a result later confirmed experimentally (Nour et al. 1998; Török 1993; Török and Tóth 1999). The analysis of the Ghent data also showed that great tit mean wing length and mean body mass decreased considerably from 1962 to 1975, an effect that we attributed to changes in the intensity of intraspecific competition (Dhondt et al. 1979). We hypothesized that this change in great tit measurements would reflect an evolutionary change as the first publication in which heritability of morphological characters in a wild bird population had been documented had just been published (Boag and Grant 1978).
I decided that it would be important to experimentally test ideas about the existence of intra- and interspecific competition and their effects on demography, behavior and possibly on measurable changes in selection pressures. Creating replicate plots in which density of great and blue tits were manipulated by varying nestbox configurations would provide the basis for long-term experimental studies to explore the existence and importance of competition in general and measure its possible effects on individuals and populations (Dhondt and Eyckerman 1980a, 1980b). As always, the field experiments generated some unexpected results.
This long-term experiment asked multiple questions.
-
1.
How did the demographic parameters vary between sites in which the intensity of intra- and interspecific competition differed?
-
2.
What resources were limiting; could they be manipulated?
-
3.
Did effects of intra- and interspecific competition vary with habitat quality?
-
4.
Were all effects of density negative, or could the manipulation of nestboxes and breeding population density have other effects too?
-
5.
Did experimental differences in the strength of competition lead to changes in selective pressures and possibly to evolutionary responses.
Experimental evidence for the existence of intra- and interspecific competition in tits
Following my “discovery” that it was possible to manipulate great and blue tits numbers separately by using nestboxes with different entrance hole size, I created in 1979 two replicates of each of three nestbox configurations (Table 1). One set were a new series of study plots near Antwerp, Belgium in high quality mainly oak habitat; the second series were inserted about 60 km away among the study plots around Ghent, Belgium that Jan Hublé had initiated in 1958. These were in much poorer habitats, and all of them were in isolated fragments (Dhondt and Hublé 1968). Configuration LARGE was the traditional configuration with large-holed (32 mm) wooden nestboxes only. Based on previous work we expected in this configuration a high great tit and an intermediate blue tit density as blue tits are outcompeted by great tits in such a configuration even when there is a surplus of nestboxes (Minot and Perrins 1986). Configuration SMALL had only small-holed (26 mm) nestboxes; based on previous work we here expected a high blue tit density and a very low great tit density as great tits were limited to natural cavities that were in short supply in managed forest (Dhondt and Eyckerman 1980b). Configuration BOTH combined high densities of large- and small-holed nestboxes. We here expected a high great tit density but we were uncertain about what blue tit density to expect. If interspecific competition for food existed in winter we expected an intermediate blue tit density as great tit density would be high and thus interspecific competition for food in winter would be increased. If interspecific competition was only for roosting sites during winter, we expected a high blue tit density, as they were released from interspecific competition for cavities. If interspecific competition was for both food and for roosting sites blue tit density would be intermediate. While this experiment did not specifically test for competition for food during the breeding season, we assumed that earlier work had provided sufficient evidence that food was limiting during the breeding season (Dhondt 1977).
The results of these manipulations on breeding density were quite clear: blue tit densities in all sites with small-holed nestboxes were high and equal in configurations BOTH and SMALL, therefore not impacted by great tit density. This implied winter competition for roosting sites but not for food with great tits (Dhondt et al. 1991). By comparing plots with different nestbox configurations we could test for effects of intra- and interspecific competition separately. Thus, comparing configuration LARGE with configuration BOTH measures the impact of interspecific competition on great tits by blue tits, as in both configurations great tit density was high, while blue tit density was low in configuration LARGE, but high in configuration BOTH; comparing configurations BOTH and SMALL measures the effect of interspecific competition on blue tits by great tits, since in configuration BOTH and SMALL blue tit density was high, while in configuration BOTH great tit density was high, but low in configuration SMALL. Difference in blue tit but not in great tit density between configuration LARGE and BOTH made it possible to measure effects on intraspecific competition in blue tits.
Initially we created two replicates of the three configurations for 5 years, but in experimental plots we changed the configuration after 5 years, while keeping configurations in control plots unchanged. This made it possible to allow for unplanned between-year variation in the comparisons (see for example Fig. 2). In all the Ghent plots in which we made changes in nestbox configurations we had data from years before 1979 in which all nestboxes were large-holed.
Experimental nestbox configuration and reproduction
I have earlier described in detail the most important correlational and experimental results of effects of competition on reproduction in great and blue tits (Dhondt 2010, 2012), and in another paper especially for blue tits (Dhondt and Adriaensen 1999). I will limit myself here to a brief summary of the results based on the experimental density manipulation only.
-
- Experimental increases in the intensity of intraspecific competition among blue tit yielded following results. In the low quality Ghent plots nest success was reduced in the experimental Plot MA compared to the control plot ZEV indicating an increase in intraspecific competition for food with density. The comparison of changes between periods in Antwerp also showed some evidence of competition for food when comparing blue tits in Plot T (experimental) and Plot B (control): breeding adult female (not male) mass was lower in the higher blue tit situation and 15 day nestling mass was 2.6% lower in the high blue tit density plot compared to the low density situation, while it did not change in the control plot. A surprising result was that in both regions laydate was delayed at high blue tit density compared to the control plot.
-
- Experimental increases in the intensity of interspecific competition of great on blue tit yielded, for the Antwerp plots, a significant decrease in nestling body mass and in adult female mass, while clutch size was marginally smaller (P = 0.07), indicating interspecific competition for food.
-
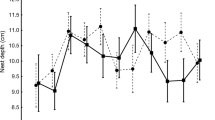
- The experimental increase in the intensity of interspecific competition of blue on great tit caused great tit clutch size and nest success to decrease in the Ghent experimental plot MA compared to the control plot SO. In Antwerp, though, the increased blue tit density did not cause a significant decrease in nestling mass across all birds, but there was a clear effect on nestling mass of the largest clutches, resulting in a selection for smaller great tit clutch sizes (Fig. 1). Condition (mass/tarsus) of both nestlings and adults were significantly lower when interspecific competition has been experimentally increased (Dhondt 2012).
15-day nestling mass of great tits as a function of clutch size during two 5-year periods during which blue density was manipulated to change the intensity of blue tit interspecific competition. In both plots and all years great tit density was high. In Plot B (triangles) blue tit density also was high throughout. In Plot T (circles) blue tit density was low in 1979–1983 (filled symbols), but high in the period 1989–1993 (open symbols) thereby increasing the intensity of interspecific competition on great tits. In that period the nestling mass in the largest broods (≥ 11) were significantly lower in Plot T than in Plot B, while that had not been the case previously. This indicates competition for food that only impacts a subset of the birds, causing a selection pressure against the largest clutches. In the 2nd period (1989–1993) overall conditions were poorer as illustrated by the lower body mass in both study plots. See also Dhondt et al. (1990a, b)
Taken together these results emphasize the importance of food as a limiting resource for blue and great tits during the breeding season, and underline that the effects are stronger in low quality habitat.
Experimental nestbox configurations and dispersal
Background on existing data on heterospecific attraction and dispersal
In Antwerp we made a major effort to individually ring, weigh and measure all nestlings at 15 days, and trap all the breeding adults of both species. This provided detailed data on survival and dispersal. In the Ghent study sites the results were not as detailed in many years, especially not for the blue tits. By the time the long-term data were being analyzed many years later a lot had changed in the thinking how birds make dispersal decisions. Several experiments had supported the existence of conspecific attraction in decisions regarding territorial settlement (Alatalo et al. 1982; Danchin et al. 2004; Stamps 1988), and ground-breaking experiments by Mikko Mönkkönen and Jukka Forsman documented effects of resident species density on settlement decisions of migrants thereby proving the importance of inadvertent public information leading to heterospecific attraction: an experimental increase in the density of resident species resulted in an increase in the density of migratory birds indicating that migrants use resident density as an indicator of habitat quality, although they might be competitors (Forsman et al. 2008; Mönkkönen et al. 1990) and that experimental manipulations of tit density might mislead the migrants as to the real habitat quality (Forsman et al. 2008). Several experiments have now documented the diversity of types of social or public information (further called Inadvertent Social Information or ISI) that influences prospecting birds in their decision where to settle. Most experiments manipulated a trait related to ISI that could impact perceived habitat quality. These included density, the number, the quality, or even the sex ratio of nestlings (Doligez et al. 2002, 1999; Nicolaus et al. 2009; Nicolaus et al. 2012; Parejo et al. 2007a, 2007b). Many manipulations influenced settlement or dispersal. Most experiments measured the use of ISI on conspecifics, although sometimes on heterospecifics. Thus, Parejo and colleagues performed experiments in which they manipulated the number and the quality of nestling blue tits to determine if great tits used that as ISI when selecting where to settle. They found that great tit adult dispersal decisions seemed to be influenced by con- and heterospecific density but not by reproductive success (Parejo et al. 2008). Age sometimes also influenced the response to experimental manipulations (Doligez et al. 2004; Parejo et al. 2007b). These results underline the complexity of dispersal and settlement decisions in birds and that conspecific and/or heterospecific attraction can play an important role. Note that although most examples of effects of heterospecific attraction on dispersal stem from birds they are also observed in other taxa, as, for example, in newts (Cayuela et al. 2018).
Density manipulations and dispersal
To test the effect of density on dispersal I compared local recruitment and breeding dispersal between plots that differed in conspecific and/or heterospecific density. Unfortunately the data collected in the replicate plots in Ghent, especially as concerns the identity of the breeding birds, were less complete than in Antwerp and I will therefore only discuss the Antwerp data in this section on dispersal. The experimental configurations made it possible to compare effects of blue tit density on great tit dispersal and vice-versa, and effects of blue tit density on blue tit dispersal. No configurations made it possible to study effects of great tit density on great tit dispersal. I will report on two components of dispersal: (1) breeding dispersal measured as the proportion of adults that bred in one year and were recaptured alive in the following year and either remained in the same study plot or bred in a different plot; and (2) local recruitment, i.e., the proportion of the breeding population in a plot that was born locally and had remained in the same plot and hence had been recruited locally. In these analyses I only included yearling birds to avoid bias (Matthysen et al. 2011). With the three nestbox configurations (LARGE, BOTH or SMALL) following three comparison were possible.
-
- Effect of blue tit density on great tit dispersal. In the control plot B with configuration BOTH great and blue tit density was high for 10 years. In the experimental Plot T the configuration was LARGE in 5 years and BOTH in another 5 years. Great tit density was high throughout, but blue tit density was low in the 5-year period with configuration LARGE, but high in configuration BOTH.
-
- Effect of great tit density on blue tit dispersal. In the control plot B we maintained configuration BOTH, while in the experimental plot T the configuration was LARGE in one 5-year period (with a high great tit and a low blue density) but SMALL in the other 5-year period. In this latter period great tit density was (very) low as there were no nestboxes for them and they needed to use natural cavities, but blue tit density was high.
-
- Effect of blue tit density on blue tit dispersal. In the control plot B we maintained configuration BOTH for 10 years (high great tit, high blue tit density), while in the experimental plot T the configuration was LARGE (high great tit, but low blue tit density) for 5 years, but BOTH in the other 5-year period.
-
I report on the effect of changes in configuration first for breeding dispersal, and then for local recruitment. I used a 3-way contingency table analysis for each sex separately to test to what extent the change in nestbox configuration leading to a change in breeding density of one species had an effect on either breeding dispersal or local recruitment. I performed the analyses separately for males and females because it is well known that both in great end blue tits females disperse further than males (Dhondt 1979; Matthysen et al. 2001). If the 3-way interaction is significant I report the significance of the 2-way interaction of interest, i.e. the Period x Movement (corrected for Plot) interaction. I could not test intraspecific effects for great tits as in the plots with small-holed nestboxes only we did not have information on great tit breeding. An earlier correlational analysis of great tit juvenile dispersal in Plot B showed a significant increase in the proportion juveniles dispersing with the number of fledged juveniles, indicating the impact of intraspecific competition on dispersal for this species (Dhondt 2012).
Effects on breeding dispersal
As the experimental configuration in the experimental plot was changed every 5 years we could only use the first 4 years of the experimental configuration.
Experimental changes of blue tit density and great tit breeding dispersal
The 3-way contingency analysis for males yields a significant three-way interaction (G2 = 10.6, df = 4, P = 0.031). The two-way interaction of interest Period x Movement(Plot) is not significant (G2 = 4.52, df = 2, P = 0.10), a result most likely resulting from the lack of any dispersal in the control plot. The proportion of dispersing female great tits, however, decreased from 14.1% at low blue tit density to 0% at high blue tit density, while remaining unchanged in the control plot, a very significant result (G2 = 12.72, df = 2, P = 0.0017). Combining the P-values of males and females using Fisher’s test for combining P-values of independent tests (Fisher 1954; Sokal and Rohlf 1995) yields a very significant result (χ2 = 17.36, df = 4, P < 0.01).
This strongly suggests that great tit breeding dispersal is less likely when blue tit density is high, suggesting that heterospecific attraction does operate and that great tits use blue tit density as an indicator of habitat quality (Table 2).
Experimental changes of great tit density and blue tit breeding dispersal
Neither for males (3-way interaction G2 = 3,8 df = 4, P = 0.43) nor for females (3-way interaction G2 = 2.88, df = 4, P = 0.58) does the breeding dispersal of blue tits change significantly between periods when great tit density was manipulated. The combined probability confirmed that (χ2 = 3.88, df = 4, P < 0.50).
Blue tit breeding dispersal was not influenced by great tit breeding density (Table 3).
Experimental changes of blue tit density and blue tit breeding dispersal
Neither for males (3-way interaction G2 = 7.52, df = 4, P = 0.11) nor for females (3-way interaction G2 = 5.62, df = 4, P = 0.23) does the breeding dispersal of blue tits change significantly between periods when blue tit density was manipulated.
Blue tit breeding dispersal was not influenced by blue tit breeding density (Table 4).
Density manipulations and local recruitment
As the experimental configuration in the experimental plot was changed every 5 years we could only use the last 4 years of the experimental configuration.
Experimental changes in blue tit density and great tit local recruitment (LR)
In control Plot B male great tit LR was around 31% in both periods while in the experimental Plot T great tit LR almost doubled from 9.8% in the low blue tit density period to 18.9% in the high blue tit period when small-holed boxes had been added. In females, we found a much weaker but similar effect. A 2-way contingency analysis yields a very significant result for the Period x Origin (Plot) in males (G2 = 18.2, df = 2, P = 0.0001) and a smaller but still significant effect in females (G2 = 6.42, df = 2, P = 0.04). When blue tit density was experimentally increased great tit LR increased, and this both for males and for females, indicating that heterospecific attraction influences great tit juvenile dispersal decisions that are positively influenced by blue tit density (Table 5).
Experimental changes in great tit density and blue tit local recruitment
Female blue tit LR was very low and did not vary significantly between periods or plots (2-way interaction Period x Origin (Plot) G2 = 0.06, df = 2, P = 0.97). Male blue tit LR did vary significantly between periods (two-way interaction Period x Origin(Plot) (G2 = 11.1, df = 2, P = 0.004). Male LR increased about 2.3 times when great tit density had been increased, while the increase in the control plot was about fivefold. Increasing great tit density reduced blue tit male LR (Table 6) suggesting that blue tits are more likely to disperse at high great tit density. Detailed behavioral observations of interactions between blue and great tits in the fall would need to confirm that juvenile blue tis are outcompeted by great tits at that time.
Experimental changes in blue tit density and blue tit local recruitment
In Plot T, after the nestbox configuration was changed from LARGE to BOTH, blue tit breeding density increased and LR of blue tit males increased eightfold. In the control plot B (configuration BOTH) the proportion local born males remained very high at 22% (Table 7). A 3-way contingency analysis yields a significant result (3-way interaction G2 = 12.16, df = 4, P = 0.016); the two-way interaction Period x Origin (Plot) was significant (G2 = 7.18, df = 2, P = 0.028) indicating that the increase in blue tit density coincided with an increase in blue tit LR. Blue tit female LR was very low and did not significantly increase when nestbox configuration was changed (3-way interaction G2 = 0.96, df = 4, P = 0.92; two-way interaction Period x Origin (Plot) G2 = 0.4, df = 2, P = 0.82).
Blue tit males were more likely to recruit in their birth plot when blue tit density was higher indicating conspecific attraction; in female blue tits density did not influence LR. The mechanism involved more than likely was the presence of small-holed nestboxes that provided blue tits with high quality roosting sites. However, it is unclear if it is the presence of small-holed nestboxes that leads to an increase in LR, which then leads to an increase in breeding density, or if both together cause this. The increase in breeding density was also the result of an increase in the number of immigrants.
Overview of effects of density manipulations on tit dispersal
Effects on great tit—When blue tit density was experimentally increased great tit adult breeding dispersal decreased and LR of great tit juveniles increased, and this for both sexes. Great tits of all ages and both sexes use blue tit density as an indication of habitat quality implying heterospecific attraction as a mechanism. This result is similar to that obtained by Parejo et al. (2007a).
Effects on blue tit—Changes in great tit density did not influence blue tit breeding dispersal for either sex. Adult blue tits did not use great tit breeding density as a measure of habitat quality. As regards local recruitment, most juvenile blue tit females dispersed with no effect of the changes in nestbox configuration. Blue tit males, though, used conspecific density and/or the presence of small-holed boxes for local recruitment decisions and were much more likely to breed locally in sites with high blue tit density and/or a large number of protected roosting sites. On the other hand, they were more likely to disperse when great tit density was increased.
It is interesting that in this system great tits use blue tit density as an index of habitat quality, while the subordinate blue tit either does not, or actually avoids areas with high great tit density which results in an increase in juvenile dispersal pre-empting interspecific competition.
How do interspecific competition and heterospecific attraction interact?
One of the referees commented that the paper does not fully discuss the consequences for the existence of social attraction next to negative competition. They write “the data now point in the direction that there are not only negative effects, but also positive effects of blue tit density on great tit performance. This makes the effects interpreted earlier as competition possibly a mixture between negative competition effects and positive attraction effects”.
To address this interesting remark it is necessary to separate heterospecific effects on reproduction on the one hand and on dispersal on the other. The experimental nestbox manipulations that result in an increase in breeding density have either a negative or no effect on reproduction for the other tit species implying that interspecific competition for food occurs during the breeding season and that this varies by habitat quality (Dhondt 2010). This could be considered a traditional effect of interspecific competition. Experimental increases of great tit density did not influence breeding dispersal of blue tits but caused a reduction in blue tit local recruitment. This would also represent an adverse effect of interspecific competition on blue tit juvenile dispersal.
The experimental increase in blue tit density following the increase in the number of small-holed nestboxes had an effect both on great tit breeding dispersal (reduced at high blue tit density) and local recruitment (increased at high blue tit density). Great tits, therefore, seemed to use blue tit density as an indicator of habitat quality that influenced their dispersal behavior. Any increase in great tit density resulting from this change in dispersal behavior would have impacted interspecific competition, but while their dispersal behavior was impacted their density was not influenced, although the origin of the birds was. Hence, this experiment did not impact possible effects of interspecific competition.
Changes in selection pressures and possible evolutionary effects of the long-term experiments
One of the objectives of the long-term experiment was to experimentally test the idea that selection pressures in experimental populations would differ from those in control populations and test the hypothesis that this would result in divergent changes, even in open plots in which a high proportion of the breeding populations were immigrants. One example possibly documenting such an (evolutionary) change was that observed for blue tit body size in plot T, although opposite to what we had expected. Note that this result is not replicated.
During the 1984–1988 breeding seasons the mean 15-day tarsus length and the mean 15-day mass of all first brood blue tit nestlings in Plots T (configuration SMALL) and B (configuration BOTH) remained largely similar (Fig. 2). After we added large-holed boxes in Plot T before the winter 1988–89, thereby increasing great tit density, things gradually changed over the next 5 breeding seasons 1989–1993: body mass tended to become smaller in Plot T than in Plot B, probably caused by the increase in interspecific competition for food, while tarsus length gradually increased in Plot T compared to Plot B. By 3 years after the change mean tarsus length in Plot T was the largest of the plots (Including Plot C), and by 5 years after the change they were significantly larger than in Plot B and larger than in any previous year (Fig. 2).
Mean tarsus length and mean body mass of 15-day blue tits nestlings across 10 years in Plot B (open symbol) and T (filled symbol). In Plot B (control) nestbox configuration BOTH (high great and high blue tit density) remained unchanged, while in Plot T the nestbox configuration in 1984–1988 SMALL (low great, high blue tit density) was changed to BOTH in 1989–1993 leading to an increase in the intensity of interspecific competition with great tits. Note how, compared to Plot B, blue tit nestling tarsus length increased during the second 5-year period in Plot T, while the body mass in the second 5 year period decreased
This result came as a complete surprise. First, it indicated that even in a high quality oak plot there was competition for food during the breeding season. Second, it suggested that the increase in interspecific competition would cause selection resulting in an increase in body size (tarsus length in tits, a structural size, no longer increases after nestlings are 15 days old). The question was: does this change reflect a genetic change, and if so, how did it come about? Heritability of blue tit tarsus length is pretty high. When in 1979, I exchanged clutches between females in Plot C to separate genetic and environmental effects on nestling size the mid-parent/mid-offspring heritability between nestlings and their biological parents that had not raised them was h2 = 0.62, with no additional influence of the foster parents. (Dhondt 1982). In the same year the estimate was h2 = 0.61 in Plots T and B where parents had raised their own young (Dhondt 1988). In the breeding seasons 1981–1986 the average heritability in Plot C, when parents raised their own young, was h2 = 0.62 (Dhondt 1988). The observed considerable change in tarsus length over a 5-year period therefore reflected a genetic change, especially since the nestlings in Plot T had become lighter, although they were larger. Note that on average tarsus length and body mass are positively correlated in tits (Garnett 1981). Furthermore, the adults breeding in 1993 also had a larger tarsus than in 1988, although in 1988 they were very similar to the adults in Plot B (Fig. 3) (and also in Plot C).The change in nestling tarsus length is the result of a change in the tarsus length of their parents (Fig. 4.) While in 1988, the year before the change in nestbox configuration, the measures of birds from both plots were intermingled, by 1991, the third breeding season following the change, many of the dots representing the Plot T birds had moved to the right indicating they were larger than the birds in Plot B; by 1993, both parent and offspring of Plot T birds were firmly on the right indicating that this strongly suggests an evolutionary change at the population level.
Blue tit mean tarsus length of first time breeding immigrants in Plot B (open symbol) and T (filled symbol) over two consecutive 5-year periods during which the intensity of interspecific competition with great tits was changed. In Plot B (control) nestbox configuration BOTH (high great and high blue tit density) remained unchanged, while in Plot T the nestbox configuration SMALL in 1984–1988 (low great, high blue tit density) was changed to BOTH leading to an increase in the intensity of interspecific competition with great tits. Note how, compared to Plot B, breeding immigrants became gradually larger during the second 5-year period in Plot
Mid-parent mid-offspring plots of blue tit tarsus length in Plot T (dark circles) and in Plot B (open triangles). Each point represents the measurements of one pair and its young. Note how the dots for plot T that were firmly embedded among those of plot B in 1988, gradually moved to the right and up within 3 years of the change in nestbox configuration, and were firmly to right after 5 years. This indicates that the increase in tarsus size of immigrant led to an increase in the size of their offspring
Textbook theory says that when two species compete for a limiting resource they will evolve to avoid competition by becoming more different. Blue tits compete with great tits, their larger counterpart, and is was therefore expected that an increase in the strength of interspecific competition would lead to blue tits becoming smaller, i.e., more different. But, we observe the opposite. How is this possible?
One hint comes from the observation that the change happened gradually and did not start until year 3 of the experiment. A second hint comes from the observation that blue tits not only compete for nestboxes as winter roosting sites with great tits (that mostly exclude them when the nestbox opening is 32 mm), but also compete with one another for this resource. Tits sleep alone in a box which in winter they defend also in day time (Drent 1987). As Kluyver (1957) found for great tits, blue tits roosting in nestboxes are also mostly males. As an example in December we found over the years 158 roosting blue tits in Plot B (82% males), 291 in Plot C (70% males) and after 1984, when small-holed boxes were available, 108 birds in Plot T (77% males). Blue tit values are actually more sex biased than for great tits in our Antwerp study sites with large-holed boxes in which 59% of 835 great tits found roosting were males. We can assume that in competition for this discrete resource larger individuals will usually win, so that when small-holed boxes are provided, the males occupying them will, on average, be larger. This would gradually increase the size of the breeding birds, leading to an increase in the size of their offspring. The provision of small-holed nestboxes in a site where they were previously absent, will, on the one hand, reduce the intensity of interspecific competition with great tits for cavities in general, but, on the other, increase the intensity for intraspecific competition for small-holed boxes benefiting the larger individuals. I am at a loss of what to call this. At first sight we cannot call this this evolution through natural selection, because although the trait selected for (body size) has considerable variation and has a high heritability, the mechanism is differential immigration not differential survival or reproduction. Unless, of course, larger individuals that use safe roosting sites in winter survive better than smaller individuals as was found by Piet Drent (1987) for great tits. Note also that plots B and T are located in the same forested area of about 200 ha and that a fair number of birds move between the plots as shown above. The fact that the size of the immigrant breeders was similar during the 1984–1988 period, but became so different between Plot T and B in the next 5 years (Fig. 4) indicates that the change in nestbox configuration in Plot T did, to some extent at least, drive the increasing difference between the sizes of the blue tits in the two plots, and might indicate that blue tits use the availability of protected roosting sites as one measure of habitat quality. It is unfortunate that I was not able to continue this experiment in which I manipulated nestbox types and hence density, nor that I had a second replicate plot.
Demographic and possible evolutionary effects of sparrowhawks on blue tit
Sparrowhawks Accipiter nisus, the main predators of great and blue tits (Opdam et al. 1987), were abundant in Europe until the late 1950s. The wide use of organochlorine pesticides caused their rapid decline, resulting in the absence of sparrowhawks from large parts of their range from the 1960s onwards. After the ban of DDT populations of sparrowhawks made a gradual comeback across Europe and the UK as organochlorine residues decreased (Newton and Wyllie 1992; Newton et al. 1999). In Wytham Woods, near Oxford, the first pair bred again in 1973 (Gosler et al. 1995). In The Netherlands and in Flanders (northern Belgium) the sparrowhawk made a gradual comeback from the late 1960s onwards https://nl.wikipedia.org/wiki/Sperwer_(vogel). Sparrowhawks prey year-round on tits (Opdam 1979). One of the many interesting impacts of sparrowhawks on tits was that great tits carried more fat in winter in the absence of the predator (Gosler et al. 1995) which was the result of individual adjustments (Gentle and Gosler 2001). When I started the nestbox studies near Antwerp in 1979 sparrowhawks were present in the large forested area north of Antwerp (Peerdsbos) in which Plots B and Plot T were situated, but absent in the isolated Plot C, just a few km away. Plot C was assigned to the “small-holed nestbox” treatment where blue tit breeding densities were very high at around 2.5 pairs/ha (Dhondt et al. 1990a, 1990b).
At the onset of the study we observed multiple important differences in the demographic traits of blue tits between Plot C, and both other plots in the Peerdsbos (Plot B and Plot T) in which sparrowhawks had been present before we started our studies in 1979 (Adriaensen et al. 1998; Dhondt et al. 1998). Given an average annual sparrowhawk survival of 69% (Newton et al. 1983) multiple different sparrowhawk individuals must have been present during the 15-year study and later also in Plot C. Following the settlement of a pair of sparrowhawks in Plot C rapid changes in the blue tit population occurred there. One of the reasons that we can evaluate these is that we had detailed observations both during a pre- and a post-sparrowhawk period in Plot C, while we had a control situation with sparrowhawks throughout in Plot B in which blue tit density was also high because of the presence of small-holed nestboxes. I here summarize these effects based on published sources and some novel analyses.
Effect of sparrowhawks on blue tit adult survival and age structure
In the period 1979–1988 blue tit adult annual survival rate in plot C (49%) was significantly higher than in plots B (34%) and T (36%). After the establishment of a pair of sparrowhawks in Plot C survival rate decreased there to 38%, while it did not significantly change in the other two plots (B: 33%; T: 34%) (Dhondt et al. 1998). In Plot C in the pre-sparrowhawk period blue tit annual survival rates of males (49%) and females (47%) were not different, but while in males there was no age effect, female annual survival rates varied between age classes: age 1: 49%; age 2: 59%; age ≥ 3: 30% (Dhondt et al. 1998). Not surprisingly the establishment of a sparrowhawk pair resulted in a substantial change in the age structure of the population. In the period 1979–1989 the proportion yearlings was 46.6% in males (n = 388) and 46.9% in females (n = 439). In the period 1990–1993 this increased to 52.0% in males (n = 179) and 59.3% in females (n = 182). The large difference in females compared to that in males suggests that the sparrowhawk effect on female survival was larger than that for males. Annual adult survival for males was 42% and for females 30%.
Effect of sparrowhawks on blue tit mating system
Blue tits in high quality habitat are frequently polygynous, but intense studies of color-banded birds are required to detect that. Although already in 1969 Lars Von Haartman listed the blue tit as a polygynous species (Von Haartman 1969) there were no detailed descriptions of polygyny in blue tits until we started our studies in Antwerp (Dhondt et al. 1983). Because we made every attempt to identify all nesting males in the Antwerp plots we were able to document that across all plots the minimum percentage of polygynous males was 8%, as we had observed those males feeding nestlings at 2 (once at 3) nests simultaneously. If we assumed that the nests at which we had not been able to detect a male were actually nests of secondary females that had been deserted by their partner the percentage polygynous males rose to 17% for the period between 1979 and 1985 (Dhondt 1987b). The same data showed that females in plot B followed the polygyny threshold model and preferentially settled in higher quality parts of a plot in which monogamous pairs recruited more offspring (Dhondt 1987a). We also found that in Plot C a high proportion of males did not breed as yearlings, implying there was a male surplus (Dhondt et al. 1990b). Two things happened in Plot C after those analyses: in 1990 a sparrowhawk pair settled in Plot C and Bart Kempenaers started fieldwork for his Ph.D. One of the things he did not ever observe were floater males as evidence of surplus males. This led to sometimes heated discussions in which he questioned my analyses, and in which I questioned his field observations. It turned out the we were both right because things had changed in Plot C following the settlement of a sparrowhawk. Through very careful and intense observations during 1990–1992 he did disentangle the multiple ways in which polygyny came about, a fascinating story (Kempenaers 1994, 1995). Bart discovered that polygyny could arise behaviorally in three very distinct ways: (1) year-round polygyny in which a male was paired to two females from early winter through the breeding season and sometimes longer. In this system both females used the entire male territory and did not show mutual aggression; (2) successive polygyny in which, after considerable strife, a 2nd female settled on the territory of a monogamous pair, but used only part of the male’s territory; often this was only possible after the primary female had started incubation; (3) replacement polygyny which arose after a female became widowed before laying and joined a neighboring male on his territory and settled after a period of strife with the primary female. We hypothesized that this latter type of polygyny in particular was the result of a sparrowhawk having taken her partner, and the lack of surplus males, because blue tits are frequently taken by sparrowhawks in early spring (Opdam 1979).
Effect of sparrowhawks on blue tit nestling and adult mass
The sparrowhawk pair had several more effects on this blue tit population. Whereas all great tit studies in the absence of sparrowhawks found that the heaviest fledglings were the most likely to be recruited (Perrins 1965; Dhondt 1971a; Garnett 1981) as was also the case for blue tits in Plot C before the sparrowhawks had settled, we were surprised to discover that there was selection against the heaviest nestling mass classes once sparrowhawks had settled (Adriaensen et al. 1998). The fact that such a result was also found in all four tit studies carried out across Europe after 1975, when sparrowhawks had become re-established (Julliard et al. 1996; Lindèn et al. 1992; Nur 1984; Tinbergen and Boerlijst 1990) implies that this was a causal effect. During the sparrowhawk period in Plot C, there was also a clear tendency for blue tits to lay a smaller clutch, for the nestlings to have a significant lower average fledgling mass (although no change in tarsus length) and a significant reduction in fledging success. As found by Gosler et al. (1995) for great tits adult winter body mass in blue tits also differed significantly between the two periods (Adriaensen et al. 1998).
Effect of sparrowhawks on the trade-off between reproduction and survival and considerations on life-history theory
A basic assumption in life-history theory is that a trade-off between reproduction and survival exists: species that invest more in reproduction generally suffer a higher mortality or vice versa. This has been documented across birds in general (Saether 1988) and also when limiting the analysis to tit species (Paridae) (Dhondt 2001) and even when comparing different populations of the same species (Dhondt 2001). In this latter comparison I documented a significant negative relationship between productivity (fledglings pair−1 season−1) and adult annual survival rate when comparing 8 great tit and 10 blue tit populations across Belgium and France. At the within-population level, however, a positive rather than a negative relationship between reproduction and survival can often be found because at that level differences in individual quality or territory quality can be sufficiently large that the amount of resources individuals can spend on life history traits varies substantially so as to override this inverse relationship (Van Noordwijk and Dejong 1986). These authors made the point that one of the reasons why producing more offspring may lead to fewer offspring reaching breeding age, is because intermediate clutches can be more productive. They illustrate their model with a graph in which the within population variation between reproduction and survival is perpendicular to the expected trade off line that has a negative slope.
The settlement of a breeding pair of sparrowhawks in Plot C resulted in (1) a substantial decrease in in adult survival rate; (2) a considerable decrease in average clutch size and number of young fledged, together with a reduction in their fledging weight; and (3) selection against the heaviest fledglings, leading to selection against the largest clutches. What is important to underline is that in Plot C, during the pre-sparrowhawk years 1979–1989, we observed large differences in survival rate between females that laid different clutch sizes: 142 blue tit females of 1 or 2 years of age that laid a first brood clutch of ≤ 11 eggs had a survival rate of 47%, while 151 females that laid a clutch ≥ 12 eggs had a substantially and significantly larger survival rate at 61% [data from (Dhondt et al. 1990b). Furthermore, when winters were warm females that laid a larger clutch survived substantially better than females that laid a smaller clutch while the opposite was true, but to a lesser extent, when winters were cold (i.e. with extended periods of frost) (Table 8) (Dhondt et al. 1990b). In the sparrowhawk years things changed. Not only did adult mortality of both sexes combined on average decrease by about 12% per year, and did the proportion of breeding yearling birds substantially increase, female survival as a function of clutch size also changed. Whereas in the period 1979–89 females laying a larger clutch survived better this was no longer the case during the period when sparrowhawks bred in Plot C (Table 8). Including all females, survival of the birds laying a clutch larger than modal decreased from 55 to 26%, a 53% decline, while in females laying a clutch smaller than modal survival decreased from 41 to 33%, a decline of 20%. The results are similar for yearling females only (Table 9): in pre-sparrowhawk years birds laying a clutch ≤ 11 survived less well than those laying a clutch ≥ 12, but the latter suffered more in the presence of the newly established predator. The predator pressure of the sparrowhawk has an effect quantitatively similar to that of a cold winter.
What is interesting about all this, although these results are not replicated and are not technically experimental, is that the position of the Plot C population in the trade-off diagram has now moved below the tradeoff line that I calculated previously using 10 blue tit populations from Belgium and France (Fig. 5). This can help us understand how populations move between different positions on the trade-off line when selection pressures change. When conditions deteriorate—as caused by cold winters or the arrival of a predator—fewer young survive to breed to the next year and adult survival is also impacted. If the new conditions persist these changes will select for different phenotypes: in many tit populations in high quality habitat there is selection for a larger clutch as long as this leads to a higher recruitment rate and there is no survival cost to the adult female. That was the case in Plot C in pre-sparrowhawk years (Dhondt et al. 1990b). When conditions deteriorated the previously “high quality” females that survived well and were able to lay a large clutch resulting in many recruits, not only lost their advantage of raising more offspring, but are also paid an individual survival cost; this was larger than the cost paid by the pre-sparrowhawk “low quality” females that survived less well and produced fewer young. This resulted in selection for females that laid a smaller clutch. This will eventually lead to a change in the reproductive output at the population level, that may allow the survival rate of the population to increase towards he trade-off line to the left of where it started. This will be a relatively slow process because female blue tits disperse relatively far (Matthysen et al. 2001), but might be accelerated because, as shown many years ago by Arie Van Noordwijk in the pre-climate change period, annual variations are often sufficient to cause a detectable evolutionary change from one year to the next as regards laydate (Van Noordwijk et al. 1980).
Trade-off line between reproduction (number of young fledged female−1 year−1) and adult (both sexes combined) annual survival rate calculated using SURGE, a capture-mark-recapture software (Lebreton et al. 1992) for 10 populations of blue tits in Belgium and France: from left to right: Pirio: Corsica, F; CI: Citadelpark, Ghent, B; Ventoux, southern France; MA: Maaltepark, Ghent, B; HU: Hutsepot, Ghent, B; ZE:Zevergem, Ghent, B; C-a: Plot C, Antwerp, before sparrowhawk period; C-b: Plot C with sparrowhawk; T: Plot T, Antwerp; B: plot B, Antwerp, B; GO: Gontrode, Ghent, B. The trade-off line is calculated using all the sites represented by an open triangle, thus without C-b; The line connecting the dots of C-a and C-b represents the sparrowhawk effect that reduced both productivity and adult survival. More detailed information on study plots in (Dhondt et al. 1996)
It is interesting to underline that there are possible direct maternal sparrowhawk effects on blue tits. Thus nestlings of great tit females that were exposed experimentally to a perceived predation risk, but raised by non-exposed foster parents, were smaller than controls but showed a higher growth rate of the wings, leading to recruits with longer wings than control offspring of control birds (Coslovsky and Richner 2011).
While this report on effects of a new predator is a “natural experiment” and hence not a real experiment (although in this case there is a control plot) and its results need to be handled carefully, such natural experiments can provide interesting results as illustrated by a somewhat similar non-experimental study. It too illustrates the non-lethal impact of predators on a previously predator free population of Audouin’s gulls (Ichthyaetus audouinii) colony. When predators settled the gulls moved closer to the water, reduced clutch size, and decreased egg volume (Payo-Payo et al. 2018).
The way forward
I will limit myself here to a couple of examples of what could be done to gain a better understanding of competition and heterospecific attraction by using new techniques or by addressing poorly studied systems. In what follows, I limit myself to one example of each.
In many cases interspecific competition in birds is for food. To obtain large samples one needs to use a non-lethal method. As already shown by MacArthur’s observational studies of foraging niches such observations made it possible to calculate overlap in diet between potentially competing species, and by combining this with observed foraging technique determine the extent to which individuals compete (MacArthur 1958). By combining such observational foraging studies with metabarcoding of fecal samples it now becomes posisble to identify what birds eat, not just where and how they find their food. The method provides very detailed results as illustrated by studies of diet overlap between coexisting species (Hoenig et al. 2022; Trevelline et al. 2018a; Trevelline et al. 2018b; Zurdo et al. 2023). If I were to repeat my experimental manipulations of tits this technique would deepen our understanding of what intra- and interspecific competition is for exactly. I can even imagine comparing the feces of different nestlings in the same nest in species like great and blue tit that are mostly single loaders, to determine how food choice of the parents changes with nestling age, season, year, habitat, or even by nestling sex within a brood in species where parents selectively feed male or female offspring as found in eastern bluebirds Sialia sialis (Droge et al. 1991).
An example of a problem that needs to be studied urgently concerns how resident tropical birds are impacted by the billions of birds that migrate to join them each year during the northern winter. While many excellent studies exist on behavior and ecology of temperate zone bird when wintering in the tropics it is surprising how few studies have addressed the impact of these billions of overwintering birds on the tropical residents, a point I made earlier (Dhondt 2012). Now 11-year later there are still surprisingly few such studies and most are local or short-term (Leisler 1992; O'Donnell et al. 2014; Randler 2013; Randler et al. 2010, 2015; Salewski et al. 2002, 2003). We absolutely need to understand if and how and which species are impacted so as to develop better conservation strategies. By adding metabarcoding of feces to observational studies we can determine if the diet of residents changes when migrants arrive, to what extent their diets overlap enough for them to compete, if tropical residents delay breeding until migrants have left, etc. A challenge will certainly be to carry out experimental studies to validate any conclusions and also as we can expect that diffuse competition could be frequent.
Data availability
All data are availble in the SPI-Birds data hub (Culina et al 2021).
References
Adriaensen F, Dhondt AA, Van Dongen S, Lens L, Matthysen E (1998) Stabilizing selection on blue tit fledgling mass in the presence of sparrowhawks. Proc Royal Soc Lond Series B-Biol Sci 265:1011–1016
Alatalo RV, Lundberg A, Bjorklund M (1982) Can the song of male birds attract other males—an experiment with the pied flycatcher Ficedula hypoleuca. Bird Behav 4:42–45
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. The University of Chicago Press, Chicago
Baeyens G (1979) Description of the social behavior of the magpie (Pica pica). Ardea 67:28–41
Birch LC (1971) The role of environmental heterogeneity and genetical heterogeneity in deterining distribution and abundance. In: den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations' Oosterbeek 1970. PUDOC, Wageningen, pp 109–128
Boag PT, Grant PR (1978) Heritability of external morphology in Darwin finches. Nature 274:793–794
den Boer PJ (1971) Stabilization of animal numbers and the heterogeneity of the environment: the problem of the persistence of sparse populations. In: Den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations' Oosterbeek 1970. PUDOC, Wageningen, pp 77–97
Brown JL (1969) Territorial behavior and population regulation in birds. Wilson Bull 81:293–329
Cayuela H, Grolet O, Joly P (2018) Context-dependent dispersal, public information, and heterospecific attraction in newts. Oecologia 188:1069–1080. https://doi.org/10.1007/s00442-018-4267-3
Chitty D (1967) What regulates bird populations. Ecology 48:698–701
Cockle KL, Martin K, Drever MC (2010) Supply of tree-holes limits nest density of cavity-nesting birds in primary and logged subtropical Atlantic forest. Biol Cons 143:2851–2857
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on ‘Dynamics of Numbers in Populations’ Oosterbeek 1970. PUDOC, Wageningen, pp 298–312
Connell JH (1980) Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35:131–138
Cornelius C et al (2008) Cavity-nesting birds in neotropical forests: cavities as a potentially limiting resource. Ornitologia Neotropical 19:253–268
Coslovsky M, Richner H (2011) Predation risk affects offspring growth via maternal effects. Funct Ecol 25:878–888. https://doi.org/10.1111/j.1365-2435.2011.01834.x
Culina A et al (2021) Connecting the data landscape of long-term ecological studies: the SPI-Birds data hub. J Anim Ecol 90:2147–2160. https://doi.org/10.1111/1365-2656.13388
Danchin E, Giraldeau LA, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Dhondt AA (1971a) The regulation of numbers in Belgian populations of great tits. In: Den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations' Oosterbeek 1970. PUDOC, Wageningen, pp 532–547
Dhondt AA (1971b) Some factors influencing territory in the great tit Parus major L. Gerfaut - Giervalk 61:125–135
Dhondt AA (1977) Interspecific competition between great and blue tit. Nature 268:521–523
Dhondt AA (1979) Summer dispersal and survival of juvenile great tits in Southern Sweden. Oecologia 42:139–157
Dhondt AA (1982) Heritability of blue tit tarsus length from normal and cross-fostered broods. Evolution 36:418–419
Dhondt AA (1987a) Polygynous blue tits and monogamous great tits—does the polygyny-threshold model hold. Am Nat 129:213–220. https://doi.org/10.1086/284631
Dhondt AA (1987b) Reproduction and survival of polygynous and monogamous blue tit Parus caeruleus. Ibis 129:327–334. https://doi.org/10.1111/j.1474-919X.1987.tb03176.x
Dhondt AA (1988) The necessity of population genetics for understanding evolution: an ecologist’s view. In: De Jong G (ed) Population Genetics and Evolution. Springer-Verlag, Berlin, pp 14–18
Dhondt AA (2001) Trade-offs between reproduction and survival in Tits. Ardea 89:155–166
Dhondt AA (2010) Effects of competition on great and blue tit reproduction: intensity and importance in relation to habitat quality. J Anim Ecol 79:257–265
Dhondt AA (2012) Interspecific competition in birds. Oxford University Press, Oxford
Dhondt AA, Adriaensen F (1999) Experiments on competition between great and blue tit: effects on blue tit reproductive success and population processes. Ostrich 70:39–48
Dhondt AA, Eyckerman R (1980a) Competition and the regulation of numbers in great and blue tit. Ardea 68:121–132
Dhondt AA, Eyckerman R (1980b) Competition between the great tit and the blue tit outside the breeding season in field experiments. Ecology 61:1291–1296
Dhondt AA, Hublé J (1968) Fledging date and sex in relation to dispersal in young great tits. Bird Stud 15:127–234
Dhondt AA, Eyckerman R, Hublé J (1979) Will great tits become little tits. Biol J Lin Soc 11:289–294
Dhondt AA, Eyckerman R, Schillemans J (1983) Polygyny by blue tits. British Birds 76:34–36
Dhondt AA, Adriaensen F, Matthysen E, Kempenaers B (1990a) Nonadaptive clutch sizes in tits. Nature 348:723–725
Dhondt AA, Matthysen E, Adriaensen F, Lambrechts MM (1990b) Population dynamics and regulation of a high density Blue Tit population. In: Blondel J, Gosler A, Lebreton J-D, McCleery R (eds) Population Biology of Passerine Birds. Springer Verlag, Berlin, Heidelberg, pp 39–53
Dhondt AA, Kempenaers B, De Laet J (1991) Protected winter roosting sites as a limiting resource for Blue Tits. Acta XX Congressus Internationalis Ornithologici New Zealand Ornithological Congress trust Board, Christchurch, New Zealand. 1436–1443
Dhondt AA, Adriaensen F, Plompen W (1996) Between- and within-population variation in mate fidelity in the Great Tit. In: Black JM (ed) Partnerships in Birds. Oxford University Press, Oxford, pp 235–248
Dhondt AA, Kempenaers B, Clobert J (1998) Sparrowhawk Accipiter nisus predation and blue tit Parus caeruleus adult annual survival rate. Ibis 140:580–584. https://doi.org/10.1111/j.1474-919X.1998.tb04702.x
Doligez B, Danchin E, Clobert J, Gustafsson L (1999) Tbe use of conspecific reproductive success for breeding habitat selection in a non-colonial, hole-nesting species, the collared flycatcher. J Anim Ecol 68:1193–1206. https://doi.org/10.1046/j.1365-2656.1999.00362.x
Doligez B, Danchin E, Clobert J (2002) Public information and breeding habitat selection in a wild bird population. Science 297:1168–1170
Doligez B, Part T, Danchin E (2004) Prospecting in the collared flycatcher: gathering public information for future breeding habitat selection? Anim Behav 67:457–466. https://doi.org/10.1016/j.anbehav.2003.03.010
Drent PJ (1984) Mortality and dispersal in summer and its consequences for the density of great tits Parus major at the onset of autumn. Ardea 72:127–162
Drent PJ (1987) The importance of nestboxes for territory settlement, survival and density of the great tit. Ardea 75:59–71
Droge DL, Gowaty PA, Weathers WW (1991) A test for differences in field metabolic rates of nestling Eastern Bluebirds. Condor 93:793–798. https://doi.org/10.2307/3247713
East ML, Perrins CM (1988) The effect of nestboxes on breeding populations of birds in broadleaved temperate woodlands. Ibis 130:393–401
Ens BJ, van de Pol M, Goss-Custard JD (2014) The study of career decisions: oystercatchers as social prisoners. In: Naguib M, Barrett L, Brockmann HJ, Healy S, Mitani JC, Roper TJ, Simmons LW (eds) Advances in the Study of Behavior, vol 46. pp 343–420
Fisher RA (1954) Statististical methods for research workers, 12th edn. Edinburgh, Oliver and Boyd
Forsman JT, Hjernquist MB, Taipale J, Gustafsson L (2008) Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav Ecol 19:539–545
Fretwell SD, Lucas HL (1969) On territorial behavior and other factors influencing habitat distribution in birds. 1 theoretical development. Acta Biotheor 19:16–36
Garnett MC (1981) Body size, its heritability and influence on juvenile survival among great tits, Parus major. Ibis 123:31–41
Gause GF (1934) The struggle for existence. The Williams & Wilkins company, Baltimore
Gentle LK, Gosler AG (2001) Fat reserves and perceived predation risk in the great tit, Parus major. Proc Royal Soc Lond Ser B-Biol Sci 268:487–491
Gosler AG, Greenwood JJD, Perrins C (1995) Predation risk and the cost of being fat. Nature 377:621–623
Goymann W, Landys MM (2011) Testosterone and year-round territoriality in tropical and non-tropical songbirds. J Avian Biol 42:485–489. https://doi.org/10.1111/j.1600-048X.2011.05464.x
Hairston NG (1989) Ecological experiments : purpose, design, and execution. Cambridge University Press, Cambridge
Hoenig BD, Trevelline BK, Kautz A, Latta SC, Porter BA (2022) Two is better than one: coupling DNA metabarcoding and stable isotope analysis improves dietary characterizations for a riparian-obligate, migratory songbird. Mol Ecol 31:5635–5648. https://doi.org/10.1111/mec.16688
Hogstad O (1989) The presence of non-territorial males in willow warbler Phylloscopus trochilus populations—a removal study. Ibis 131:263–267
Howard H (1920) Territory in bird life. Murray, London
Huxley JS (1934) A natural experiment on the territorial instinct. British Birds 27:207–277
Jaakkonen T, Kivela SM, Meier CM, Forsman JT (2015) The use and relative importance of intraspecific and interspecific social information in a bird community. Behav Ecol 26:55–64. https://doi.org/10.1093/beheco/aru144
Julliard R, Perret P, Blondel J (1996) Reproductive strategies of philopatric and immigrant blue tits. Acta Oecol-Int J Ecol 17:487–501
Keddy PA (1989) Competition. Chapman and Hall, London, New York
Kempenaers B (1994) Polygyny in the blue tit—Unbalanced sex-ratio and female aggression restrict mate choice. Anim Behav 47:943–957. https://doi.org/10.1006/anbe.1994.1126
Kempenaers B (1995) Polygyny in the blue tit—intra-sexual and inter-sexual conflicts. Anim Behav 49:1047–1064. https://doi.org/10.1006/anbe.1995.0134
Kempenaers B, Dhondt AA (1991) Competition between blue and great tit for roosting sites in Winter—an aviary experiment. Ornis Scand 22:73–75
Kluijver HN (1951) The population ecology of the great tit. Parus m major L Ardea 39:1–135
Kluijver HN, Tinbergen L (1953) Territory and the regulation of density in titmice. Arch Néerl De Zool 10:265–289
Kluyver HN (1957) Roosting habits, sexual dominance and survival in the great tit. Cold Spring Harb Symp Quant Biol 22:281–285
Kluyver HN (1971) Regulation of numbers in populations of great tits (Parus m. major). In: den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations, Oosterbeek 1970. PUDOC, Wageningen, pp 507–531
Knapton RW, Krebs JR (1974) Settlement patterns, territory size, and breeding density in song sparrow (Melospiza melodia). Can J Zool 52:1413–1420
Krebs JR (1971) Territory and breeding density in great tit, Parus major L. Ecology 52:2–22
Krebs JR (1977) Song and territory in the great tits Parus major. In: Stonehouse B, Perrins CM (eds) Evolutionary Ecology. University Park Press, Baltimore, pp 43–62
Lack D (1954) The natural regulation of animal numbers. Clarendon Press, Oxford
Lack D (1966) Population studies of birds. Clarendon Press, Oxford
Lack D (1971) Ecological isolation in birds. Harvard University Press, Cambridge
Lack D, Lack L (1933) Territory review. British Birds 27:179–199
Lambrechts M, Dhondt AA (1988) Male quality and territory quality in the great tit Parus major. Anim Behav 36:596–601
Law R, Watkinson AR (1989) Competition. In: Cherrett JM (ed) Ecological Concepts. Blackwell Scientific Publications, Oxford, pp 243–284
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals—a unified approach with case-studies. Ecol Monogr 62:67–118. https://doi.org/10.2307/2937171
Leisler B (1992) Habitat selection and coexistence of migrants and Afrotropical residents. Ibis 134:77–82
Lindèn M, Gustafsson L, Part T (1992) Selection on fledging mass in the collared flycatcher and the great tit. Ecology 73:336–343
Löhrl H (1977) Nistökologische und ethologische anpassungserscheinungen bei höhlenbrütern. Die Vogelwarte 29:92–101
MacArthur RH (1958) Population ecology of some warblers of northeastern coniferous forests. Ecology 39:599–619
Matthysen E (1990) Nonbreeding social organisation in Parus. In: Power DM (ed) Current Ornithology, vol 7. Plenum Press, New York, pp 209–249
Matthysen E, Adriaensen F, Dhondt AA (2001) Local recruitment of great and blue tits (Parus major, P. caeruleus) in relation to study plot size and degree of isolation. Ecography 24:33–42
Matthysen E, Adriaensen F, Dhondt AA (2011) Multiple responses to increasing spring temperatures in the breeding cycle of blue and great tits (Cyanistes caeruleus, Parus major). Glob Change Biol 17:1–16. https://doi.org/10.1111/j.1365-2486.2010.02213.x
Medina-Estrada J, Remolina-Figueroa D, Ramirez-Bastida P, Vazquez-Reyes LD (2022) Nesting resources availability for cavity adopter birds in a tropical dry forest of Central Mexico. Rev Mex De Biodivers. https://doi.org/10.22201/ib.20078706e.2021.93.3836
Minot EO, Perrins CM (1986) Interspecific interference competition—nest sites for blue and great tits. J Anim Ecol 55:331–350
Mönkkönen L, Forsman J (2002) Heterospecific attraction among forest birds: a review. Ornithol Sci 1:41–51
Mönkkönen M, Helle P, Soppela K (1990) Numerical and behavioral responses of migrant passerines to experimental manipulation of resident tits (Parus spp): heterospecific attraction in northern breeding bird communities. Oecologia 85:218–225
Moore IT, Vernasco BJ, Escallon C, Small TW, Ryder TB, Horton BM (2019) Tales of testosterone: advancing our understanding of environmental endocrinology through studies of neotropical birds. Gen Comp Endocrinol 273:184–191. https://doi.org/10.1016/j.ygcen.2018.07.003
Newton I (1972) Finches. Collins, London
Newton I (1992) Experiments on the limitation of bird numbers by territorial behavior. Biol Rev Camb Philos Soc 67:129–173. https://doi.org/10.1111/j.1469-185X.1992.tb01017.x
Newton I (1994a) Experiments on the limitation of bird breeding densities—a review. Ibis 136:397–411
Newton I (1994b) The role of nest sites in limiting the numbers of hole-nesting birds—a review. Biol Cons 70:265–276
Newton I, Wyllie I (1992) Recovery of a sparrowhawk population in relation to declining pesticide contamination. J Appl Ecol 29:476–484. https://doi.org/10.2307/2404515
Newton I, Marquiss M, Rothery P (1983) Age structure and suvival in a sparrowhawk popularion. J Anim Ecol 52:591–602
Newton I, Wyllie I, Dale L (1999) Trends in the numbers and mortality patterns of sparrowhawks (Accipiter nisus) and kestrels (Falco tinnunculus) in Britain, as revealed by carcass analyses. J Zool 248:139–147. https://doi.org/10.1111/j.1469-7998.1999.tb01190.x
Nice MM (1941) The role of territory in bird life. Am Midl Nat 26:441–487
Nicolaus M, Both C, Ubels R, Edelaar P, Tinbergen JM (2009) No experimental evidence for local competition in the nestling phase as a driving force for density-dependent avian clutch size. J Anim Ecol 78:828–838
Nicolaus M et al (2012) Social environment affects juvenile dispersal in great tits (Parus major). J Anim Ecol 81:827–837. https://doi.org/10.1111/j.1365-2656.2012.01959.x
Nour N, Currie D, Matthysen E, Van Damme R, Dhondt AA (1998) Effects of habitat fragmentation on provisioning rates, diet and breeding success in two species of tit (great tit and blue tit). Oecologia 114:522–530. https://doi.org/10.1007/s004420050476
Nur N (1984) The consequences of brood size for breeding blue tits.2. nestling weight, offspring survival and optimal brood size. J Anim Ecol 53:497–517. https://doi.org/10.2307/4530
O’Donnell S, Kumar A, Logan CJ (2014) Do nearctic migrant birds compete with residents at army ant raids? A geographic and seasonal analysis. Wilson J Ornithol 126:474–487. https://doi.org/10.1676/13-109.1
Opdam P (1979) Feeding ecology of a sparrowhawk population (Accipiter-nisus). Ardea 66:137–155
Opdam P, Burgers J, Muskens G (1987) Population trend, reproduction, and pesticides in Dutch sparrowhawks following the ban on DDT. Ardea 75:205–212
Parejo D, Aviles JM (2016) Social information use by competitors: resolving the enigma of species coexistence in animals? Ecosphere. https://doi.org/10.1002/ecs2.1295
Parejo D, White J, Clobert J, Dreiss A, Danchin E (2007a) Blue tits use fledgling quantity and quality as public information in breeding site choice. Ecology 88:2373–2382
Parejo D, White J, Danchin E (2007b) Settlement decisions in blue tits: difference in the use of social information according to age and individual success. Naturwissenschaften 94:749–757
Parejo D, Danchin E, Silva N, White JF, Dreiss AN, Aviles JM (2008) Do great tits rely on inadvertent social information from blue tits? A habitat selection experiment. Behav Ecol Sociobiol 62:1569–1579
Payo-Payo A et al (2018) Predator arrival elicits differential dispersal, change in age structure and reproductive performance in a prey population. Sci Rep 8:1971. https://doi.org/10.1038/s41598-018-20333-0
Perrins CM (1965) Population fluctuations and clutch-size in the great tit, Parus major L. J Anim Ecol 34:601–647
Randler C (2013) Do migrants influence the foraging behaviour of the insectivorous Cyprus Wheatear, Oenanthe cypriaca, at a stopover site? (Aves: Passeriformes). Zool Middle East 59:196–202. https://doi.org/10.1080/09397140.2013.841421
Randler C, Teichmann C, Pentzold S (2010) Breeding habitat preference and foraging of the Cyprus Wheatear Oenanthe cypriaca and niche partitioning in comparison with migrant Oenanthe species on Cyprus. J Ornithol 151:113–121
Randler C, Pentzold S, Pentzold C (2015) Foraging behaviour of insectivorous migrants and a resident songbird at a stopover site. Biologia 70:141–149. https://doi.org/10.1515/biolog-2015-0015
Reynoldson TB, Bellamy PE (1971) The establishement of interspecific competition in field populations, with an example of competition in action between Polycelis nigra (Mull.) and P. tenuis (Ijima) (Turbellaria, Tricladida). In: den Boer J, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations' Oosterbeek 1970. PUDOC, Wageningen, pp 282–297
Robles H, Ciudad C, Matthysen E (2012) Responses to experimental reduction and increase of cavities by a secondary cavity-nesting bird community in cavity-rich pyrenean oak forests. For Ecol Manage 277:46–53. https://doi.org/10.1016/j.foreco.2012.04.017
Saether BE (1988) Pattern of covariation between life-history traits of European birds. Nature 331:616–617. https://doi.org/10.1038/331616a0
Salewski V, Falk KH, Bairlein F, Leisler B (2002) A preliminary assessment of the habitat selection of two Palaearctic migrant passerine species in West Africa. Ostrich 73:114–118. https://doi.org/10.1080/00306525.2002.11446739
Salewski V, Bairlein F, Leisler B (2003) Niche partitioning of two Palearctic passerine migrants with Afrotropical residents in their West African winter quarters. Behav Ecol 14:493–502. https://doi.org/10.1093/beheco/arg021
Samplonius JM, Both C (2019) Climate change may affect fatal competition between two bird species. Curr Biol 29:327. https://doi.org/10.1016/j.cub.2018.11.063
Smith SM (1967) Seasonal changes in the survival of the black-capped chickadee. Condor 69:344–359
Smith SM (1978) Underworld in a territorial sparrow—adaptive strategy for floaters. Am Nat 112:571–582
Smith SM (1987) Responses of floaters to removal experiments on wintering chickadees. Behav Ecol Sociobiol 20:363–367
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Solomon ME (1971) Elements in the development of population dynamics. In: Den Boer PJ, Gradwell GR (eds) Proceedings of the Advanced Study Institute on 'Dynamics of Numbers in Populations' Oosterbeek 1970. PUDOC, Wageningen. PUDOC, Wageningen
Stam CW (1968) Gecoordineerd nestkastenonderzoek in benelux. Het Vogeljaar 16:585–599
Stamps JA (1988) Conspecific attraction and aggregation in territorial species. Am Nat 131:329–347
Tarazona-Tubens FL, Abadi F, Britt CR, Muschamp M, Desmond MJ (2022) Nest box placement influences occupancy by yellow-headed (Amazona oratrix) and white-fronted (Amazona albifrons) parrots in the pine savannas of Belize. Avian Conserv Ecol. https://doi.org/10.5751/ace-02234-170230
Tinbergen JM, Boerlijst MC (1990) Nestling weight and survival in individual great tits (Parus major). J Anim Ecol 59:1113–1127. https://doi.org/10.2307/5035
Török J (1993) The predator-prey size hypothesis in 3 assemblages of forest birds. Oecologia 95:474–478
Török J, Tóth L (1999) Asymmetric competition between two tit species: a reciprocal removal experiment. J Anim Ecol 68:338–345
Trautz AC, Illangasekare TH, Rodriguez-Iturbe I (2017) Role of co-occurring competition and facilitation in plant spacing hydrodynamics in water-limited environments. Proc Natl Acad Sci USA 114:9379–9384. https://doi.org/10.1073/pnas.1706046114
Trevelline BK, Nuttle T, Hoenig BD, Brouwer NL, Porter BA, Latta SC (2018a) DNA metabarcoding of nestling feces reveals provisioning of aquatic prey and resource partitioning among neotropical migratory songbirds in a riparian habitat. Oecologia 187:85–98. https://doi.org/10.1007/s00442-018-4136-0
Trevelline BK et al (2018b) Stream acidification and reduced aquatic prey availability are associated with dietary shifts in an obligate riparian neotropical migratory songbird. PeerJ 6:1. https://doi.org/10.7717/peerj.5141
Van Noordwijk AJ, Dejong G (1986) Acquisition and allocation of resources - their influence on variation in life-history tactics. Am Nat 128:137–142
Van Noordwijk AJ, Van Balen JH, Scharloo W (1980) Heritability of ecologically important traits in the great tit. Ardea 68:193–203
Von Haartman L (1969) Nest-size and polygamy in European passerine birds. Ornis Fennica 16:1–12
Watson A, Jenkins D (1968) Experiments on population control by territorial behaviour in red grouse. J Anim Ecol 37:595–614
Watson A, Miller GR (1971) Territory size and aggression in a fluctuating red grouse population. J Anim Ecol 40:367. https://doi.org/10.2307/3251
Wesolowski T (2007) Lessons from long-term hole-nester studies in a primeval temperate forest. J Ornithol 148:S395–S405. https://doi.org/10.1007/s10336-007-0198-1
Wesolowski T, Czeszczewik D, Hebda G, Maziarz M, Mitrus C, Rowinski P (2015) 40 years of breeding bird community dynamics in a primeval temperate forest (Bialowieza National Park, Poland). Acta Ornithologica 50:95–120. https://doi.org/10.3161/00016454ao2015.50.1.010
Wiens JA (1989) The ecology of bird communities. Cambridge University Press, Cambridge
Wolda G (1913) Kultuur van in ’t wild levende vogels. 1912. Tijdschrift over Plantenziekten 19:68–90
Zurdo J et al (2023) Dietary niche overlap and resource partitioning among six steppe passerines of Central Spain using DNA metabarcoding. Ibis 165:905
Acknowledgements
I am very grateful to the multiple colleagues, students and other Ghent and Antwerp staff who over the years have helped with collecting, proofing, discussing and organizing the data. The work would not have been possible without repeated funding by the Belgian National Fund for Scientific Research. I am grateful to the three referees who provided extensive and critical feedback leading to extensive edits.
Funding
Given that this paper is mostly based on published sources any required permissions for work with live animals is reported in those papers. For the same reason no new funding sources were acquired to write this paper. The work would not have been possible without repeated funding by the Belgian National Fund for Scientific Research.
Author information
Authors and Affiliations
Contributions
AAD conceived and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interests
I declare that there are no conflicts of interest.
Additional information
Communicated by Andreas Nord.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhondt, A.A. Effects of competition and predation operating at individual and population levels: an overview of results from a long-term field experiment. Oecologia 203, 277–296 (2023). https://doi.org/10.1007/s00442-023-05448-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05448-0