Abstract
Seabirds breeding in the high Arctic contend with variable annual sea ice conditions, with important consequences depending on a species’ unique reproductive and foraging ecology. We assessed the influence of sea ice extent and phenology on seabird breeding biology using monitoring data collected for northern fulmar (Fulmarus glacialis), glaucous gull (Larus hyperboreus), black-legged kittiwake (Rissa tridactyla), and thick-billed murre (Uria lomvia) breeding at Prince Leopold Island, Nunavut, Canada over 4 decades. We expected that years of later sea ice break-up and greater ice cover around the colony would create greater challenges to foraging and could result in delayed nest initiation, decreased colony attendance, and lower nesting success, but with distinct responses from each species. We also tested for time-lagged effects of ice conditions, where sea ice in a given year could impact food availability or juvenile recruitment in later years. Ice conditions around the colony exhibited no significant overall temporal trends or changepoints over the past 50 years (1970–2021), while counts of kittiwakes and murres increased over the study period 1975–2013. No trends were evident in counts of fulmars or gulls or in egg-laying dates or nest success for any species. However, three species (all but glaucous gulls) exhibited unique responses between breeding metrics and sea ice, highlighting how breeding decisions and outcomes may differ among species under the same environmental conditions in a given year. Time-lagged effects were only detected for kittiwake nest counts, where the date of spring ice break-up around the colony was negatively associated with counts at a 5-year lag. Greater distances to open water were associated with lower colony attendance by fulmars and later nest initiation by kittiwakes and murres. Our analyses provide additional insights to effects of sea ice on high-Arctic seabird breeding ecology, which will be useful in predicting and planning for the complex effects of a changing climate and changing human pressures on this high-latitude ecosystem and for the management of high-Arctic marine-protected areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is transforming the Arctic, where temperatures have warmed three times faster than the global average over the past 50 years (AMAP 2021). In the Canadian Arctic Ocean, the marine environment is shifting at an unprecedented rate in response to the changing climate (Bush and Lemmen 2019; Niemi et al. 2019). Understanding ecological relationships in this system is hindered by a lack of long-term scientific monitoring of ocean conditions and wildlife. Fortunately, the single, most influential feature of this marine environment, sea ice, has been monitored by satellite since the 1970s (Niemi et al. 2019). The presence and structure of ice dictates how the atmosphere interacts with the ocean, ocean water biogeochemistry (e.g., light and nutrient availability, acidity, salinity), availability of habitat for flora and fauna above and below the ice surface, the timing and location of annual events (i.e., many species coordinate breeding and feeding with seasonal productivity blooms), and in turn how energy is transferred through the food web (e.g., Post et al. 2013). In the Canadian Arctic, regional variation is evident in changes to sea ice phenology, but overall, sea ice is breaking up earlier in the summer and forming later in the fall (Niemi et al. 2019). This longer open-water period affects the dynamics of ice algae and phytoplankton at the foundation of food webs (Arrigo et al. 2008), which in turn influences upper trophic levels.
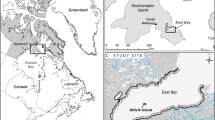
Feeding high in the Arctic marine food web, seabirds act as important sentinels of the health of the environment (Parsons et al. 2008). They are also valued by communities in Inuit Nunangat for both subsistence and traditional cultural practises (e.g., Priest and Usher 2004). Several million seabirds rely on a marine region in the northern Canadian Arctic Archipelago made up of the waterways of Tallurutiup Imanga/Lancaster Sound (Gaston et al. 2012; Fig. 1). Within this region, Prince Leopold Island Migratory Bird Sanctuary (74.01° N, 90.02° W, herein ‘PLI’; Fig. 1) supports the main, multispecies seabird colony, located at the center of the soon-to-be established Tallurutiup Imanga National Marine Conservation Area (Government of Canada 2020). Positioned at the southern boundary of a region identified as the ‘Last Ice Area’, the extent and duration of summer sea ice in the waters around PLI are predicted to outlast ice losses throughout the rest of the Arctic, providing a last refuge for pagophilic (ice-loving) biota as climate change accelerates elsewhere (Loewen and Michel 2018). Despite this predicted relative long-term stability, the region experiences high year-to-year variability and periodic extremes in ice conditions where ice cover during the spring and summer can range from completely ice free to entirely ice bound (Nettleship et al. 1984; Gaston et al. 2005a; Hipfner et al. 2005). Evaluating how seabirds breeding in this region have coped with variable ice conditions through the past will provide a critical baseline for evaluating responses in the future.
Examples of four ice variables from 3 years of contrasting ice conditions around Prince Leopold Island, Nunavut (74.01° N, 90.02° W; identified as ‘PLI’ in d)). Left panel a, d, g depicts ice coverage in the week of 25 June from Canadian Ice Service archival data (Icegraph 2.0, https://iceweb1.cis.ec.gc.ca/IceGraph/) with distance from the colony to open water during the week of 25-Jun (nearest occurrence of < 80% ice cover) as a red dashed line. Middle panel b, e, h depicts satellite imagery for the same week from NASA worldview (worldview.earthdata.nasa.gov/). Right panel c, f, i depicts seasonal change in ice coverage in the region around the colony (equivalent to full extent of left panes) with the 25-Jun identified by a solid red circle and the date of spring break-up (i.e., first week of 50% ice cover) identified by a dashed red circle
The seabird breeding season at PLI is constrained by the ice-free period when access to marine prey is possible (Gaston et al. 2005a, b). Foraging opportunities are dictated by seasonal sea ice conditions through effects of ice on the timing and magnitude of two consecutive pulses of marine autotrophs at the base of the food web (sea-ice algae followed by pelagic phytoplankton) which in turn impact the distribution, abundance, and availability of different types of seabird prey (e.g., Ramírez et al. 2017). Sea ice also influences the seasonal productivity of ice-dependent plankton and fish which can lead to impacts on seabird prey abundance some years later (Provencher et al. 2012, Niemi et al. 2019).
Seabird foraging is further affected by the physical condition of the sea ice within foraging range; years of partial ice cover comprised of fractured flows interspersed with openings provide different foraging opportunities and levels of inter- and intraspecific competition than years with larger expanses of open water (Pratte et al. 2019). In contrast, years of persistent solid ice cover with little to no openings demand longer foraging transits or dietary shifts (Gaston et al. 2005b). Sea ice conditions then can have immediate direct effects on foraging efficiency which in turn can impact breeding timing, propensity, and success. The effects of sea ice on seabird populations can also be delayed in time, either through effects on productivity at lower trophic levels, or through future recruitment of seabird offspring (e.g., Zador et al. 2013, Descamps and Ramírez 2021). For example, if challenging sea ice conditions affect fledging success or fledgling survival, then future recruitment into the population would influence breeding population size at time lags relative to age of sexual maturity.
We studied thick-billed murres (Uria lomvia, herein ‘murres’), black-legged kittiwakes (Rissa tridactyla, ‘kittiwakes’) and northern fulmars (Fulmarus glacialis, ‘fulmars’), all of which occur in thousands on PLI, as well as a smaller number of glaucous gulls (Larus hyperboreus, ‘gulls’; Gaston et al. 2005a). All species breed at PLI under the same broad environmental conditions, but each has unique ecological adaptations for feeding and reproduction. For all, most nutrients transferred to the egg by the female are acquired locally after arrival at PLI (‘income breeders’ (Mallory et al. 2008; Jacobs et al. 2009; Moe et al. 2009). The timing of egg production varies across species, with colony return happening in May for fulmars and gulls and in June for murres and kittiwakes (Gaston et al. 2005b). Clutch size also varies from a single egg for fulmars and murres (Gaston and Hipfner 2020; Mallory et al. 2020), and one to three eggs for gulls and kittiwakes (Hatch et al. 2020; Weiser and Gilchrist 2020). Murres begin to return to the colony as non-breeders after 2 years and breed around 5 to 6 years (Gaston and Hipfner 2020), and fulmars return after 4 years but first breed at 8 to 12 years (Mallory et al. 2020). Kittiwakes and glaucous gulls return to first attempt breeding as early as 4 years but on average at 5 (Gaston et al. 2009; Hatch et al. 2020; Weiser and Gilchrist 2020).
Each species generally forages on prey at a different position in the food web. Fulmars are opportunistic surface feeders, and can travel more than 500 km from the colony in search of lower trophic level prey including small squid, copepods, and polychaete worms (Mallory et al. 2010, 2020). Glaucous gulls are also opportunists, but employ a wide range of foraging strategies to acquire mostly higher trophic level prey including scavenging and depredating the eggs and chicks of murres and kittiwakes, and even adult kittiwakes (Weiser and Gilchrist 2020). They will also surface feed for schooling fish and zooplankton, but all foraging occurs close to the nest site (Weiser and Gilchrist 2020). In contrast, kittiwakes are mainly piscivorous surface feeders, generally accessing mid-trophic level fish prey within 50–150 km (Hatch et al. 2020; AJ Gaston, unpubl. data). The murres also feed on mid-trophic level prey including mainly fish 3 years old and younger as well as macro-zooplankton, but use pursuit-diving to depths up to 150 m, generally within 150 km of the colony (Gaston et al. 2003; Gaston and Hipfner 2020).
In light of these diverse ecologies, combined with prior research at PLI, we expected unique responses from each species to local sea ice conditions. Populations of fulmars, murres, and kittiwakes were monitored at PLI discontinuously since 1975 (Nettleship 1977; Gaston and Nettleship 1981), and glaucous gulls since 2000, but comprehensive assessments of these time series (ending in 2014) have yet to be undertaken. The effect of contrasting sea ice conditions at PLI on these four species was investigated in a series of earlier papers, based on limited runs of years (Gaston et al. 2005a, 2005b; Hipfner et al. 2005). More recent work employing biochemical analyses have investigated the importance of ice-derived resources to egg production for fulmars and murres at PLI across 4 years of contrasting ice conditions (Cusset et al. 2019), and the differing responses in trophic niche of all four species breeding at PLI to variable ice conditions across 3–5 years (Moody et al. 2012; Pratte et al. 2019). Overall, this body of work has indicated that years of greater sea ice cover around the colony (indicated by higher ice concentration, greater distance to open water, and later date of ice clearance) create challenges to foraging and result in delayed nest initiation, decreased colony attendance, and lower nesting success, although the strength of effects varies widely among species.
In this paper, we use an expanded time frame and dataset which encompasses a wide range of ice conditions to assess which relationship is most important for each species, as well as evaluate the potential for changepoints and time-lagged responses. The significant efforts undertaken to generate these datasets have produced the best available baseline monitoring for these species’ populations in the Canadian high Arctic where breeding is uniquely impacted by the local sea ice dynamics. We make use of them to provide further insight into the influence of high interannual variability of sea ice on the breeding biology of a community of high-Arctic seabirds in support of evidence-based policy and decision making in the northern reaches of the Canadian Arctic Ocean ecosystem.
Methods
Monitoring seabird breeding biology
Beginning in 1975, populations of seabirds at PLI, Nunavut, were monitored during the breeding period in years when seasonal, logistic and financial conditions allowed (Mallory et al. 2018). The sample size differed for each species for each of four breeding metrics because not all information was obtained in all years (sample size varied among species and breeding metrics from 8 to 20 years, Table 1). For each of the four species, an index of population size in a given year was derived from standardized, species-specific counts of birds observed in study plots on identifiable nests (kittiwakes and gulls) or on nesting ledges (murres and fulmars) during the timing of peak laying and hatching (‘count’). Median lay date (MLD) of the first egg was estimated as the median of observed laying dates based on onset of incubation or by back-calculating from observed dates of first hatching, for breeding sites within the study plots (Gaston et al. 2005b). For gulls and kittiwakes only, nest success in a given year was estimated based on the median number of chicks fledged per monitored nest (‘NS’). Methods used to monitor each breeding metric were consistent within species and were generally similar across all four species but varied in specifics. Gaston et al. (2005b) provides further methodological details of the long-term seabird population monitoring program.
Sea ice conditions
Four annual sea ice variables in the vicinity of the colony were evaluated for each year between 1970 and 2021 based on weekly regional ice maps from the Barrow Strait to Lancaster Sound area, Eastern Region (Canadian Ice Service, Icegraph 2.0, https://iceweb1.cis.ec.gc.ca/IceGraph/): (1) mean percent ice coverage (between 0 and 100) during May; (2) mean percent ice coverage during June; (3) timing of ice clearance, or date of spring break-up, defined as the ordinal date of 50% ice coverage in spring; and (4) distance from the colony to open water, defined as the distance (in km) to the nearest occurrence of < 80% ice cover in the week of 25-Jun. Ice variables were selected based on review of previous work conducted at PLI, as well as a colony of murres at Coats Island, Nunavut (Gaston et al. 2005a, b, 2012; Cusset et al. 2019; Pratte et al. 2019). We provide examples of ice variables for each of 3 model years exemplifying the wide range of ice conditions which can occur at PLI (Fig. 1). In light of the possibility of time lag effects of ice conditions, the date of spring break-up at time lags of 1 to 5 years was also determined. Lastly, for species with nest success data (kittiwakes and gulls), percent ice coverage during the week of hatch was evaluated for each year (based on MLD + average incubation period of 4 weeks for both species; Hatch et al. 2020; Weiser and Gilchrist 2020).
Trends in breeding metric and sea ice condition time series
All analyses were conducted using the software R (v.3.6.1, R Core Team 2020). Time series for breeding metrics and ice conditions were assessed for the presence and strength of overall trends and for the possibility of temporal changepoints. The ice condition time series covered all years from 1970 to 2021 to capture a 5-year time lag from the earliest breeding data collected to the present. We used non-parametric trend, slope and changepoint test approaches for detecting statistically significant tendencies in environmental time series, including the Mann–Kendall correlation, the Theil–Sen slope, and the Lanzante’s test for shifts in the central tendency of a time series (Kocsis et al. 2020). Trends and slopes were estimated using the ‘mkac’ package (Butler 2017), which determines and adjusts for temporal autocorrelation and can handle incomplete time series (i.e., the breeding metric time series). We tested time series with a minimum of 10 data points for the presence of temporal changepoints using the ‘trend’ package via Lanzante’s test (Pohlert 2020). No significant temporal autocorrelation was detected in any time series. Significant trends and changepoints were identified based on significance levels approaching p ≤ 0.05. Correlation among the four main ice variables was tested with a correlation matrix using the ‘corrplot’ package (Wei and Simko 2017).
Relationships between ice and breeding metrics
To explain variation in breeding metrics relative to ice conditions, single predictor, linear models were fitted between the breeding metric response and relevant ice condition predictors, using the R base function ‘lm’. For each breeding metric, the number of biologically relevant models in each candidate set varied from five to seven including time lags (Table 2). High collinearity among ice variables precluded incorporating more than a single ice variable in each model. Assumptions of normality and homoscedasticity were checked using diagnostic plots of fitted and observed values and residuals. Relative model fit to the data within each candidate set were compared via differences in sample-size-corrected AIC values (ΔAICc, where the best-fit model has the lowest AICc value), as well as relative Akaike model weights (wi, which sum to 1 and provide an index of support for each model relative to the total candidate model set; Burnham and Anderson 2002). Values for differences in AICc values (ΔAICc) were computed by subtracting minimum AICc from all candidate model AICc such that the model with the lowest ΔAICc was considered the best fit and models with ΔAICc < 2 were considered similar in their ability to describe the data (Burnham and Anderson 2002). Thus, top models with ΔAICc < 2 were further examined for the strength of relationships based on relative model weight (wi), R2 to estimate the amount of variance in the breeding metric explained by the ice variable, and slope coefficient estimates to evaluate the magnitude of directional relationships (95% confidence intervals bounding zero were considered insignificant effects). We tested each dataset with a minimum of 10 data points for the presence of segmented threshold effects using the ‘chngpt.test’ function in the R package ‘chngpt’ (Fong et al. 2017). If a significant changepoint was detected, we used linear models for data split before and after the changepoint to evaluate the strength of the segmented relationships. Means are reported ± one standard deviation (SD).
Results
Variation and trends in ice conditions, timing of breeding, counts, and nest success over time
Ice conditions around the colony at PLI exhibited no significant overall temporal trends or changepoints but were highly variable over the full time series from 1970 to 2021 (Fig. 2). Ice cover in May was > 80% in 38 of 52 years, and ranged from a low of 31% (in 1976) to 100% (in 6 years), with an average of 84 ± 16% (Fig. 2). Ice cover in June varied more widely, ranging from a low of 16% (in 1976) to 100% (in 5 years), with an average of 63 ± 25% (Fig. 2). The date of spring ice break-up was on average 29-Jun ± 29 days, with the earliest break-ups occurring on 08-May in 1974 and 1976 and the latest occurrence on 27-Aug in 1978. The average distance from the colony to open water on 25-Jun was 47 ± 77 km, with open water at the colony in 32 of the past 52 years. Open water was > 50 km from the colony in 16 years, and > 150 km from the colony in 9 years.
Time series of four annual ice variables representative of sea ice conditions in the waters surrounding Prince Leopold Island, Nunavut, during the seabird breeding season between 1970 and 2021. Variables were derived from data archived by the Canadian Ice Service for the Barrow Strait–Lancaster Sound Eastern region. No significant trends or changepoints were detected. The line and shaded areas depict a LOESS smoother and associated 95% confidence interval
Counts of fulmars on ledges in study plots varied by 142 birds between 1976 and 2014 (n = 10 years, 299 ± 53, 225–367, Fig. 3) and no temporal trends were detected. For glaucous gulls, the total number of active nests in the colony varied by 11 nesting pairs between 2000 and 2013 (n = 9, 20 ± 4, 15–26, Fig. 3). Of those nests, nest success varied by 1.14 chicks fledged per monitored nest (0.82 ± 0.40, 0.33–1.47, Fig. 3). Gull counts and nest success did not exhibit any detectable temporal trends. Counts of kittiwakes on nests within study plots varied by 318 between 1975 and 2012 (n = 10 years, 359 ± 122, 225–543) and significantly increased over time (Mann–Kendall Z = 2.95, p < 0.01) at a rate of 7.9 birds per year (Fig. 3). A changepoint was detected at 1988 (Wilcoxon–Mann–Whitney U = 0, p < 0.001) whereby the rate of increase accelerated to 54 birds per year. For years where kittiwake nest fates could be determined, nest success varied by 0.96 chicks fledged per monitored nest (n = 8 years, 0.77 ± 0.29, 0.38–1.34, Fig. 3). For murres, counts of birds occupying nesting ledges in study plots varied by 557 between 1976 and 2013 (n = 16 years, 1198 ± 159, 849–1406), steadily increasing over time (Mann–Kendall Z = 3.24, p < 0.001) at a rate of 10 birds per year (Fig. 3).
Change in Median Laying Date (MLD; first column a, c, f, i, day of year), counts (second column b, d, j, g) and nest success (third column e, h) over time for four species of breeding seabirds (species by row; northern fulmar Fulmarus glacialis (a, b), glaucous gull Larus hyperboreus (c–e), black-legged kittiwake Rissa tridactyla (f–h) and thick-billed murre Uria lomvia (i, j)) at Prince Leopold Island, Nunavut between 1975 and 2014. The line and shaded areas represent the linear trend or LOESS smoother (where significant linear trends were not detected) and associated 95% confidence interval. Trends were not evaluated over data gaps of 15 years of more (e.g., kittiwake MLD (f))
Estimated MLD were earliest and varied least for fulmars, ranging 8 days between 1975 and 2009 (n = 11 years, 11-Jun ± 2.0 days, 8-Jun–16-Jun, Fig. 3). Glaucous gulls also nested in June with little variation in MLD, varying by 10 days between the earliest and latest recorded MLD between 1976 and 2014 (n = 11 years, 12-Jun ± 3.6 days, 9-Jun–19-Jun, Fig. 3). Kittiwakes and murres initiated nesting on average 3 weeks later than gulls and fulmars (kittiwakes: n = 11, 3-Jul ± 4.9 days, 27-Jun–12-Jul, 1976–2014; murres: n = 20, 4-Jul ± 6.0 days, 25-Jun–20-Jul, 1975–2014; Fig. 3). Murres varied most in MLD, with dates varying by 25 days between 1975 and 2014. No temporal trends were detected in MLD for any species.
Sea ice and seabird breeding metrics
Correlation coefficients among the four main ice variables were highest between mean June ice cover and date of spring ice break-up (0.93), and between mean June ice cover and distance to open water in the week of 25-Jun (0.81). Mean May ice cover was also highly correlated with the date of ice break-up (0.79). Thus, we did not include mean May or June ice cover in our models as they were well captured by ice break-up and distance to open water whereby years of high ice cover in May or June have later spring ice break-up and further distances between the colony and open water. We did retain both ice break-up and distance to open water in models to explain temporal variation in breeding metrics, despite being correlated (0.76), as each of these ice variables corresponds to distinct temporal and spatial aspects of ice conditions that differ in how they may influence each species. Our assessment of species-specific responses between each of three breeding metrics and relevant ice condition predictors revealed at least one significant relationship for three species (all but glaucous gulls), of varying strength (Table 2).
Fulmar counts declined with increasing distance to open water, with this model garnering 45% of support among seven candidate models and explaining 41% of the variance in counts (slope estimate = − 0.4 ± 0.17 SE, 95% CI [− 0.8, − 0.01]; Fig. 4, Table 2). The only significant lag effects detected for any species were for kittiwake nest counts where the number of nests was best explained (> 99% of model support from a candidate set of seven; Table 2) by a 5-year lag effect of the date of spring ice break-up. Kittiwake counts exhibited a strong response to ice break-up five seasons earlier whereby counts in year t decreased with later ice break-up in year t-5 (R2 = 0.77, slope estimate = − 3.11 ± 0.6 SE, 95% CI [− 4.49, − 1.73]; Fig. 4, Table 2).
Breeding–ice relationships from top-ranked models for seabird species breeding at Prince Leopold Island, Nunavut (northern fulmar Fulmarus glacialis (a), black-legged kittiwake Rissa tridactyla (b, c) and thick-billed murre Uria lomvia (d, e)). Breeding metrics include counts of birds observed in study plots on identifiable nests (kittiwakes, c) or on nesting ledges (fulmars, a), and median laying date (MLD as Day of Year) estimated as median date of start of incubation (b, d, e). The line and shaded areas represent the estimated trajectory from linear regression and associated 95% confidence interval. Models where significant breakpoints were detected are shown as linear models on either side of a threshold. Full model results indicated in bold in Table 2
Kittiwakes laid eggs later in years with increased distance to open water, with the majority (84%) of model weight supporting this model from the candidate set of five, explaining 53% of the variance in MLD (slope estimate = 0.05 ± 0.01 SE, 95% CI [0.01, 0.08]; Fig. 4, Table 2). Like kittiwakes, murre MLD also became later with increasing distance to open water (67% of model support, R2 = 0.62, slope estimate = 0.05 ± 0.009 SE, 95% CI [0.03, 0.07]; Fig. 4, Table 2) and with increasing date of spring ice break-up (the remaining 33% of model support, R2 = 0.59, slope estimate = 0.15 ± 0.03 SE, 95% CI [0.09, 0.21]; Fig. 4, Table 2).
Significant changepoints were detected in the relationships between murre MLD with distance to open water (maximal statistic = 7.6, p < 0.05) and spring break-up (maximal statistic = 12.3, p < 0.05). Egg-laying by murres became gradually later until a break-up date of 30-Jul (± 14 d SE; R2 = 0.38, slope estimate = 0.09 ± 0.03 SE, 95% CI [0.03, 0.15]) and distance to open water of 210 km (± 40 km SE; R2 = 0.27, slope estimate = 0.03 ± 0.01 SE, 95% CI [0.01, 0.05]), after which laying delayed at a more rapid rate for both spring break-up (R2 = 0.74, slope estimate = 0.58 ± 0.14 SE, 95% CI [0.23, 0.91]) and distance to open water (R2 = 0.75, slope estimate = 0.24 ± 0.09 SE, 95% CI [0.18, 0.65]; Fig. 4). All remaining candidate model sets had top ranking models with poor predictive relationships (Table 2).
Discussion
Our work features a comprehensive long-term, multispecies and multi-metric investigation of seabird responses to annual variation in sea ice in a high-latitude polar environment. Most previous investigations of seabird breeding or foraging at PLI have been limited to 3 to 5 years of contrasting ice conditions characterized by a relatively limited number of ice variables (Gaston et al. 2005a; Hipfner et al. 2005; Moody et al. 2012; Cusset et al. 2019; Pratte et al. 2019). Our study tests how the previously identified relationships stand up in a larger time series of seabird monitoring and ice conditions characterized by multiple ecologically relevant ice variables. Importantly, the region around our study colony has yet to experience consistent directional loss of sea ice during the breeding period, in contrast with other Arctic locations (e.g., Svalbard, Descamps and Ramirez 2021; Alaska, Divoky et al. 2021), thus confirming the present and future importance of the Tallurutiup Imanga MPA for seabirds in the high Arctic.
Sea ice and population size
Population size was only associated with sea ice conditions in the current year for fulmars, where counts of birds attending the colony were slightly reduced by increasing distance to open water and the edge of the ice pack. Fulmars at higher latitudes generally take a longer pre-laying exodus and spend less time at the colony before egg laying than those at more southern latitudes (due to heavier ice cover and less food availability; Mallory and Forbes 2007, Mallory et al. 2020), presumably requiring this extra time to locate sufficient resources to form the egg (female) or store reserves for the first, very long incubation shift (male; Mallory et al. 2008). These resources can be very distant and highly limited early in the season, and thus prey availability is likely reduced or delayed in years with larger expanses of solid ice between the colony and open water where fulmars forage (Welch et al. 1992). At a high-latitude fulmar colony in Greenland, egg neglect occurred in 18% of nests (Falk and Møller 1997), a phenomenon likely to increase in response to greater travel distances between the colony and foraging areas. Since we recorded fulmar colony counts during incubation, lower numbers in heavier ice years likely represents a combination of lower attendance by pre-breeding birds, fewer birds initiating nesting because they could not acquire resources to breed, and perhaps birds abandoning nesting attempts prior to our counts because marine food resources were insufficient to sustain their condition during incubation (Mallory et al. 2020). Despite the relatively low proportion of variance in fulmar counts explained by distance to open water, this relationship is likely still ecologically important. As long-lived, K-selected species (e.g., Wooller et al. 1992), fulmars should forego or abandon breeding in “poor” years to maximize adult survival for future breeding attempts (Brooke 2004).
Kittiwakes, on the other hand, did not exhibit an association between nest counts and sea ice conditions in the current year. In some parts of the range, this species nests even when conditions are not favorable. In Shetland, UK, kittiwakes bred in years of poor food supply rather than forego breeding to benefit their own survival at the cost of not rearing chicks, given years of lower food availability were associated with reduced adult survival (Oro and Furness 2002). Adult kittiwakes possess behavioral plasticity in response to interannual variation in prey composition, distribution and abundance, by shifting to forage at lower trophic levels in years of high ice cover at PLI (Pratte et al. 2019), or by increasing foraging effort and prey-switching in Alaska without negative impacts on reproductive success (Suryan et al. 2000). In contrast, kittiwake population sizes in some colonies in Svalbard, Norway, showed a weak positive association with ice cover at a two-year time lag, where kittiwake numbers are reduced when less ice results in lower food availability in later breeding seasons (Descamps and Ramirez 2021), but the extent of sea ice around Svalbard, ranging only from 15 to 40%, is very different from the situation at PLI. Together these past studies highlight that seabird–ice relationships can vary between colonies of the same species, where prey dynamics and foraging adaptations are shaped by local drivers. Adult kittiwakes at PLI are capable of successful nesting attempts under varying ice conditions, but a lagged response of nest counts to late spring break-up (and associated high ice cover later into the breeding season) 5 years prior also suggests that inexperienced fledglings may not cope as well with challenging foraging conditions. Given the average age of first breeding in kittiwakes is 5 years (Suryan et al. 2000), the strength of the lagged response indicates that hatch-year sea ice plays an important role in recruitment of kittiwakes to the breeding population at PLI, where natal philopatry may be quite high (Suryan and Irons 2001).
Unlike Gaston et al. (2005b), we did not find any significant effects of ice on counts of murres on nesting ledges, perhaps because our 16-year time series encompassed a broader spectrum of fluctuating ice dynamics rather than 4 years of contrasting conditions, or because it was much more affected by the positive regional population trend. Murres possess an even greater ability than kittiwakes to adopt a variety of diets under extreme high and low ice conditions, as they can dive to feed on both ice-associated prey (e.g., Arctic cod Boreogadus saida; Bradstreet 1980), and prey in open waters (e.g., boreo-Atlantic armhook squid Gonatus fabricii; Gaston and Hipfner 2020). This dietary flexibility is also supported by biochemical analyses (Cusset et al. 2019; Pratte et al. 2019).
Like kittiwakes, murres breeding in areas with generally lower ice cover show differing degrees of adaptability in response to interannual variation in foraging conditions (e.g., Provencher et al. 2012; Descamps and Ramirez 2021), once again highlighting that seabird-prey dynamics are regionally-specific. As suggested by Gaston et al. (2005a), we expect that continued global warming may benefit murres at PLI in the short term, as the availability of open water during the early breeding season in the high Arctic increases. At the same time, climate amelioration may result in an eventual northward displacement of populations from farther south (Frederiksen et al. 2013; Boertmann et al. 2020; Patterson et al. 2021). The steady growth we report in the murre breeding population at PLI since 1975 does not appear to be related to ice given the lack of consistent directional change in ice conditions around the colony, and warrants further study to determine its cause.
Glaucous gull nest counts, as well as timing of breeding and nest success, were not responsive to ice conditions. As generalist predators, these gulls are considerably less ice-reliant than their sympatrics at PLI (Gauthier et al. 2015). They likely scavenge terrestrial resources and forage along leads and coastlines early in the season to form their eggs and sustain incubation. By the time their chicks hatch in early July, there are abundant kittiwake and murre eggs available (Weiser and Gilchrist 2020). Gulls at PLI are able to maintain a high trophic position regardless of sea ice conditions, even in years of late ice break-up (Pratte et al. 2019). Bearing in mind that the relatively small population of glaucous gulls at PLI has been monitored for less than 10 total breeding seasons, the lack of association between breeding metrics and ice conditions could reflect the small sample sizes and limited variance in breeding metrics, or the highly flexible nature of this species in an unpredictable environment.
Sea ice and breeding phenology
At a global scale, sea surface temperatures in waters around seabird colonies in the pre-breeding period significantly increased between 1952 and 2015 (Keogan et al. 2018), and an overall advancement of spring ice break-up around Arctic seabird colonies has occurred over the same period (Descamps et al. 2019). Yet, seabirds at high northern latitudes, and diving species in particular, have shown little phenological change (Descamps et al. 2019). Although colonies at high latitudes tend to have less between-year variance in timing of breeding than those at lower latitudes (Keogan et al. 2018), some Arctic surface-feeding species in the Pacific (where spring onset has advanced most rapidly) have shown a strong phenological response to the advancement of spring (Descamps et al. 2019).
On the other hand, our findings support the contention that both kittiwakes (range of c. 2 weeks), and murres (range of c. 4 weeks) breeding at PLI vary their phenology in response to variation in ice conditions (Gaston et al. 2005a, b). For murres especially, the timing of nest initiation was highly responsive to ice around the colony; linear relationships and breakpoints clearly indicate that years of heavy ice conditions have the greatest impact on timing of breeding. Given that kittiwakes are obligate surface feeders (Hatch et al. 2020), a delay in breeding associated with late ice break-up is predictable, as the availability of prey would likely be higher and earlier in years with earlier ice retreat and warmer sea surface temperatures (Welch et al. 1992; Yamamoto et al. 2016). Satterthwaite et al. (2012) found that kittiwakes in Alaska had higher stress levels in cold years compared to warm years, likely as a result of food stress. Moreover, delayed breeding can reduce kittiwake clutch size and overall reproductive success (Moe et al. 2009). Indeed, Gaston et al. (2005b) also reported decreased nest success with higher distance to open water for kittiwakes at PLI based on 4 years of varying ice cover. We did not detect an association between kittiwake nest success and ice conditions over 9 years of monitoring, despite detecting an association with nesting phenology. These conflicting results may be due to the limited number of years where kittiwake nests have been monitored for fledging success, or perhaps because adult kittiwakes at PLI can tolerate highly variable foraging conditions, so that breeding outcomes are not tightly linked with the impacts of sea ice on their prey.
Murres are also known to be sensitive to ice cover and resultant access to prey in their timing of breeding (Gaston et al. 2005a, b). Delays in nest initiation can reduce adult mass and chick growth during the breeding season, which can result in reduced reproductive success (Gaston et al. 2005b). Hipfner et al. (2005) showed that murres breeding at PLI laid smaller eggs in years when laying was delayed, whereas those breeding at a low Arctic colony did not and suggested that murres breeding in more variable environments display a greater variability in life history traits. This flexibility may prove beneficial in the face of climate warming in the Arctic, if earlier ice clearance and decreasing ice cover during breeding eventually lead to earlier and more successful breeding attempts. However, we have only documented responses of breeding seabirds to the range of ice conditions experienced over the past 50 years, while the acceleration of impacts from climate change are sure to bring fundamental shifts to this ice-dominated ecosystem beyond the conditions we have measured (e.g., Post et al. 2013).
While kittiwakes and murres showed high flexibility in breeding phenology, glaucous gulls and especially fulmars did not. At this extreme limit of their range, fulmars have already adapted to breed early, adjust their breeding schedule, and travel long distances to open water to forage, and, as we have shown, exhibit less variation in reproductive metrics with differences in sea ice conditions than other seabird species (Mallory and Forbes 2007). Like all Procellariiformes, fulmars have a long breeding period (May to September at PLI) as a result of long incubation (~ 48 d) and chick-rearing (~ 53 d) times (Mallory et al. 2020), thus may have less plasticity in nest initiation date to complete breeding in time for fall migration and for the nestlings to be able to depart before becoming snowed-in (Brooke 2004; Mallory and Forbes 2007).
The future of seabirds and sea ice at Prince Leopold Island
Collectively, the seabirds at PLI show behavioral plasticity to cope with unpredictable conditions. With earlier spring break-up and increasing pockets of open water created by ongoing climate change, behavioral plasticity will benefit the seabird community breeding at PLI, particularly murres (Gaston et al. 2005a; Hipfner et al. 2005), at least in the short-term, by reducing the frequency of extreme high ice years and their impact on breeding. Indeed, our results show that murre and kittiwake populations at PLI have grown over the past 40 years.
Ultimately, the species-specific combination of foraging strategy, degree of reliance on ice-associated food, and natural history traits will dictate how flexible each species will be to unpredictable ice conditions in a given year, and how each species may be able to adapt to an eventual climate-change-driven shift toward reduced ice cover around the colony.
The variable responses by breeding seabirds to sea ice extent and the acceleration of shifting ice conditions in the Arctic (Perry et al. 2005; Hazen et al. 2013) have substantial implications for conservation, notably for marine protected areas (MPAs). As the distribution of both seabirds and their prey shift with future ice changes, it is essential to understand how species use MPAs both now and under future climate conditions (Davies et al. 2017). As ocean temperatures continue to increase, seabird distributions are projected to shift northward (Frederiksen et al. 2013; Boertmann et al. 2020; Patterson et al. 2021). This highlights the importance of MPAs such as Tallurutiup Imanga that protect northern areas, but also long-term monitoring studies like ours that can inform future MPA networks (Carr et al. 2017). Further, as seabird populations from more southern regions shift northward, colonies like PLI will represent the core of the breeding range for Arctic seabirds and may become one of their last refugia. This reinforces the significance of the Tallurutiup Imanga MPA, but also the importance of science-informed adaptive management strategies to ensure MPAs can remain effective at protecting the species and ecosystems they were originally designed to preserve (Hopkins et al. 2016; Carr et al. 2017). Long-term monitoring programs, in conjunction with MPAs that protect species as their distributions change, are essential to understand and ensure the preservation of these ecologically and culturally significant Arctic ecosystems under a changing climate (Carr et al. 2017).
Data and code availability statement
The data supporting the results and custom code will be readily available from the lead author on reasonable request.
References
AMAP (2021) Arctic Climate Change Update 2021: Key Trends and Impacts Summary for Policy- makers. Arctic Monitoring and Assessment Programme (AMAP). Tromsø, Norway, p 16
Arrigo KR, van Dijken G, Pabi S (2008) Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett. https://doi.org/10.1029/2008GL035028
Boertmann D, Merkel F, Gilg O (2020) Seabird breeding colonies in East and North Greenland: a baseline. Arctic 73:20–39
Bradstreet MSW (1980) Thick-billed murres and black guillemots in the Barrow Strait area, N.W.T., during spring: diets and food availability along ice edges. Can J Zool 58:2120–2140. https://doi.org/10.1139/z80-292
Brooke ML (2004) Albatrosses and Petrels across the world. Oxford University Press, Oxford
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin
Bush E, Lemmen D (2019) Canada’ s changing climate report. Ontario, Ottawa
Butler K (2017) mac: Mann-Kendall correlation and Theil-Sen slope for possibly autocorrelated time series. R Package Version 1:1
Carr MH, Robinson SP, Wahle C et al (2017) The central importance of ecological spatial connectivity to effective coastal marine protected areas and to meeting the challenges of climate change in the marine environment. Aquat Conserv Mar Freshw Ecosyst 27:6–29. https://doi.org/10.1002/aqc.2800
Cusset F, Fort J, Mallory M et al (2019) Arctic seabirds and shrinking sea ice: egg analyses reveal the importance of ice-derived resources. Sci Rep 9:1–15. https://doi.org/10.1038/s41598-019-51788-4
Davies TE, Maxwell SM, Kaschner K et al (2017) Large marine protected areas represent biodiversity now and under climate change. Sci Rep 7:9569. https://doi.org/10.1038/s41598-017-08758-5
Descamps S, Ramírez F (2021) Species and spatial variation in the effects of sea ice on Arctic seabird populations. Divers Distrib 27:2204–2217. https://doi.org/10.1111/ddi.13389
Descamps S, Ramírez F, Benjaminsen S et al (2019) Diverging phenological responses of Arctic seabirds to an earlier spring. Glob Chang Biol 25:4081–4091. https://doi.org/10.1111/gcb.14780
Divoky GJ, Brown E, Elliott KH (2021) Reduced seasonal sea ice and increased sea surface temperature change prey and foraging behaviour in an ice-obligate Arctic seabird, Mandt’s black guillemot (Cepphus grylle mandtii). Polar Biol 44(4):701–715
Falk K, Møller S (1997) Breeding ecology of the Fulmar Fulmarus glacialis and the Kittiwake Rissa tridactyla in high-arctic northeastern Greenland, 1993. Ibis (Lond 1859) 139:270–281. https://doi.org/10.1111/j.1474-919x.1997.tb04625.x
Fong Y, Huang Y, Gilbert P, Permar S (2017) chngpt: threshold regression model estimation and inference. BMC Bioinform 18:454. https://doi.org/10.1186/s12859-017-1863-x
Frederiksen M, Anker-Nilssen T, Beaugrand G, Wanless S (2013) Climate, copepods and seabirds in the boreal Northeast Atlantic - current state and future outlook. Glob Chang Biol 19:364–372. https://doi.org/10.1111/gcb.12072
Gaston AJ, Hipfner JM (2020) Thick-billed Murre (Uria lomvia). In: Billerman SM (ed) Birds of the world. Cornell Lab of Ornithology
Gaston AJ, Nettleship DN (1981) The Thick-billed Murres of Prince Leopold Island: a study of the breeding ecology of a colonial High Arctic seabird. Monograph Series No. 6. Canadian Wildlife Service, Ottawa, p 350
Gaston AJ, Woo K, Hipfner JM (2003) Trends in forage fish populations in northern hudson bay since 1981, as determined from the diet of nestling thick-billed murres Uria lomvia. Arctic 56:227–233. https://doi.org/10.14430/arctic618
Gaston AJ, Gilchrist HG, Hipfner JM et al (2005a) Climate change, ice conditions and reproduction in an Arctic nesting marine bird: Brunnich’s guillemot (Uria lomvia L.). J Anim Ecol 74:832–841. https://doi.org/10.1111/j.1365-2656.2005.00982.x
Gaston AJ, Gilchrist HG, Mallory ML (2005b) Variation in ice conditions has strong effects on the breeding of marine birds at Prince Leopold Island, Nunavut. Ecography 28:331–344
Gaston AJ, Gilchrist HG, Mallory ML, Smith PA (2009) Changes in seasonal events, peak food availability, and consequent breeding adjustment in a marine bird: a case of progressive mismatching. Condor 111(1):111–119
Gaston AJ, Smith PA, Provencher JF (2012) Discontinuous change in ice cover in Hudson Bay in the 1990s and some consequences for marine birds and their prey. ICES J Mar Sci 69:1218–1225
Gauthier G, Legagneux P, Valiquette MA et al (2015) Diet and reproductive success of an Arctic generalist predator: Interplay between variations in prey abundance, nest site location, and intraguild predation. Auk 132:735–747. https://doi.org/10.1642/AUK-14-273.1
Government of Canada. 2020. Tallurutiup Imanga National Marine Conservation Area Inuit Impact and Benefit Agreement. Available from https://www.pc.gc.ca/en/amnc-nmca/cnamnc-cnnmca/tallurutiup-imanga [Accessed 27 April 2020].
Hatch SA, Robertson GJ, Baird PH (2020) Black-legged Kittiwake (Rissa tridactyla). In: Billerman SM (ed) Birds of the World. Cornell Lab of Ornithology
Hazen EL, Jorgensen S, Rykaczewski RR et al (2013) Predicted habitat shifts of Pacific top predators in a changing climate. Nat Clim Chang 3:234–238. https://doi.org/10.1038/nclimate1686
Hipfner JM, Gaston AJ, Gilchrist HG (2005) Variation in egg size and laying date in thick-billed murre populations breeding in the Low Arctic and High Arctic. Condor 107:657–664
Hopkins CR, Bailey DM, Potts T (2016) Perceptions of practitioners: managing marine protected areas for climate change resilience. Ocean Coast Manag 128:18–28. https://doi.org/10.1016/j.ocecoaman.2016.04.014
Jacobs SR, Elliott KH, Gaston AJ, Weber JM (2009) Fatty acid signatures of female Brünnich’s guillemots Uria lomvia suggests reliance on local prey for replacement egg production. J Avian Biol 40:327–336. https://doi.org/10.1111/j.1600-048X.2008.04625.x
Keogan K, Daunt F, Wanless S et al (2018) Global phenological insensitivity to shifting ocean temperatures among seabirds. Nat Clim Chang 8:313–317. https://doi.org/10.1038/s41558-018-0115-z
Kocsis T, Kovács-Székely I, Anda A (2020) Homogeneity tests and non-parametric analyses of tendencies in precipitation time series in Keszthely, Western Hungary. Theor Appl Climatol 139:849–859. https://doi.org/10.1007/s00704-019-03014-4
Loewen TN, Michel C (2018). In: Proceedings of the Multidisciplinary Arctic Program (MAP)—Last Ice: Science Planning Workshop, January 16–17, 2018. Winnipeg, Canada
Mallory ML, Forbes MR (2007) ) Does sea ice constrain the breeding schedules of High Arctic northern fulmars? Condor 109:894–906
Mallory ML, Forbes MR, Ankney CD, Alisauskas RT (2008) Nutrient dynamics and constraints on the pre-laying exodus of High Arctic northern fulmars. Aquat Biol 4:211–223. https://doi.org/10.3354/ab00113
Mallory ML, Karnovsky NJ, Gaston AJ et al (2010) Temporal and spatial patterns in the diet of northern fulmars Fulmarus glacialis in the Canadian High Arctic. Aquatic Biol 10:181–191. https://doi.org/10.3354/ab00277
Mallory ML, Gilchrist HG, Janssen M et al (2018) Financial costs of conducting science in the Arctic : examples from seabird research. Arct Sci 633:624–633
Mallory ML, Hatch SA, Nettleship DN (2020) Northern Fulmar (Fulmarus glacialis). In: Billerman SM (ed) Birds of the World. Cornell Lab of Ornithology
Moe B, Stempniewicz L, Jakubas D et al (2009) Climate change and phenological responses of two seabird species breeding in the high-Arctic. Mar Ecol Prog Ser 393:235–246. https://doi.org/10.3354/meps08222
Moody AT, Hobson KA, Gaston AJ (2012) High-arctic seabird trophic variation revealed through long-term isotopic monitoring. J Ornithol 153:1067–1078. https://doi.org/10.1007/s10336-012-0836-0
Nettleship DN (1977) Studies of seabirds at Prince Leopold Island and vicinity, Northwest Territories: Preliminary report of biological investigations in 1975. Fisheries & Environment Canada. Canadian Wildlife Service
Nettleship DN, Birkhead TR, Gaston AJ (1984) Breeding of Arctic seabirds in unusual ice years: the thick-billed murre Uria lomvia in 1978. Bedford Inst Oceanogr Rev 84:35–38
Niemi A, Ferguson S, Hedges K et al (2019) State of Canada’s Arctic Seas. Canadian Technical Report of Fisheries and Aquatic Sciences 3344. Ottawa, Canada
Oro D, Furness RW (2002) Influençes of food availability and predation on survival of kittiwakes. Ecology 83:2516–2528. https://doi.org/10.2307/3071811
Parsons M, Mitchell I, Butler A, Ratcliffe N, Frederiksen M, Foster S, Reid JB (2008) Seabirds as indicators of the marine environment. ICES J Marine Sci 65:1520–1526. https://doi.org/10.1093/icesjms/fsn155
Patterson A, Gilchrist HG, Gaston A, Elliott KH (2021) Northwest range shifts and shorter wintering period of an Arctic seabird in response to four decades of changing ocean climate. Mar Ecol Prog Ser 679:163–179. https://doi.org/10.3354/meps13890
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. https://doi.org/10.1126/science.1111322
Pohlert T (2020) Trend: Non-parametric trend tests and change-point detection. R package version 1.1.4. https://CRAN.R-project.org/package=trend
Post E, Bhatt US, Bitz CM, Brodie JF, Fulton TL, Hebblewhite M, Kerby J, Kutz SJ, Stirling I, Walker DA (2013) Ecological consequences of sea-ice decline. Science 341:519–524
Pratte I, Braune BM, Hobson KA, Mallory ML (2019) Variable sea-ice conditions influence trophic dynamics in an Arctic community of marine top predators. Ecol Evol 9:7639–7651. https://doi.org/10.1002/ece3.5313
Priest H, Usher PJ (2004) The Nunavut Wildlife Harvest Study, August 2004. Nunavut Wildlife Management Board, Iqaluit, NU, p 814
Provencher JF, Gaston AJ, O’Hara PD, Gilchrist HG (2012) Seabird diet indicates changing Arctic marine communities in eastern Canada. Mar Ecol Prog Ser 454:171–182. https://doi.org/10.3354/meps09299
Ramírez F, Tarroux A, Hovinen J et al (2017) Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. Sci Rep 7:4500. https://doi.org/10.1038/s41598-017-04775-6
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Satterthwaite WH, Kitaysky AS, Mangel M (2012) Linking climate variability, productivity and stress to demography in a long-lived seabird. Mar Ecol Prog Ser 454:221–235. https://doi.org/10.3354/meps09539
Suryan RM, Irons DB (2001) Colony and population dynamics of black-legged kittiwakes in a heterogeneous environment. Auk 118:636–649
Suryan RM, Irons DB, Benson J (2000) Prey switching and variable foraging strategies of black-legged kittiwakes and the effect on reproductive success. Condor 102:374–384
Wei T, Simko V (2017) Corrplot: visualization of a correlation matrix. R package version 0.84.
Weiser E, Gilchrist HG (2020) Glaucous Gull (Larus hyperboreus). In: Billerman SM (ed) Birds of the World. Cornell Lab of Ornithology
Welch HE, Bergmann MA, Siferd TD et al (1992) Energy flow through the marine ecosystem of the Lancaster Sound region, arctic Canada. Arctic 45:343–357
Wooller RD, Bradley JS, Croxall JP (1992) Long-term population studies of seabirds. Trends Ecol Evol 7:111–114
Yamamoto T, Kokubun N, Kikuchi DM et al (2016) Differential responses of seabirds to environmental variability over 2 years in the continental shelf and oceanic habitats of southeastern Bering Sea. Biogeosciences 13:2405–2414. https://doi.org/10.5194/bg-13-2405-2016
Zador S, Hunt G, TenBrink T, Aydin K (2013) Combined seabird indices show lagged relationships between environmental conditions and breeding activity. Mar Ecol Prog Ser 485:245–258. https://doi.org/10.3354/meps10336
Acknowledgements
David Nettleship originally designed and supervised biological work at Prince Leopold Island and organized follow-up work in 1988. We are very grateful to all those who have helped to collect these data over the past 40 years. Special thanks to Christine Eberl for arranging supplies and shipping and for acting as our southern agent, to Myra Robertson for handling permits, and to the Polar Continental Shelf Project of Natural Resources Canada and the Nunavut Research Institute for logistical support.
Funding
Financial support was provided by Environment Canada-Canadian Wildlife Service and the Northern Ecosystem Initiative, Natural Sciences and Engineering Research Council of Canada, the Northern Studies Trust program, and the W. Garfield Weston Foundation. SEG was supported by a Weston Family Award in Northern Research Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Contributions
AJG and MLM originally formulated the idea, AJG developed the methodology, AJG and MLM conducted the fieldwork, SEG curated and analyzed the data, SEG, JB and MLM wrote the manuscript and AJG provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
All applicable institutional and national guidelines for the care and use of animals were followed, including all appropriate annual permits (e.g., Government of Nunavut Research License WL 2012-049, Canadian Wildlife Service permits NUN-SCI-12-04, NUN-MBS-12-03, Animal Care EC-PN = 12-020).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Thomas Koert Lameris.
Rights and permissions
About this article
Cite this article
Gutowsky, S.E., Baak, J.E., Gaston, A.J. et al. Sea ice extent and phenology influence breeding of high-Arctic seabirds: 4 decades of monitoring in Nunavut, Canada. Oecologia 198, 393–406 (2022). https://doi.org/10.1007/s00442-022-05117-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05117-8